Abstract
Xenotransplantation is considered to be a solution for the human donor shortage. However, there is a potential risk of transmitting animal infections from the transplanted organ. The known transmissibility and clinical significance of human cytomegalovirus (HCMV) infection after allotransplantation led us to evaluate whether baboon cytomegalovirus (BCMV) transmission could occur after a baboon-to-human liver xenotransplant. We examined serial blood samples from a baboon liver recipient and isolated replication-competent CMV-like agents on days 29, 36, and 42 after xenotransplantation. BCMV and HCMV DNAs were detected in the day 29 isolate, while only HCMV DNA was detected in the other isolates. This is the first report of detecting a replication-competent virus from a source animal after xenotransplantation and is a concern with regard to potential zoonotic transmission to others.
Solid organ transplantation is an established treatment for a number of end-stage organ disorders. However, its use is limited by the number of available human donors. This shortage has been a key impetus for the use of organs from animals, including baboons, for xenotransplantation (3, 10). A major concern with xenotransplantation is that infectious organisms can be transmitted from the animal donor to the recipient of the organ. This is particularly important for cytomegalovirus (CMV), since transmission of human CMV (HCMV) after allotransplantation can lead to serious disease (2, 4). While CMV is considered to be species specific, we previously showed that baboon CMV (BCMV) can replicate on human fibroblasts in vitro (6). In addition, there is a high seroprevalence of BCMV in adult baboon populations (7, 12). Accordingly, we investigated whether BCMV could be transmitted via baboon-to-human liver xenotransplantation.
(This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26–29 September 1999).
MATERIALS AND METHODS
A 35-year-old man with end-stage liver disease secondary to infection with hepatitis B virus received a baboon liver xenotransplant as previously described (9). The patient was known to be infected with human immunodeficiency virus and had undergone a splenectomy following a motor vehicle accident. The patient was seropositive for HCMV before transplantation but did not have active HCMV disease. He received a liver from a baboon that was seropositive for BCMV. Prophylaxis against HCMV consisted of ganciclovir administered intravenously from 0 to 18 days after transplantation. Ganciclovir administration was reinstituted on day 30 in response to fever, esophagitis, and a viral culture positive for CMV from the blood and was continued until the patient's death 70 days after xenotransplantation. Acute hemorrhage of the brain secondary to disseminated aspergillus infection was seen at autopsy. There was no pathologic evidence of active CMV disease at the time of death.
The donor animal was an adult male baboon (Papio anubis) born and raised in captivity in the United States. It was screened for known human and primate microbial pathogens by available serologic assays. In addition, bacterial and viral cultures were obtained from the blood, urine, throat, and stool prior to transplantation and at autopsy. Parasitic infestation was evaluated by examination of the stool and peripheral blood smears. Serial tuberculin skin testing was performed to evaluate for Mycobacterium tuberculosis. Samples from the human patient's blood, urine, and throat were obtained prior to transplantation and every 1 to 2 weeks after transplantation. In addition, biopsy and autopsy specimens were subjected to viral culturing.
PCR analyses.
PCR analysis for BCMV DNA was performed using primers specific for the major immediate-early (MIE) gene and consisted of 5′-TACGTCATTGGTACCCTCC-3′ (LGH1966) (provided by Gary Hayward) and 3′-TAGTACATTGGCAGTACTCC-5′ (BCMV002). The amplified product was 249 bp. Amplifications were performed in a thermal cycler (Techne, Princeton, N.J.) under the following conditions: one cycle at 94°C for 5 min followed by 40 cycles of 95°C for 1 min, 52°C for 30 s, and 72°C for 2 min. PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. Primer sequences for PCR detection of HCMV DNA (specific for the HCMV MIE gene) were 5′-CCACAATTACTGAGGACAGAGG-3′ (CMVA) and 3′-CGGGGGCATGTACCAGTAGTAT-5′ (CMVB). The amplified product was 377 bp. Thermal cycler conditions for HCMV amplification were the following: 40 cycles of 92°C for 1 min, 55°C for 1 min, and 72°C for 1.25 min followed by a final extension at 72°C for 7 min. PCRs were carried out in a 50-μl total volume containing PCR Supermix (Gibco BRL Life Technologies, Grand Island, N.Y.), 0.25 μg of each oligonucleotide primer, and 100 to 400 μg of total DNA. Extractions from paraffin-embedded tissue were also subjected by PCR analysis to detection of glyceraldehyde-3-phosphate dehydrogenase as previously described to ensure adequate extraction of DNA and the absence of PCR inhibitors. Overnight hybridization with 32P-labeled species-specific internal probes was performed as previously described (6) with the exception that for HCMV MIE, the internal probe sequence was 5′-TCATCTGACTCCTCG-3′ (CMVP) and hybridization was done at 41°C.
Ganciclovir plaque reduction assays were performed as follows. Confluent layers of MRC5 cells (American Type Culture Collection, Manassas, Va.) in 24-well Falcon tissue culture plates (Fisher Scientific Co, Pittsburgh, Pa.) were inoculated in triplicate with 100 μl of each virus that was predetermined to contain approximately 50 PFU. Virus was allowed to adsorb to fibroblasts under conditions of 37°C and 5% CO2 for 90 min. Wells were overlaid (1.5 ml/well) with medium consisting of a 1:1 mixture of 0.8% SeaPlaque agarose (FMC Bioproducts, Rockland, Maine) and modified Eagle medium (Gibco BRL Life Technologies) supplemented with 2% serum supreme (BioWhittaker, Walkersville, Md.) and l-glutamine. Overlay media contained media supplemented with ganciclovir concentrations ranging from 1 to 50 μM; six wells received media without ganciclovir. Plates were allowed to gel at ambient temperature and then were placed in a 37°C humidified incubator with 5% CO2 for 10 days. The 50% inhibitory concentration (IC50) was determined by measuring the ganciclovir concentration that correlated with a 50% reduction in the number of viral plaques compared with the number of plaques in the absence of ganciclovir.
RESULTS
BCMV was isolated from a throat swab that was obtained from the baboon donor animal 4 days before transplantation; all other specimens collected from the animal at the time of transplantation, including cultures obtained from the liver, blood, spleen, lymph node, and lung, were negative for viruses. A total of 41 specimens were obtained from the recipient for viral culture: blood leukocytes (buffy coat) (n = 12), throat (n = 9), urine (n = 6), and body tissue (n = 14). Virus with a cytopathic effect characteristic of CMV was isolated from human foreskin fibroblasts and MRC5 human fetal lung fibroblast cultures that were inoculated with the patient's peripheral blood leukocytes (PBL) obtained on days 29, 36, and 42 after transplantation. A CMV-like virus was also isolated from a culture of a duodenal biopsy specimen obtained 54 days after transplantation; however, this virus isolate was not available for further study.
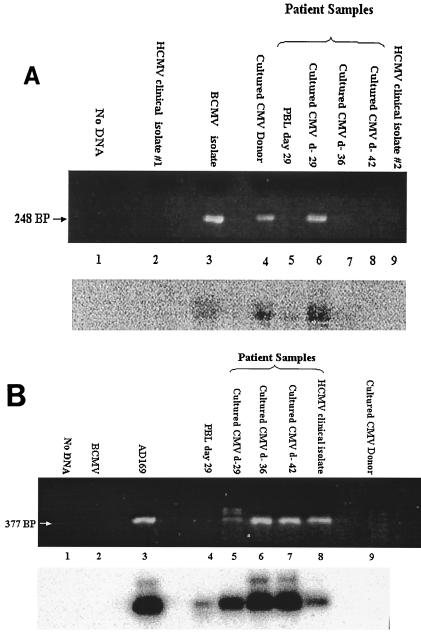
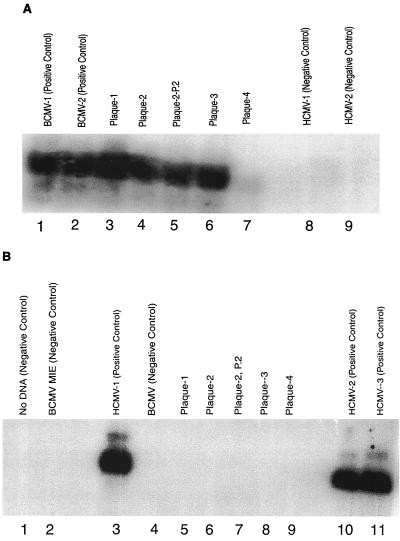
To determine the identity of the CMV isolates, DNA was extracted from the three PBL culture isolates as well as from the original PBL cryopreserved on day 29 and subjected to PCR analysis for both BCMV and HCMV DNA (Fig. 1). The DNA isolated from the cell culture isolate of day 29 PBLs showed amplification with primers directed against the BCMV MIE gene (Fig. 1A, lane 6) and with those directed against the HCMV MIE gene (Fig. 1B, lane 5). Hybridization with 32P-radiolabeled internal oligonucleotide probes confirmed the identity of these products. Serial passage of this isolate, for up to five times in human foreskin fibroblasts and MRC5 cells, continued to yield positive PCR results with the BCMV-specific primers (data not shown). Plaque purification of virus was likewise performed. Positive PCR results were found from four purified plaques using BCMV-specific primers and probes but not with HCMV-specific primers or probes (Fig. 2). An aliquot of the original day 29 PBL specimen was also positive by PCR for BCMV DNA and HCMV DNA with a 32P-labeled probe (Fig. 1A, lane 5, and Fig. 1B, lane 4). Insufficient sample material was available to repeat these studies, and original PBL samples from the other two isolates (days 36 and 42) were not available.
FIG. 1.
(A) PCR amplification of DNA samples with BCMV-specific primers. The upper panel shows PCR products electrophoresed on a 2% agarose gel and visualized with ethidium bromide. The lower panel is a DNA blot hybridization of PCR products using a 32P-labeled oligonucleotide probe specific for BCMV DNA. Lane 1, no DNA; lane 2, DNA from unrelated HCMV (negative control); lane 3, BCMV isolated from an unrelated baboon (positive control); lane 4, BCMV isolated from the oropharynx of the donor animal prior to transplantation; lane 5, patient PBL from day 29; lane 6, CMV isolate from patient PBL from day 29; lane 7, CMV isolate from patient PBL from day 36; lane 8, CMV isolate from patient PBL from day 42; lane 9, unrelated HCMV isolate (negative control). (B) PCR amplification of DNA samples with HCMV-specific primers. The upper panel shows PCR products electrophoresed on a 2% agarose gel and visualized with ethidium bromide. The lower panel is a DNA blot hybridization of PCR products using a 32P-labeled oligonucleotide probe specific for HCMV DNA. Lane 1, no DNA; lane 2, DNA from unrelated BCMV (negative control); lane 3, laboratory HCMV strain AD169 (positive control); lane 4, patient PBL from day 29; lane 5, CMV isolate from patient PBL from day 29; lane 6, CMV isolate from patient PBL from day 36; lane 7, CMV isolate from patient PBL from day 42; lane 8, unrelated HCMV clinical isolate (negative control); lane 9, BCMV isolated from the oropharynx of the donor animal prior to transplantation.
FIG. 2.
(A) PCR amplification of DNA samples with BCMV-specific primers and probe. The PCR product was electrophoresed on a 2% agarose gel and transferred for DNA blot hybridization using a 32P-labeled oligonucleotide probe specific for BCMV DNA. Lanes 1 and 2 have BCMV positive controls. Lane 3, purified plaque-1 from day 29 virus; lane 4, purified plaque-2 from day 29 virus; lane 5, P-2 passage of purified plaque-2; lane 6, purified plaque-3 from day 29 virus; lane 7, purified plaque-4; lanes 8 and 9, HCMV negative controls. (B) PCR amplification of DNA samples with HCMV-specific primers and probe. PCR product was electrophoresed on a 2% agarose gel and transferred for DNA blot hybridization using a 32P-labeled oligonucleotide probe specific for HCMV DNA. Lane 1, no DNA; lane 2, BCMV negative control; lane 3, HCMV positive control; lane 4, BCMV negative control; lane 5, purified plaque-1 from day 29 virus; lane 6, purified plaque-2 from day 29 virus; lane 7, P-2 passage of purified plaque-2; lane 8, purified plaque-3 from day 29 virus; lane 9, purified plaque-4; lanes 10 and 11, HCMV positive controls.
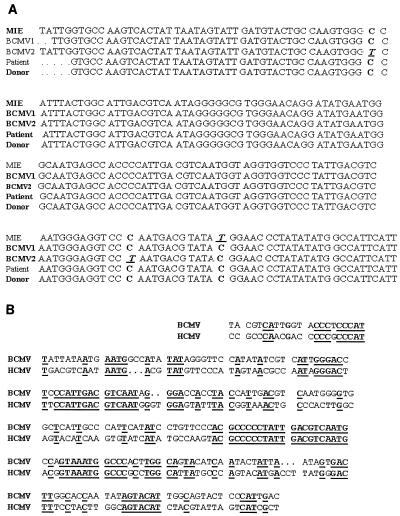
DNA sequencing of the PCR products confirmed the presence of BCMV in the patient's CMV isolate on day 29 postxenotransplantation. The PCR products from the donor's BCMV isolate, the patient's day 29 CMV isolate, three other unrelated BCMV isolates, and a BCMV MIE plasmid clone (kindly provided by Gary Hayward) were separately sequenced two to four times. Comparison of the different DNA sequences demonstrated 98 to 100% homology (Fig. 3A). Comparison of the BCMV MIE plasmid clone sequence with the homologous region in HCMV revealed a significantly lower 50% homology (Fig. 3B). These results, in conjunction with the PCR results, confirm the presence of BCMV in the patient's CMV isolate from 29 days after transplantation.
FIG. 3.
(A) DNA sequences of PCR amplification products from BCMV MIE plasmid clone (MIE); unrelated BCMV isolate 1 (BCMV1); unrelated BCMV isolate 2 (BCMV2); BCMV isolate from day 29 from the patient (Patient); BCMV isolate from the donor animal's throat 4 days prior to transplantation (Donor). Boxes indicate the locations of DNA sequences that vary among the different strains. Sequence alignments were performed using the program Pileup from the GCG Sequence Analysis Package. (B) Comparison of the DNA sequences of the MIE gene from the amplified product of the BCMV plasmid clone and the HCMV AD169 strain. Sequence alignment was performed using the program Bestfit from the GCG Sequence Analysis Package.
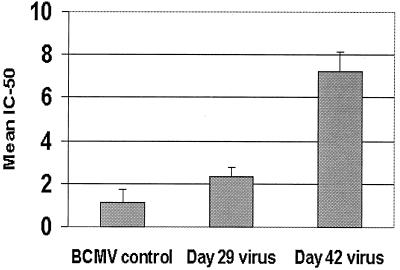
The replication-competent BCMV appeared in the peripheral blood of the xenotransplant recipient 10 days after the cessation of ganciclovir therapy and disappeared from the blood following the reinitiation of therapy on day 30 (Fig. 1A, lanes 6 to 8). In contrast, HCMV continued to be detected in the blood (Fig. 1B, lanes 5 to 7). These results suggest that the patient's BCMV isolate is more susceptible to ganciclovir than his HCMV isolate. To test this hypothesis, ganciclovir susceptibility was determined by plaque reduction assays on the patient's day 29 isolate (a mixture of BCMV and HCMV) and the day 42 isolate (HCMV alone) along with BCMV from an unrelated baboon. The results shown in Fig. 4 demonstrate that the IC50s of ganciclovir for BCMV from the unrelated baboon and the patient's day 29 isolate are significantly less than the IC50 for the patient's day 42 HCMV isolate (1.14 ± 0.622 M, 2.4 ± 0.38 M, and 7.2 ± 0.91 M ganciclovir, respectively).
FIG. 4.
Plaque reduction assay: IC50 of ganciclovir with standard deviation is shown for the control strain of BCMV, the CMV isolate from the patient from day 29, and the HCMV from the patient from day 42. Results are based on three different experiments, each using ganciclovir dilutions tested in triplicate. The numbers indicate molar concentrations.
DISCUSSION
This is the first report of the isolation of a replication-competent virus from a source animal in a patient who underwent xenotransplantation. A previous report on the same recipient found evidence by PCR of a simian foamy retrovirus in sites distant from the baboon liver (1). However, simian foamy virus could not be cultured from the patient, and the virus DNA was always found in conjunction with similar or greater quantities of DNA from baboon mitochondria. Accordingly, true infection of the patient with simian foamy virus could not be established. A recent report found porcine endogenous retrovirus to be transmitted across species in an immunosuppressed nonobese diabetic severe combined immunodeficiency mouse model (11). However, investigations of human recipients of porcine xenotransplant grafts failed to find evidence of porcine endogenous retrovirus transmission or other overt infections from donor animals, although evidence of microchimerism was present for as long as 8.5 years (8).
We previously reported that BCMV replicates in human cells in culture (6). These results are confirmed in this study by the isolation and serial passage of the patient's BCMV isolate in human foreskin fibroblasts and MRC-5 cells. The isolation of BCMV from the patient's PBL suggests that human cells were infected with the virus in vivo. Furthermore, replication competence was demonstrated by serial passages and plaque purification that separated BCMV from the primary isolate that contained both BCMV and HCMV. However, since microchimerism has been demonstrated in this patient's blood (9), it is also possible that baboon leukocytes, infected with BCMV, were circulating in peripheral blood and represent the source of BCMV in the day 29 PBL sample. Nonetheless, it is notable that replication-competent BCMV, capable of establishing a lytic infection in human cells in vitro, was present in the peripheral blood of a human 4 weeks after receipt of a baboon liver.
BCMV was isolated only once from the patient's PBL (day 29), while HCMV was isolated on two additional occasions (days 36 and 42). The BCMV isolation occurred while the patient was off intravenous ganciclovir therapy. The inability to recover BCMV on subsequent occasions, after reinitiation of antiviral therapy, almost certainly is attributable to the isolate's susceptibility to ganciclovir. The IC50 for the day 29 isolate being in between the IC50 for the BCMV control and for HCMV isolated on day 42 is likely a secondary effect of the presence of both BCMV and HCMV on day 29.
In summary, BCMV was isolated from the peripheral blood of a recipient of a baboon liver transplant 4 weeks after transplantation and 10 days after discontinuation of prophylactic ganciclovir therapy. This is the first time that an infectious virus from a donor animal has been isolated from a recipient of a xenotransplant. In this case the BCMV was susceptible to ganciclovir and responded to treatment. However, the isolation of the virus highlights the importance of potential cross-species transmission of disease to the recipient. In addition, isolation of BCMV from the patient's blood represents a risk to others who might have accidental contact with the blood.
ACKNOWLEDGMENT
This work was supported in part by a Public Health Service grant from the National Institute of Allergy and Infectious Diseases (KO8 AI01437).
REFERENCES
- 1.Allan J S, Broussard S R, Michaels M G, Starzl T E, Leighton K L, Whitehead E M, Comuzzie A G, Lanford R E, Leland M M, Switzer W M, Heneine W. Amplification of simian retroviral sequences from human recipients of baboon liver transplants. AIDS Res Hum Retrovir. 1998;14:821–824. doi: 10.1089/aid.1998.14.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delmonico F L, Snydman D R. Organ donor screening for infectious diseases. Transplantation. 1998;65:603–610. doi: 10.1097/00007890-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hammel J M, Prentice E, Fox I J. Current status of xenotransplantation. Probl Gen Surg. 1998;15:189–201. [Google Scholar]
- 4.Ho M, Suwansirikul S, Dowling J, Youngblood L A, Armstrong J. The transplanted kidney as a source of cytomegalovirus. N Engl J Med. 1975;293:1109–1112. doi: 10.1056/NEJM197511272932201. [DOI] [PubMed] [Google Scholar]
- 5.Manez R, St. George K, Linden P, Martin M, Kusne S, Grossi P, Ho M, Rinaldo C. Diagnosis of cytomegalovirus infections by shell vial assay and conventional cell culture during antiviral prophylaxis. J Microbiol. 1994;32:2655–2659. doi: 10.1128/jcm.32.11.2655-2659.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaels M G, Alcendor D, St. George K, Rinaldo C R, Ehrlich G D, Becich M J, Hayward G S. Distinguishing baboon cytomegalovirus from human cytomegalovirus: importance for xenotransplantation. J Infect Dis. 1997;176:1476–1483. doi: 10.1086/514144. [DOI] [PubMed] [Google Scholar]
- 7.Michaels M G, McMichael J, Brasky K, Kalter S, Peters R L, Starzl T E, Simmons R L. Screening donors for xenotransplantation: the potential for xenozoonoses. Transplantation. 1994;57:1462–1465. doi: 10.1097/00007890-199405000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer W M, Chapman L E, Lockey C, Onions D, Otto E The XEN 111 Study Group. Search for cross-species transmission of porcine endogenous retroviruses in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 9.Starzl T E, Fung J, Tzakis A, Todo S, Demetris A J, Marino I R, Doyle H, Zeevi A, Warty V, Michaels M G, Rudert W A, Trucco M. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Institute of Medicine Committee on Xenograft Transplantation. Xenotransplantation: science, ethics and public policy. Washington, D.C.: National Academy Press; 1996. [PubMed] [Google Scholar]
- 11.Van der Laan L J W, Lockey C, Griffeth B C, Frasier F S, Wilson C A, Onions D E, Hering B J, Long Z, Otto E, Torbett B, Salomon D R. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature. 2000;407:90–94. doi: 10.1038/35024089. [DOI] [PubMed] [Google Scholar]
- 12.Van der Riet F D S J, Human P A, Cooper D K C, Reichart B, Fincham J E, Kalter S S, Kanki P J, Essex M, Madden D L, Lai-Tung M T, Chalton D, Sever J L. Virological consideration of the use of primates in xenotransplantation. Transplant Proc. 1987;19:4068–4069. [PubMed] [Google Scholar]