Abstract
Introduction
Non-alcoholic fatty liver disease, now known as metabolic dysfunction-associated steatotic liver disease (MASLD), is a phenotype of the metabolic syndrome in the liver and is clearly associated with metabolic abnormalities such as hyperglycaemia and dyslipidaemia. Although the prevalence of MASLD is increasing worldwide, there is currently no consensus on the efficacy and safety of the drugs used to treat MASLD/metabolic dysfunction-associated steatohepatitis (MASH). Pemafibrate, a selective peroxisome proliferator-activated receptor alpha modulator, was designed to have higher peroxisome proliferator-activated receptor alfa (PPARα) agonist activity and selectivity than existing PPARα agonists, and in development trials, without increasing creatinine levels, lipid parameters and alanine aminotransferase (ALT) were significantly improved. Thus, pemafibrate may effectively ameliorate the pathogenesis and metabolic abnormalities in MASLD/MASH. In this trial, we evaluated the efficacy and safety of pemafibrate in patients with MASLD/MASH.
Methods and analysis
This trial was designed as an open-label, three-arm, randomised controlled study. After obtaining informed consent, patients aged 20–80 years who met the selection criteria were enrolled. Patients were randomised to receive pemafibrate 0.4 mg/day, 0.2 mg/day or fenofibrate (n=120 per group). The duration of treatment was 48 weeks. The primary endpoint was a change in ALT levels after 24 weeks of administration. Secondary endpoints included changes from baseline in liver fibrosis markers (fibrosis-4 index, type IV collagen 7s, enhanced liver fibrosis and Mac-2 binding protein glycosylation isomer) at 48 weeks as well as changes in liver fat mass and liver stiffness measured by MRI and ultrasound (US) at centres equipped with MRI and US capabilities.
Ethics and dissemination
Ethical approval was obtained from the Yokohama City University Certified Institutional Review Board before participant enrolment (CRB20-014). The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conferences. Participants wishing to understand the results of this study will be contacted directly on data publication.
Trial registration number
This trial was registered in the Japan Registry of Clinical Trials (number: jRCTs031200280).
Protocol version
V.1.9, 23 November 2023
Keywords: Hepatology; Other metabolic, e.g. iron, porphyria; Lipid disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first study to compare the effects of high-dose pemafibrate, low-dose pemafibrate and fenofibrate on hepatic pathology in patients with metabolic dysfunction-associated steatotic liver disease.
Secondary endpoints will include changes in liver stiffness measured by elastography and fibrosis markers.
The limitations of the study include open-label and no liver biopsy.
Introduction
Non-alcoholic fatty liver disease (NAFLD), which has recently been reclassified as metabolic dysfunction-associated steatotic liver disease (MASLD),1 is a clinical condition diagnosed in the presence of steatosis by liver biopsy and imaging studies when alcoholism and other liver diseases are excluded. It is a hepatic manifestation of metabolic syndrome and is often associated with obesity, diabetes mellitus, dyslipidaemia, hypertension and other disorders. The prevalence of NAFLD is increasing worldwide. In Japan, it increased from 12.9% in 1994 to 34.7% in 2000.2 NAFLD is classified as non-alcoholic fatty liver (NAFL) or non-alcoholic steatohepatitis (NASH), which includes inflammation and progressive disease associated with liver cancer or cirrhosis, affecting 10%–20% of patients.3 According to previous epidemiological reports, the old name NAFLD and the new name MASLD are identical in 97.6%–99.7% of patients.4,6 NAFLD should be reworded as MASLD and NASH as metabolic dysfunction-associated steatohepatitis (MASH). In this paper, the terminology is unified as MASLD/MASH. As a treatment for MASLD/MASH, diet and exercise therapy with a low-calorie diet are effective, and it has been reported that weight loss improves liver function and liver histology.7 However, there is no consensus on the efficacy of any drug for MASLD/MASH, and none are covered by insurance globally, including Japan.
MASLD is often complicated by lifestyle-related diseases, such as dyslipidaemia and diabetes mellitus. The prevalence of MASLD in patients with dyslipidaemia is high, ranging from 26% to 58%, and both are closely related.8 Fibrates are often used in drug therapy for MASLD complicated by hypertriglyceridaemia. Fibrates have been used clinically since the 1930s, and their ability to activate peroxisome proliferator-activated receptor (PPAR) was discovered in 1990. The PPAR family is composed of three molecules, PPARα, PPARγ and PPARδ. PPARα is highly expressed in the liver, skeletal muscle, and brown adipocytes, increases β-oxidation and inhibits fatty acid synthesis. Bezafibrate, which activates the PPAR family in general, was released in 1995, and fenofibrate, which selectively activates PPARα, was released in 2011. Among fibrates, fenofibrate is commonly used in Japan. In a previous pilot clinical trial, 16 patients with MASLD treated with 200 mg of fenofibrate for 48 weeks showed improvements in alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (γ-GTP) levels and hepatocellular ballooning on histological evaluation.9 In addition, a randomised controlled trial (RCT) using ultrasound (US) elastography (USE) showed improvements in ALT and liver stiffness.10 11 However, although the efficacy of fenofibrate in MASLD has been reported in many clinical trials, none have examined it in a large enough sample size, and no consensus has been reached at this time.
Pemafibrate is a selective PPARα modulator (SPPARMα) created by Kowa Company and is designed to have higher PPARα agonist activity and selectivity than existing PPARα agonists.12 Pemafibrate selectively binds to PPARα and causes a ligand-specific conformational change in PPARα. In a phase III study in patients with dyslipidaemia, pemafibrate significantly improved lipid parameters and ALT levels without increasing creatinine (Cr) levels.13 Based on the above data, pemafibrate is expected to have MASLD-improving action. Pemafibrate is a drug that has been approved in Japan since 2017 as well as in Thailand, Singapore and Malaysia.14
In this study, we investigated the efficacy and safety of pemafibrate administered for 48 weeks, using fenofibrate as a control. In addition, since the therapeutic efficacy of 0.4 mg/day pemafibrate and 0.2 mg/day pemafibrate may be different, the participants in this study will be divided into high-dose (0.4 mg/day) and low-dose (0.2 mg/day) pemafibrate groups.
Methods and analysis
Trial design
The Standard Protocol Items for Randomised Trials statement and checklist were used to prepare the study protocol. This trial was designed as an open-label, three-arm, RCT to investigate the efficacy and safety of high-dose pemafibrate, low-dose pemafibrate and fenofibrate.
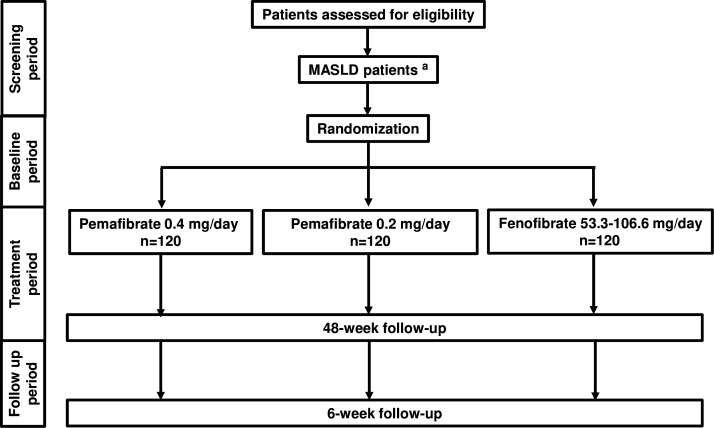
The study period was from 23 December 2020 to 31 March 2026. The study protocol is described in online supplemental document 1. All treatments were administered daily for 48 weeks to patients with MASLD. The experimental groups will be as follows: high-dose pemafibrate group, low-dose pemafibrate group and fenofibrate group (figure 1). Fenofibrate 53.3 mg tablets shall be administered orally once daily, once per dose, after breakfast. Thereafter, the dose may be carefully increased to two tablets per dose at the physician’s discretion. In the next phase, we planned to examine targets and stages. MRI or US examination will be performed at baseline and 48 weeks after the intervention, and the data will be evaluated by a blinded independent liver specialist (MY).
Figure 1. Study design. aN = 360 enrolled. MASLD, metabolic dysfunction-associated steatotic liver disease.
Study endpoints and rationale
The primary endpoint was a change in ALT levels after 24 weeks of administration. Serum aminotransferase levels are sensitive indicators of hepatocellular damage and inflammation.15,17 In particular, ALT is abundantly distributed in the liver and is a useful indicator of hepatopathy in MASLD because it is elevated during MASLD. However, changes, such as hepatic necrosis and fibrosis, do not correlate with ALT levels.18 The degree of hepatocellular damage can be monitored by measuring the ALT level, a parameter of hepatopathy. Since not only advanced but also primary and secondary medical institutions were scheduled to participate in this study, changes in ALT levels were selected as the primary endpoint because they can be performed within health insurance coverage and are highly versatile. The ALT-lowering effect of pemafibrate has been observed as early as 4 weeks,19 with stable reductions reported from 24 to 52 weeks.20 To assess the sustained, long-term impact of ALT, the study was conducted in both the USA and Japan. A 24-week period was deemed sufficient to evaluate the stable, long-term effect on ALT levels. Our secondary endpoint will determine the amount and rate of change from baseline (table 1). Other variables monitored included adverse events (AEs), standard laboratory analysis results, physical examination results, vital signs and compliance rates.
Table 1. Study endpoints.
| Primary endpoint | Secondary endpoints | |
| Efficacy endpoint | Efficacy endpoint | Safety endpoint |
| Change in ALT level from baseline after 24 weeks of administration | Amount and rate of change in the following parameters:
|
Occurrence rate of adverse events |
ALT, alanine aminotransferase; HDL-C, high-density lipoprotein cholesterol
Rationale for treatment dose, mode and duration
To analyse the efficacy of pemafibrate and fenofibrate, doses approved for essential dyslipidaemia were used. The dose of pemafibrate was set in compliance with the Drug Information for Drug Administration in Japan, referring to the phase III clinical trial in patients with dyslipidaemia.13 Because of the risk of hepatotoxicity with fenofibrate, the Japanese guidelines state that for patients with abnormal liver function test results or a history of hepatotoxicity, the daily dose of fenofibrate should be started at 53.3 mg. The dosage of fenofibrate was set based on this information. In a study focusing on the effects of 0.4 mg/day pemafibrate and 0.2 mg/day pemafibrate in dyslipidaemia complicated by patients with type 2 diabetes, the ALT change at 24 weeks was −13.1 IU/L in the 0.4 mg/day pemafibrate group and −6.6 IU/L in the 0.2 mg/day pemafibrate group, suggesting that there may be a dose-dependent difference in hepatopathy improvement.21 The treatment period was set at 48 weeks because we thought that long-term administration would allow us to evaluate the efficacy and safety of pemafibrate and fenofibrate at high and low pemafibrate doses.
In this study, patients were randomised in a 1:1:1 ratio to receive high-dose pemafibrate (0.4 mg/day), low-dose pemafibrate (0.2 mg/day) or fenofibrate.
High-dose pemafibrate group
Parmodia 0.1 mg tablets are to be administered orally two times per day, two tablets per dose, after morning and evening meals. If necessary, the drug can be substituted by extended-release pemafibrate, either 0.2 mg (two tablets once daily) or 0.4 mg (one tablet once daily).
Low-dose pemafibrate group
Parmodia 0.1 mg tablets are to be administered orally two times per day, one tablet per dose, after morning and evening meals. If necessary, the drug can be substituted by 0.2 mg extended-release pemafibrate (one tablet once daily).
Fenofibrate group
Lipidil, Tricore or fenofibrate 53.3 mg tablets are to be administered orally once daily, one tablet per dose, after breakfast. Subsequently, the dose may be carefully increased to two tablets per dose at the physician’s discretion.
Drug supply
This clinical trial will be open, and the patient registration centre, doctors and patients will be informed of the allocation results. Pemafibrate and fenofibrate were manufactured and marketed by the Kowa Company (Tokyo, Japan). Physicians prescribe these drugs to patients receiving insurance reimbursements.
Sample size estimation
The target number of patients in the study was 360 (120 patients in the high-dose pemafibrate group, 120 patients in the low-dose pemafibrate group and 120 patients in the fenofibrate group).
A phase III study comparing high and low doses of pemafibrate with fenofibrate showed that ALT levels at baseline and 24 weeks were 30.3±14.5 and 25.5±12.5 (high-dose pemafibrate), 32.0±19.5 and 23.7±12.0 (low-dose pemafibrate) and 30.5±14.6 and 33.4±23.0 (fenofibrate), respectively. The SD for these values in each group were calculated to be 17.1, 20.8 and 24.7, respectively, assuming a correlation of 0.2 between the ALT levels at baseline and 24 weeks. In this study, a fixed-order test was conducted by comparing the high-dose pemafibrate group with the fenofibrate group using a two-tailed t-test with a 5% significance level. If a statistically significant difference was found, the low-dose pemafibrate group was compared with the fenofibrate group using a two-tailed t-test with a 5% significance level. Assuming that the expected change in ALT level at week 24 is −4.8 (high-dose pemafibrate), −8.3 (low-dose pemafibrate) and 2.9 (fenofibrate) and that their SD is 20 in the fixed-order test above, 109 patients were required in each group to achieve a power of at least 80% in the two comparisons (two-sample t-test) between the high-dose pemafibrate group and the fenofibrate group, and between the low-dose pemafibrate group and the fenofibrate group, as calculated via a simulation. Thus, 120 patients per group were required to account for the dropouts.
Eligibility
The physician entered the consenting patients into a screening list, assigned an identification code to each patient and determined eligibility according to the inclusion and exclusion criteria (table 2). Only patients between the ages of 20 and 80 years were included after informed consent was obtained. This is because (1) until March 2022, the legal age for obtaining consent in Japan was 20 years and (2) patients over 80 years of age are generally physiologically impaired and more prone to AEs. The inclusion criteria for hypertriglyceridaemia encompassed triglyceride (TG) levels of 150–500 mg/dL, based on a phase III trial evaluating the efficacy of Palmodia in patients with hypertriglyceridaemia.13 The lower limit of elevated ALT levels, as an inclusion criterion, was set at 43 for males and 24 for females, in accordance with the Japanese Committee for Clinical Laboratory Standards (JCCLS).22 Furthermore, given that hepatotoxicity associated with fenofibrate has been reported in the Drug Information for Fenofibrate in Japan, it is recommended that administration should be discontinued if aspartate aminotransferase (AST) or ALT consistently exceeds 2.5 times the upper limit of normal or 100 units. In reference to this, the upper limit of ALT in the participation criteria was set at 100.
Table 2. Patient inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria | |
| 1 | Men and women aged at least 20 years and under 80 years at the time of obtaining consent | Patients taking contraindications.Ciclosporin, rifampicin, steroids (excluding topical and inhaled drugs), amiodarone and breast cancer drugs. |
| 2 | Patients with fatty liver diagnosed histologically within 1 year prior to obtaining consent or imaging examination within 6 months prior to obtaining consent and who have failed exercise and diet therapy for at least 3 months. | Patients with body mass index <18.5 kg/m2 at the time of obtaining consent. |
| 3 | Patients with hypertriglyceridaemia (150–500 mg/dL) within 91 days prior to obtaining consent. | Patients who have been diagnosed with liver cirrhosis at the time of obtaining consent |
| 4 | Patients with elevated alanine aminotransferase (43–100 IU/L for men, 24–100 IU/L for women) within 91 days prior to obtaining consent. | Patients with findings of portal hypertension (varicose veins, ascites, encephalopathy and splenomegaly) at the time of obtaining consent. |
| 5 | Patients whose daily alcohol consumption (ethanol equivalent) is less than 30 g/day for men and less than 20 g/day for women at the time of obtaining consent | Patients with total bilirubin >2× the upper limit of normal within 91 days prior to obtaining consent, excluding Girbert syndrome. |
| 6 | Patients with hepatitis C, hepatitis B (excluding inactive carriers), autoimmune hepatitis, primary biliary cholangitis or other hepatic complications that have been ruled out at the time of obtaining consent. | Platelet count 80×109/L within 91 days prior to obtaining consent. |
| 7 | Patients whose written consent to participate in this study has been obtained. | Serum creatinine level of 1.5 mg/dL or higher within 91 days prior to obtaining consent. |
| 8 | Patients with gallstones or biliary obstruction at the time of obtaining consent. | |
| 9 | Patients with severe infection, preoperative or postoperative or severe trauma at the time of obtaining consent. | |
| 10 | Patients who have used fibrates within 91 days prior to obtaining consent. | |
| 11 | Patients with 10% weight change in 91 days prior to obtaining consent. | |
| 12 | Patients who have undergone bariatric surgery or are scheduled for surgery during the study period. | |
| 13 | Patients with a history of type I diabetes mellitus. | |
| 14 | Patients with HbA1c >9.5% within 91 days prior to obtaining consent(If HbA1c >9.5%, re-entry will be possible after improvement by treatment.). | |
| 15 | Patients with psychosis, alcoholism, drug addiction or narcotic addiction that would affect compliance with the research protocol. | |
| 16 | Patients who participated in other clinical trials in 100 days prior to obtaining consent. | |
| 17 | Pregnant women or patients who may be pregnant. | |
| 18 | Patients with complications of malignant tumoursHowever, patients who have undergone radical surgery or completed anticancer drug administration may enrol. Patients under observation and evaluation for malignant tumours are excluded. | |
| 19 | Other patients who are judged by the principal investigator to be inappropriate as participants of this study. |
HbA1c, haemoglobin A1c
If there were no eligibility issues, the investigator entered the necessary information for registration in the electronic data capture system. Registration was completed by assigning a registration number to the patient.
Randomisation and masking
The patients will be randomly assigned to one of the three groups in a 1:1:1 ratio using an application under the supervision of the Saga University Clinical Research Centre. A stratified permuted block method using the presence of diabetes mellitus, serum TG level (cut-off: 300 mg/dL), and FIB-4 index (cut-off: 1.3) as adjustment factors were used for random assignment to avoid large biases in the factors. The detailed procedure of the random assignment will not be communicated to the investigators at the participating centres. Adjustment factors were set because they were related to the primary endpoint, MASLD/MASH progression and degree of hepatic fibrosis. Treatment assignments were not fully masked to the patients and physicians.
Keycode break
Not applicable.
Harm and AE monitoring
AEs are any unwanted or unintended side effects, including abnormal laboratory test values, vital signs, symptoms or illnesses that occur during the trial. A causal relationship with the investigational drug was not considered. The principal or subinvestigator will assess the severity of AEs. Any AE that meets any of the following criteria will be considered a serious AE (SAE): death, life-threatening condition, hospitalisation requirement or prolonged hospitalisation for treatment, disability, disability threat, other serious conditions, congenital disease or anomaly in the offspring. If an SAE occurs, the principal or subinvestigator will appropriately treat the SAE and immediately report the details to the hospital director and study drug supplier.
Study procedures
The investigator or subinvestigator will observe, examine and investigate according to the instructions listed in table 3. If blood samples are drawn at the time of the visit, the patient should fast for 8 hours prior to blood collection. Blood samples will be collected and stored at visits 2 and 8 after obtaining additional consent (table 4).
Table 3. Schedule for observations, tests and assessments.
| Perioditem | Screening period | Administration period | Follow-up period | |||||||
| −91st to date of registration | 0 weekStart date of administration | 4 weeks | 8 weeks | 12 weeks | 24 weeks | 36 weeks | 48 weeks(the time of discontinuation)*† | 6 weeks after completion of administration | ||
| Tolerance (days) | – | – | ±7 | ±7 | ±14 | ±14 | ±14 | ±14 | ±14 | |
| Visit | Visit 1 | Visit 2‡ | Visit 3 | Visit 4¶¶ | Visit 5 | Visit 6 | Visit 7 | Visit 8 | Visit 9 | |
| Obtainment of consent | 〇 | |||||||||
| Registration | 〇 | |||||||||
| Study drug administration§ |

|
|||||||||
| Patient background check | 〇 | |||||||||
| Survey of alcohol consumption and smoking status | 〇 | |||||||||
| Weight, BMI¶, abdominal circumference | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | ||||
| Vital signs | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | |
| ECG | Ο** | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | |
| Clinical examination†† | Haematologic test | Ο** | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 |
| Blood chemistry test‡‡ | Ο** | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | 〇 | |
| Measurement of liver fat content and fibrosis (MRE or USE) | Ο**§§ | Ο§§ | ||||||||
| Liver disease-related events |

|
|||||||||
| Cardiovascular eventspresence of carcinogenesis in other organs |

|
|||||||||
| Investigation of concomitant medications |

|
|||||||||
| Observation of disease, etc and adverse events |

|
|||||||||
At the time of discontinuation, only tests that can be performed will be conducted, and the data will be submitted.
In the discontinuation test, liver fat content and fibrosis measurements (MRE or USE) were not performed.
Visits 1 and 2 may occur on the same day. Blood collection at visit 2 may be substituted with that at visit 1, but missing values are compensated for separately.
The study drug should be prescribed every 4 weeks up to week 12 and every 12 weeks after week 12.
Body mass index was calculated based on height and weight.
Blood collection, ECG and imaging tests within 3 months prior to obtaining consent can be substituted for screening tests.
Fasting blood samples should be obtained (fasting for >8 hours).
Type IV collagen 7s, enhanced liver fibrosis and Mac-2 binding protein glycosylation isomer were measured only after 0 and 48 weeks.
MRE or USE should only be performed at facilities where they are available. The tolerable date range of liver fat content and fibrosis measurement (MRE or USE) after 48 weeks of administration is ±14 days.
At visit 4 (8 weeks after administration), an in-person visit is not required; however, a telephone safety confirmation should always be made.
MREMR elastographyUSEultrasound elastography
Table 4. Clinical laboratory items.
| Biochemical test(screening, every visit, follow-up, termination) | Residual blood for storage in blood tests(at the time of visits 2 and 8) | Haematological examination/coagulation(screening, every visit, follow-up, termination) |
| AlbuminAlanine aminotransferaseAlkaline phosphataseAmylaseAspartate aminotransferaseBlood urea nitrogenChlorineCreatinineEstimated glomerular filtration rateγ-glutamyl transpeptidaseLactate dehydrogenasePotassium (K)Sodium (Na)Calcium (Ca)Total bilirubinTotal proteinUric acidHigh-density lipoprotein-cholesterolLow-density lipoprotein-cholesterolTotal cholesterolTriglyceridesGlucoseHaemoglobin A1c (screening, visits 2,6 and 7)insulin (visits 2,6 and7) | Type IV collagen 7s,enhanced liver fibrosis,Mac-2 binding protein glycosylation isomer | White blood cell count, neutrophil count, lymphocyte count, red blood cell count haematocrit, haemoglobin and platelets |
Concomitant treatment
-
Contraindicated treatments
Ciclosporin, rifampicin.
Steroids (excluding topical and inhalation drugs), amiodarone and breast cancer drugs (tamoxifen, toremifene and raloxifene).
-
Immutable drugs
Vitamin E and ursodeoxycholic acid.
Antihypertensive drugs: angiotensin II receptor blockers.
Drugs for hyperlipidaemia: statins and small intestinal cholesterol transporter inhibitors.
Antidiabetic drugs other than those listed above can be added for poorly controlled patients with HbA1c ≥7.5%. However, insulin preparations can increase or decrease the total dose of insulin by up to 20%.
Criteria and procedure for withdrawal from the study
The principal or subinvestigator should terminate the participation of a patient enrolled in a clinical trial if any of the following applies:
A serum Cr level ≥2.5 mg/dL or higher after the start of the study.
ALT or AST >2.5 times the upper facility reference limit and 2.5 times the baseline (V2) for two consecutive prescribed visits after the start of the study.
If TG exceeds 1000 mg/dL for two consecutive prescribed visits after the start of the study.
Muscle pain, weakness and other subjective symptoms were characterised by worsening creatine kinase (CK) (CK exceeding the upper limit of the reference value by 10 times) after the start of the study.
When the study participant requests to withdraw consent.
If, after registration, the study participant does not meet the selection criteria or violates the exclusion criteria, it is inappropriate to include him/her as a participant.
If it is difficult to continue the study due to worsening symptoms and findings of MASLD/MASH or dyslipidaemia.
When the use of prohibited concomitant drugs is deemed necessary.
When it is difficult to continue the study due to the occurrence of adverse events.
In the event of a serious deviation from the research protocol.
Death.
If the study participant is found to be pregnant.
If 80% or more of the medication is not taken at each visit (see ‘ Medication Instructions’ section in online supplemental file).
When the principal investigator or subinvestigator determines that the continuation of the study is not desirable.
Efficacy evaluation
The primary efficacy endpoint was a change in ALT levels after 24 weeks of administration. Secondary endpoints are presented in table 1. MRE was performed independently by a liver specialist.
Safety assessments
The occurrence rate of AEs was monitored during each patient visit, from visit 1 to the follow-up period (visit 9). The population enrolled in the study that received the study drug at least once was defined as the safety analysis set.
Population analysis
The full analysis set (FAS) was defined as all study subjects who were enrolled in the study and received at least one dose of the study drug after randomisation and for whom efficacy data were available. However, study subjects for whom baseline data could not be obtained and those with serious violations of the study protocol (eg, failure to obtain consent and enrolment outside the contract period) were excluded. The per-protocol set is defined as the population excluding serious violations of the rules of the research protocol, including research methods and concomitant therapies, from the FAS.
(1) Selection criteria violation, (2) violation of exclusion criteria, (3) violation of concomitant use of prohibited drugs and (4) violation of concomitant use of prohibited therapies.
Statistical analysis
The main analysis will be conducted on the FAS. The following procedure was used to perform fixed-order tests: to compare the high-dose pemafibrate group with the fenofibrate group, differences between the groups were evaluated at a two-sided significance level of 5% using a t-test for the change in ALT at 24 weeks. If a statistically significant difference was found, the next step was to evaluate the difference between groups using a t-test at a two-sided significance level of 5% for comparison between the low-dose pemafibrate and fenofibrate groups.
For each secondary endpoint, only if a statistically significant difference is found at a two-sided significance level of 5% in the comparison of the high-dose pemafibrate group and the fenofibrate group by t-test, a t-test is used to compare the low-dose pemafibrate group with the fenofibrate group and the high-dose pemafibrate group with the low-dose pemafibrate group. The differences between the respective groups were evaluated by multiple comparisons using Holm’s method, with a two-sided significance level of 5% for the p-values for the two comparisons.
Amendment of the clinical trial protocol
The following procedure shall be used to change the content of the research protocol. (1) If the principal investigator determines that changes to the research protocol are necessary, the principal investigator will provide the proposed changes to the research protocol and other necessary materials and information to the investigators. (2) The principal investigator will allow the investigators the time necessary to fully review and discuss with the investigators the proposed changes to the research protocol and other materials and information provided by the principal investigator in accordance with the preceding paragraph. (3) After obtaining the agreement of the investigators, the principal investigator will prepare an implementation plan as necessary from the document describing the changes and modified research protocol. (4) The principal investigator shall obtain the opinion of the approved clinical research review committee, as stated in the implementation plan, regarding the modified research protocol and obtain approval. (5) Based on the review results, the principal investigator will report to the investigators and obtain approval for implementation from the administrator of the implementing medical institution.
Conclusion, termination or suspension of the clinical trial
After the clinical trial, the principal investigator will inform the head of the implementing medical institution that the clinical trial has ended; the head will also be provided with a written summary of the clinical trial results. Subsequently, the institutional review board will be promptly notified in writing that the head of the implementing institution has received the report. The board will also be provided with the clinical trial results outlined in the report submitted by the principal investigator.
In case of clinical trial termination or suspension, the clinical trial conductor promptly sends a written report detailing the termination or suspension and the reason to the director of the implementing medical institution and regulatory agency. The clinical trial conductor may terminate or suspend the trial under the following circumstances:
In the event of an unforeseen serious illness (please refer to the ‘Evaluation of diseases’ section in online supplemental file 1), which may be detrimental to the participant as a whole.
If a serious violation/non-compliance of the study protocol with the law and related laws or regulations is found.
If facts that undermine or may undermine the ethical validity or scientific rationality of the study are obtained.
If a significant risk to participants is identified.
When a request or recommendation for discontinuation is received from the head of the research institution or a certified clinical research review committee.
If a cancellation request or order is received from the Minister of Health, Labour and Welfare.
Interim analysis
Not applicable.
Data management, central monitoring and audit
The site at which the investigator conducts the clinical trial will be maintained as the source of data for each patient. All data, including copies of informed consent, medical records, laboratory data records and individual records, including notes, are collected by an independent data management centre. During monitoring, the principal investigator shall implement the following measures: (1) protect the human rights of research subjects and ensure their safety; (2) conduct the clinical research in compliance with the latest implementation plan, research protocol, and relevant ministerial ordinances; (3) obtain written consent from research subjects to conduct this clinical research and (4) verify the accuracy of the records and relevant data in relation to the source documents. Central monitoring will be conducted by the person in charge according to the monitoring procedures. No audits are set up.
Study flow and schedule of enrolment, interventions and assessments
A flowchart of the study is shown in figure 1. The study schedule is presented in table 3.
Medication instructions
The investigator and subinvestigator shall provide medication instructions to the patient and pay attention to the following points at the time of delivery of the study drug.
When to take the medication, how many tablets per dose and how to take the medication.
If the patient forgets to take the study drug after breakfast, the drug should be taken 12 hours after the scheduled time or by 21:00, whichever is earlier.
The study drug is to be taken in the morning when the patient comes to the hospital.
Remaining medication due to multiple reasons, including forgetfulness, must be brought to the hospital.
Whom to contact if the patient is unsure about the drug.
The patient shall be asked to bring the remaining medication upon arriving at the hospital, and the investigator and subinvestigator shall check the remaining medication.
Non-compliance with dosage form and dosage, medication failure or withdrawal of medication shall be recorded in the medical record.
Patient and public involvement
Patients and the general public will not be involved in the specific design and planning of this RCT. In this trial, patients will be involved in the recruitment and conduct of the study. In particular, the development of the research questions and outcome measures will be based on the priorities, experiences and preferences of patients. The results of this study will be disseminated via email to participants interested in the results. The burden of intervention will be assessed by the patients before the commencement of the trial, and patient satisfaction with the treatment will be assessed as part of the postintervention assessment.
Ethics and dissemination
This study will be conducted in compliance with the Declaration of Helsinki. The study protocol and relevant supporting data were approved on 18 November 2020, by the Yokohama City University Certified Institutional Review Board before participant enrolment (CRB20-014). The trial results will be reported in accordance with the 2010 Consolidated Standards of Reporting Trials guidelines. The trial was registered with the Japan Registry of Clinical Trials (jRCTs031200280). Written informed consent (see online supplemental document 2) for study participation was obtained from all enrolled participants. The results of this study will be submitted for publication in international peer-reviewed journals, and the key findings will be presented at conferences. The funder had no role in the study design, data collection or data analysis.
Health damage compensation and insurance
If a research subject suffers from health problems as a result of participation in this research, the principal investigator and subinvestigator will provide appropriate medical treatment and other necessary measures. In such cases, treatment will be provided as a medical treatment covered by insurance, and the research subject will pay the medical expenses for which he/she is responsible. No medical fees or allowances were received. In addition, clinical research liability insurance will be purchased to cover compensation in the event of liability due to health damage resulting from this research and in the event of death or permanent disability, levels 1–3 health damage to the research subject. Compensation is subject to certain conditions, and payments may be excluded or limited if the following items are identified. (1) Significant deviations from research protocol. (2) In the event of wilful misconduct or negligence on the part of the principal investigator or subinvestigator or medical malpractice. (3) In the event of illegal acts or default by a third party. (4) In the case of wilful misconduct or gross negligence on the part of the research subject.
Discussion
Worldwide, the name was changed from NAFLD to MASLD in 2023. In this study, all cases were complicated with hypertriglyceridaemia, and they met the cardiometabolic risk factor conditions for MASLD, so the notation was changed from NAFLD to MASLD. The aim of this study was to evaluate the efficacy of pemafibrate in patients with MASLD. As the drug being evaluated is a therapeutic drug for hypertriglyceridaemia and the frequency of MASLD complications in patients with hypertriglyceridaemia is high, patients with MASLD with hypertriglyceridaemia should serve as an appropriate target group to test the medical efficacy of the treatment.
Pemafibrate, a SPPARMα, enhances the expression of genes associated with fatty acid β-oxidation, such as FGF21,23 and decreases VLDL secretion from the liver.24 By inhibiting VLDL secretion and promoting TG clearance, it potentially lowers serum TG levels and increases HDL-C levels.25 In a MASLD/MASH mouse model, pemafibrate reduced liver function test values and improved fatty liver, ballooning, inflammation and fibrosis.26 27 There have been numerous studies looking at the efficacy of pemafibrate in patients with MASLD. However, only one RCT has been conducted to date.28 In that RCT, MRE-based liver stiffness was significantly reduced by pemafibrate at week 48 compared with placebo and was maintained until week 72. Additionally, significant reductions in ALT and LDL-C levels were observed. These results suggest that pemafibrate is a promising new therapeutic candidate for the treatment of MASLD/MASH.
Pemafibrate has also attracted attention owing to its safety. Because pemafibrate is metabolised in the liver and excreted in bile, long-term administration of pemafibrate has been shown to be effective and safe in patients with dyslipidaemia, including those with renal dysfunction.29 Therefore, we examined the safety of pemafibrate in the present study.
In some well-known trials, the primary endpoint was liver histology evaluated using liver biopsy specimens.30 31 Liver histology endpoints, such as the complete resolution of MASH, are considered surrogates for preventing cirrhosis in that they potentially predict clinical benefit. However, liver biopsy can pose limitations in terms of cost, possible risks, interobserver and intraobserver bias, sampling errors and healthcare resource utilisation.32 33 In this study, ALT was selected as the primary endpoint because it can monitor the degree of hepatocellular damage and is versatile enough to be performed at both primary and secondary medical institutions.
This study has several strengths. First, it was a multicentre study that included primary and secondary medical institutions, as well as tertiary institutions. Second, the sample size was relatively large, with 120 patients in each group for a total of 360 patients. Third, in addition to various fibrosis markers, USE and MRE were performed at facilities where available. However, this study had some limitations. First, the study was open-label. Second, no liver biopsies were performed. Third, factors such as food intake and physical activity, which may influence the development and progression of MASLD, have not been evaluated. Fourth, there is no standardised approach to blood testing in this study, as not all facilities use the same analysis methods, measurement principles, calibrators or reagents. Finally, liver hardness was not measured using USE or MRE in any patient. In the next phase, the efficacy will be confirmed by further evaluation, including histological assessment and long-term prognosis.
MASLD/MASH has a complex pathophysiology involving multiple metabolic pathways. This study aims to confirm the efficacy and safety of pemafibrate. After confirming the efficacy and safety of pemafibrate, we proceeded to the next phase.
supplementary material
Acknowledgements
We thank Editage (https://editage.jp) for editing this manuscript and for helping draft the abstract. We also thank all the patients, patient advisers, staff and investigators involved in this study.
Footnotes
Funding: This work was supported by Yokohama City University and funded by Kowa Company (Tokyo, Japan). We do not have an award/supply number. The funder played no role in the study design, data collection, data analysis, data interpretation or manuscript writing. The corresponding author had full access to all data and had the final responsibility for the decision to submit for publication.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-088862).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Michihiro Iwaki, Email: michihirokeidai@yahoo.co.jp.
Takashi Kobayashi, Email: tkbys@yokohama-cu.ac.jp.
Asako Nogami, Email: nogamia@yokohama-cu.ac.jp.
Yuji Ogawa, Email: yuji.ogawa01@gmail.com.
Kento Imajo, Email: kento318@yokohama-cu.ac.jp.
Eiji Sakai, Email: eiji-not_found@goo.jp.
Yoshinobu Nakada, Email: yoshinobu_nakada@tsc.u-tokai.ac.jp.
Satoshi Koyama, Email: sk581007@yahoo.co.jp.
Takeo Kurihashi, Email: kurihasi@cd5.so-net.ne.jp.
Noriko Oza, Email: ohza-n@koseikan.jp.
Toshikazu Kohira, Email: kohhi831@yahoo.co.jp.
Michiaki Okada, Email: f8388@cc.saga-u.ac.jp.
Yuki Yamaguchi, Email: yamaguchi-y@masuda.jrc.or.jp.
Shinji Iwane, Email: ssmhkiwan@gmail.com.
Fujito Kageyama, Email: kageyama-f@hmedc.or.jp.
Yuzo Sasada, Email: sasada@hospital.iwata.shizuoka.jp.
Masahiro Matsushita, Email: double-precision@shimada-gmc.jp.
Akimitsu Tadauchi, Email: a_tada@sis.seirei.or.jp.
Gou Murohisa, Email: murohisa@sis.seirei.or.jp.
Masamichi Nagasawa, Email: m-nagasa@sis.seirei.or.jp.
Shuichi Sato, Email: bbsato@outlook.jp.
Kazuhisa Maeda, Email: kaz@kitasenri-maeda-cl.com.
Koichiro Furuta, Email: f73767km@gmail.com.
Ryuta Shigefuku, Email: shigefuku@clin.medic.mie-u.ac.jp.
Yuya Seko, Email: yuyaseko@koto.kpu-m.ac.jp.
Hiroshi Tobita, Email: ht1020@med.shimane-u.ac.jp.
Kazuhito Kawata, Email: kawata@hama-med.ac.jp.
Miwa Kawanaka, Email: m.kawanaka@med.kawasaki-m.ac.jp.
Takaaki Sugihara, Email: sugitaka@tottori-u.ac.jp.
Nobuharu Tamaki, Email: nobuharu.tamaki@gmail.com.
Motoh Iwasa, Email: m.iwasa@za.ztv.ne.jp.
Takumi Kawaguchi, Email: takumi@med.kurume-u.ac.jp.
Yoshito Itoh, Email: yif1m2yy@yahoo.co.jp.
Atsushi Kawaguchi, Email: akawa@cc.saga-u.ac.jp.
Hirokazu Takahashi, Email: takahas2@cc.saga-u.ac.jp.
Atsushi Nakajima, Email: nakajima-tky@umin.ac.jp.
Masato Yoneda, Email: yoneda@yokohama-cu.ac.jp.
Data availability statement
Data are available upon reasonable request.
References
- 1.Kanwal F, Neuschwander-Tetri BA, Loomba R, et al. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology. 2024;79:1212–9. doi: 10.1097/HEP.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–95. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 3.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Hagström H, Vessby J, Ekstedt M, et al. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J Hepatol. 2024;80:e76–7. doi: 10.1016/j.jhep.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Ratziu V, Boursier J, de Ledinghen V, et al. Confirmatory biomarker diagnostic studies are not needed when transitioning from NAFLD to MASLD. J Hepatol. 2024;80:e51–2. doi: 10.1016/j.jhep.2023.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Tao X, Zeng M, et al. Clinical and histological features under different nomenclatures of fatty liver disease: NAFLD, MAFLD, MASLD and MetALD. J Hepatol. 2024;80:e64–6. doi: 10.1016/j.jhep.2023.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amarapurkar DN, Hashimoto E, Lesmana LA, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–93. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Miranda C, Pérez-Carreras M, Colina F, et al. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–5. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Yaghoubi M, Jafari S, Sajedi B, et al. Comparison of fenofibrate and pioglitazone effects on patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2017;29:1385–8. doi: 10.1097/MEG.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 11.El-Haggar SM, Mostafa TM. Comparative clinical study between the effect of fenofibrate alone and its combination with pentoxifylline on biochemical parameters and liver stiffness in patients with non-alcoholic fatty liver disease. Hepatol Int. 2015;9:471–9. doi: 10.1007/s12072-015-9633-1. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki Y, Abe K, Toma T, et al. Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor alpha agonists. Bioorg Med Chem Lett. 2007;17:4689–93. doi: 10.1016/j.bmcl.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 13.Ishibashi S, Arai H, Yokote K, et al. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J Clin Lipidol. 2018;12:173–84. doi: 10.1016/j.jacl.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Kamimura T, Hounslow N, Suganami H, et al. Drug-drug interactions between pemafibrate and statins on pharmacokinetics in healthy male volunteers: Open-label, randomized, 6-sequence, 3-period crossover studies. Clin Transl Sci. 2024;17:e13900. doi: 10.1111/cts.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violi F, Cangemi R. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;363:1185–6. doi: 10.1056/NEJMc1006581. [DOI] [PubMed] [Google Scholar]
- 16.ZIMMERMAN HJ, WEST M. SERUM ENZYME LEVELS IN THE DIAGNOSIS OF HEPATIC DISEASE. Am J Gastroenterol. 1963;40:387–404. [PubMed] [Google Scholar]
- 17.WROBLEWSKI F. The clinical significance of transaminase activities of serum. Am J Med. 1959;27:911–23. doi: 10.1016/0002-9343(59)90175-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim WR, Flamm SL, Di Bisceglie AM, et al. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 19.Arai H, Yamashita S, Yokote K, et al. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J Atheroscler Thromb . 2018;25:521–38. doi: 10.5551/jat.44412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki E, Yamashita S, Arai H, et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52‐week data from the PROVIDE study. Diabetes Obesity Metabolism. 2019;21:1737–44. doi: 10.1111/dom.13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki E, Yamashita S, Arai H, et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients With Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care. 2018;41:538–46. doi: 10.2337/dc17-1589. [DOI] [PubMed] [Google Scholar]
- 22.Ichihara K, Yomamoto Y, Hotta T, et al. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann Clin Biochem. 2016;53:347–56. doi: 10.1177/0004563215608875. [DOI] [PubMed] [Google Scholar]
- 23.Takei K, Nakagawa Y, Wang Y, et al. Effects of K-877, a novel selective PPARα modulator, on small intestine contribute to the amelioration of hyperlipidemia in low-density lipoprotein receptor knockout mice. J Pharmacol Sci. 2017;133:214–22. doi: 10.1016/j.jphs.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Kanno K, Koseki M, Chang J, et al. Pemafibrate suppresses NLRP3 inflammasome activation in the liver and heart in a novel mouse model of steatohepatitis-related cardiomyopathy. Sci Rep. 2022;12:2996. doi: 10.1038/s41598-022-06542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennuyer N, Duplan I, Paquet C, et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200–8. doi: 10.1016/j.atherosclerosis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Takei K, Han S-I, Murayama Y, et al. Selective peroxisome proliferator-activated receptor-α modulator K-877 efficiently activates the peroxisome proliferator-activated receptor-α pathway and improves lipid metabolism in mice. J Diabetes Investig. 2017;8:446–52. doi: 10.1111/jdi.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda Y, Kessoku T, Ogawa Y, et al. Pemafibrate, a novel selective peroxisome proliferator-activated receptor alpha modulator, improves the pathogenesis in a rodent model of nonalcoholic steatohepatitis. Sci Rep. 2017;7:42477. doi: 10.1038/srep42477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima A, Eguchi Y, Yoneda M, et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1263–77. doi: 10.1111/apt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokote K, Yamashita S, Arai H, et al. Long-Term Efficacy and Safety of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor-alpha Modulator (SPPARMalpha) 2019;20 doi: 10.3390/ijms20030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadranel JF. Good clinical practice guidelines for fine needle aspiration biopsy of the liver: past, present and future. Gastroenterol Clin Biol. 2002;26:823–4. [PubMed] [Google Scholar]
- 33.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]