Abstract
Introduction
Patients with type 2 diabetes (T2D) undergoing dialysis exhibit a higher mortality rate compared with those with other conditions, primarily due to vascular complications including coronary artery disease, heart failure and stroke. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a type of drug for T2D, have reportedly decreased cardiovascular and renal events in patients with heart failure and chronic kidney disease, irrespective of diabetes presence. Nevertheless, the evidence supporting the use of SGLT2 inhibitors in patients undergoing dialysis has been limited. Our study aims to evaluate the impact of SGLT2 inhibitors on cardiovascular disease in individuals with T2D undergoing peritoneal dialysis (PD).
Methods and analysis
The CARD-PD study is a multicentre, prospective, randomised, open-label comparison trial of canagliflozin treatment in patients diagnosed with T2D undergoing PD. Eligible patients meeting the criteria for participation will be randomly assigned to either the canagliflozin treatment group (100 mg/day) or the control group (delayed-start canagliflozin group) for a duration of 6 months. We set a target of 18 participants in each group (a total of 36) based on sample size calculations from a previous report. Randomisation is performed using a web-based system, wherein patients are stratified by age, sex and plasma brain natriuretic peptide (BNP) concentrations at the baseline. The primary outcome measure is the plasma BNP levels after 6-month period. Following this initial phase, patients from both groups will continue to receive canagliflozin treatment (100 mg/day) in the following manner: (1) patients in the canagliflozin group will continue canagliflozin treatment for an additional 6 months, while (2) patients initially in the placebo arm will transition to canagliflozin treatment for an additional 12 months.
Ethics and dissemination
The Ethics Review Board of Hokkaido University Hospital (CRB no. 1180001) has approved the CARD-PD study protocol. The results will be disseminated in peer-reviewed journals and summaries will be presented at scientific conferences.
Trial registration number
Japan Registry of Clinical Trials (jRCT1011210022); pre-results.
Keywords: Nephrology, Dialysis, Cardiovascular Disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The CARD-PD trial is a prospective, multicentre, randomised open-label study designed to compare treatment with and without the sodium-glucose cotransporter 2 (SGLT2) inhibitor in peritoneal dialysis (PD) patients with diabetes.
The trial incorporates a significant evaluation of key clinical outcomes, including changes in brain natriuretic peptide levels, residual renal function and PD efficacy, which will provide valuable insights into the comprehensive effects of SGLT2 inhibitors in PD patients with diabetes.
As an open-label trial, the study is vulnerable to conscious or unconscious bias, particularly in the assessment of subjective endpoints.
Variations in treatment, including the addition of antihypertensive or diuretic medications, may affect the consistency of intervention effects across patients.
Introduction
Diabetic kidney disease (DKD) manifests as proteinuria, glomerular sclerosis and progressive renal dysfunction, ultimately leading to end-stage renal disease (ESRD). Patients with DKD exhibit a higher prevalence of cardiovascular disease (CVD) due to several contributing factors, including abnormal metabolism, hypertension, exposure to uremic toxins, mineral bone disorders and anaemia. The occurrence of ESRD in patients with DKD significantly amplifies cardiovascular morbidity and mortality.1 Approximately 30% of these fatalities are attributed to heart failure, fluid overload and ischaemic heart disease,2 prompting the need for innovative therapeutic strategies in managing CVD among patients with ESRD.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors function by reducing renal glucose reabsorption, increasing urinary glucose excretion and lowering blood glucose levels. Recent studies have revealed that SGLT2 inhibitors improve cardiac and renal outcomes in individuals with heart failure and CKD, with or without diabetes.3 4 Clinical observations and basic research indicate that the mechanisms underlying nephroprotective effects extend beyond glycaemic control. These renoprotections are attributed to several mechanisms, including tubuloglomerular feedback, improved tubular oxygenation and the regulation of renal inflammation and fibrosis, potentially contributing to the prevention of cardiovascular events.5 Additionally, SGLT2 inhibitors moderately inhibit SGLT1, which is expressed in the heart as well as the proximal tubule of the kidney. SGLT1 inhibition may contribute to cardiac protection independently of renoprotection, as it is involved in the pathogenesis of cardiac oxidative stress, inflammation and fibrosis.6 Clinical studies have also revealed that SGLT inhibition improves renal outcomes in patients with DKD with severe renal dysfunction,7 8 suggesting these agents might influence damaged kidney. However, there is a limited number of evidence regarding the efficacy of SGLT2 inhibition in patients undergoing dialysis.
This study aims to assess the impact of SGLT2 inhibitors on cardiac outcomes in patients with diabetes undergoing peritoneal dialysis (PD) with residual renal function. PD eliminates unwanted substances and fluids from the blood via the peritoneum in individuals with kidney failure. Continuous PD is associated with a slower decrease in residual renal function.9 The ultrafiltration process is driven by an osmotic pressure gradient by storing dialysate solutions with high glucose concentrations in the abdominal cavity for a certain period. Whereas, in individuals with type 2 diabetes (T2D) on PD, elevated blood glucose levels could minimise the osmotic gradient, leading to ultrafiltration failure, which contributes to the development of CVD. Thus, SGLT2 inhibitors might potentially enhance PD efficiency and protect residual renal function.
In this prospective, randomised, open-label trial, we aim to investigate whether the use of SGLT2 inhibitors in diabetic PD patients influences CVD outcomes by protecting residual kidney function, alleviating cardiac stress and improving ultrafiltration in PD.
Methods
Study design
The CARD-PD is a multicentre, prospective, randomised, open-label, comparative study to evaluate the efficacy of canagliflozin in managing CVD in patients with T2D on PD. The study period will be from 16 July 2021 to 31 March 2025. Following enrolment and the provision of written informed consent, participants will undergo a series of assessments including physical examination, biochemical analysis and various imaging tests, which include peritoneal equilibration test (PET), CT, cardiac echocardiography (echo), cervical echo, Holter ECG, chest X-ray and bone mineral density. PET provides detailed information regarding peritoneal membrane function and assesses any changes in its permeability. CT is performed to evaluate coronary artery disease. Cardiac echo assesses cardiac function and structure, crucial for detecting heart diseases. Cervical echo assesses the progression of arteriosclerosis to examine its association with the risk of cardiovascular disease. Holter ECG monitors heart rhythms over 24 hours, detecting arrhythmias. Chest X-ray provides information about heart size and lung condition. Bone mineral density measurement evaluates the presence of bone mineral disorders common in patients with chronic kidney disease (CKD).
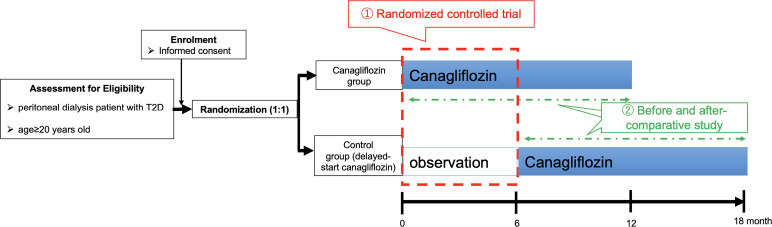
During these initial assessments, participants are randomly assigned to either the canagliflozin (100 mg once daily) treatment group or the control group for a period of 6 months. Participants are stratified by age, sex and baseline brain natriuretic peptide (BNP) concentration, and the randomisation is performed using a web-based system. The primary endpoint of this comparative study is the plasma BNP levels after 6 months between the canagliflozin treatment and the control groups (refer to figure 1). Following this initial phase, participants in both groups continue to receive canagliflozin. Specifically, patients in the canagliflozin group continue the medication for an additional 6 months, while those in the control arm are switched to canagliflozin for another 12 months as a delayed-start canagliflozin group (refer to figure 1). The efficacy of canagliflozin before and after treatment in managing CVD will be assessed for all participants in both groups (canagliflozin group and delayed-start canagliflozin group). Throughout the study, measurements including blood pressure, pulse rate, body weight, water removal during daily PD and serum and urine metabolic parameters will be taken at each study visit. Patients with end-stage CKD face multiple clinical problems, including CVD, fluid imbalance, mineral and bone disorders, inflammation and oxidative stress. To evaluate the effect of SGLT2 inhibitors on these issues, PET, CT (a coronary CT angiography), cardiac echo, cervical echo, Holter ECG, chest X-ray and bone mineral density measurement will be performed at months 0, 6 and 12 for the canagliflozin group and at months 0, 6, 12 and 18 for the delayed-start canagliflozin group (tables1 2). Throughout the study period, all adverse events related to the study drug will be reported to the head of the site and the principal researcher.
Figure 1. Patient recruitment scheme. Randomised participants will be assigned to either the canagliflozin (100 mg once daily) treatment group or the control group for a period of 6 months. Following this initial phase, participants in both groups continue to receive canagliflozin. Patients in the canagliflozin group continue the medication for an additional 6 months, while those in the control arm are switched to canagliflozin for another 12 months as a delayed-start canagliflozin group. T2D, type 2 diabetes.
Table 1. Observation, test and evaluation schedule canagliflozin treatment group.
| Visit | Observation period | ||||
| Before starting | Week 0 | Week 12 (month 3) | Week 24 (month 6) | Week 52 (month 12) | |
| Schedule window | – | – | ±6 week | ±9 week | ±12 week |
| Informed consent | X | ||||
| Enrolment | X | ||||
| Allocation | X | ||||
| Patient characteristics | X | ||||
| Physical examination | X | X | X | X | X |
| Blood test | X | X | X | X | |
| Plasma BNP | X | X | X | X | |
| Image tests* | X | X | X | ||
| Kt/Vurea, PET† | X | X | X | ||
| Use of concomitant drugs |

|
||||
| Adverse events |

|
||||
Image tests; CT scan, cardiac echo, cervical echo, Holter ECG, chest X-ray and bone mineral density measurement at week 0 should be performed 12 weeks prior to the start of administration to week 0.
Kt/Vurea, PET; week 0 should be performed 24 weeks prior to the start of administration to 0 week. Week 24 is not required.
BNPbrain natriuretic peptidePETperitoneal equilibration test
Table 2. Control group (delayed-start canagliflozin group).
| Visit | Observation period | ||||||
| Before starting | Week 0 | Week 12(month 3) | Week 24(month 6) | Week 36(month 9) | Week 48(month 12) | Week 76(month 18) | |
| Schedule window | – | – | ±6 week | ±9 week | ±12 week | ±12 week | ±12 week |
| Informed consent | X | ||||||
| Enrolment | X | ||||||
| Allocation | X | ||||||
| Patient characteristics | X | ||||||
| Physical examination | X | X | X | X | X | X | X |
| Blood test | X | X | X | X | X | X | |
| Plasma BNP | X | X | X | X | X | X | |
| Image tests* | X | X | X | X | |||
| Kt/Vurea, PET† | X | X | X | X | |||
| Use of concomitant drugs |

|
||||||
| Adverse events |

|
||||||
Image tests; CT scan, cardiac echo, cervical echo, Holter ECG, chest X-ray and bone mineral density measurement at week 0 should be performed from 12 weeks prior to the start of observation to week 0.
Kt/Vurea, PET; week 0 should be performed 24 weeks prior to the start of administration to 0 week. Week 48 is not required.
BNPbrain natriuretic peptidePETperitoneal equilibration test
Recruitment strategy
The enrolment of participants in this study will take place between 16 July 2021 and 30 June 2023, at eight hospitals and clinics located in Hokkaido, Japan. The study will be conducted from 16 July 2021 to 31 March 2025. The inclusion criteria for this trial include individuals with T2D undergoing PD as a form of renal replacement therapy, aged ≥20 years (see box 1). The exclusion criteria are defined as: within 3 months after the initiation of PD, unstable hypertension (systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥110 mm Hg) and inconsistency with the trial for other reasons, as decided by the physician (see box 2).
Box 1. Inclusion criteria.
Age ≥20 years old.
Patients with type 2 diabetes undergoing peritoneal dialysis, as a renal replacement therapy.
Patients from whom written informed consent was obtained.
Box 2. Exclusion criteria.
Within 3 months after the initiation of peritoneal dialysis.
Unstable hypertension (systolic blood pressure ≥180 mm Hg or diastolic blood pressure ≥110 mm Hg).
Inconsistency with the trial for other reasons, as decided by the physician.
Patients will be discontinued from the trial if any of the following criteria apply: (1) withdrawal of consent, (2) physician’s decision based on the patient’s condition, (3) discontinuation of this clinical study or (4) physician’s decision based on other reasons.
Intervention
All participants will be randomly assigned to the canagliflozin group that starts taking canagliflozin (100 mg/day) at month 0 of observation or the delayed start canagliflozin group that starts taking canagliflozin (100 mg/day) at month 6 of observation (figure 1). All participants in both groups will receive canagliflozin for 12 months (figure 1).
The treatments will be supervised by the appropriate medical care centres. In principle, treatments that influence the outcome, including doses of antihypertensive drugs, anti-hyperglycaemic agents and PD prescriptions, will not be adjusted during the study period. When patients’ blood pressure and fluid volume are not controlled according to clinical guidelines,10 they will be treated with antihypertensive drugs or have their PD prescription adjusted at the discretion of the attending physician. If there is an increased likelihood of hypoglycaemia or the emergence of symptoms suggestive of hypoglycaemia, as defined by relevant guidelines,11 the adjustment of diabetes medications will be considered. The patients’ conditions will be recorded when the medications are changed.
Consent to participate and withdrawal
A physician in the research team will obtain written informed consent from all eligible participants. The research committee has approved the written material, consisting of a participant information leaflet and consent documentation. There will be an opportunity for participants to freely ask questions to members of the research team, and their consent can be withheld at any time during the study period.
Randomisation and allocation
Randomisation is centrally carried out using a web-based automated system (Muzinwari; https://autoassign.mujinwari.biz/) and the monitoring is conducted independently of the participating sites. All participants will be randomly assigned to the canagliflozin treatment group or control group (delayed start canagliflozin group) at a ratio of 1:1. To minimise the imbalance of characteristics and influence on the outcome between the two groups, patients are stratified by age, sex and baseline BNP concentrations.
Patient and public involvement statement
Participants are not directly involved in the design or development of the study and will not be involved in the recruitment and conduct of the study. The results of the examinations will be given to the participants, just as in the case of a regular medical examination during a medical consultation in their participating centre.
Trial endpoints
Primary endpoint
The primary endpoint will be the plasma BNP levels after 6 months, which will be compared between the canagliflozin treatment and control groups.
Secondary endpoints
-
Comparative study between canagliflozin treatment and control groups (6 months, refer to figure 1)
changes in:
(a) BNP levels, (b) cardiac echo parameters (left ventricular ejection fraction, left atrial enlargement, ventricular filling pressure), (c) coronary artery calcification score, (d) metabolic parameters (glycated haemoglobin (HbA1c), free plasma glucose), (e) PD-related markers (Kt/Vurea, volume of dehydration, renal anaemia, dose of erythropoiesis-stimulating agents, dialysis prescription),(f) CVD-related tests (cervical echo, Holter ECG, chest X-ray), (g) bone mineral density and markers of bone turnover (bone alkaline phosphatase and tartrate-resistant acid phosphatase 5b), (h) marker of reactive oxygen species (serum 8-hydroxy-2’-deoxyguanosine), (i) blood pressure, (j) body weight and (k) body mass index.
-
Efficacy of canagliflozin treatment for 12 months in all participants (refer to figure 1)
changes in:
(a) BNP levels, (b) cardiac echo parameters, (c) coronary artery calcification score, (d) metabolic parameters (HbA1c, free plasma glucose), (e) PD-related markers (Kt/Vurea, volume of dehydration, renal anaemia, dose of erythropoiesis-stimulating agents, dialysis prescription), (f) CVD-related tests (cervical echo, Holter ECG, chest X-ray), (g) bone mineral density and markers of bone turnover, (h) marker of reactive oxygen species, (i) blood pressure, (j) body weight and (k) body mass index.
Sample size calculation
The primary endpoint of this study is the change in BNP levels from baseline after 6 months in randomised controlled trial of canagliflozin. Our sample size determination was based on previous data indicating a significant decrease in BNP levels with canagliflozin treatment compared with baseline levels (baseline: 73.5 pg/mL, 6 months after canagliflozin: 48.4 pg/mL) in patients diagnosed with T2D and heart failure.12 To demonstrate the superiority of canagliflozin concerning the primary endpoint of BNP, a total of 12 cases are required per group. This is based on a two-sided significance level (alpha error) of 5%, a power of 80% and a common SD of 20.4.
Factoring in an estimated dropout rate of six participants (30%) from each group, we have set the target number of cases at 18 individuals in each group, totalling 36 cases.
Statistical analysis
The primary and secondary endpoints in a randomised controlled trial of canagliflozin will be compared between the canagliflozin and control groups using an unpaired t-test. Additionally, comparisons of changes before and after canagliflozin administration will be conducted at each assessment point (6 and 12 months) using a paired t-test.
For the data analysis, we will employ JMP Pro V.16.1.0 statistical software.
Ethics and dissemination
Ethics approval
The trial was registered with the Japan Registry of Clinical Trials before enrolment commenced. The study protocol has been approved by the Ethics Review Board of Hokkaido University Hospital (CRB no. 1180001; approval number 020–007), and the current version is 4.0 (approval on 16 July 2024). The study will be carried out according to the principles of Declaration of Helsinki and its amendments.
The researchers will explain this study carefully to the candidate participants, confirm that they have understood the information and have written consent from them to voluntarily participate in the study.
Data protection and management
Data management, including coding, security, storage and cleaning, will be performed by researchers to ensure that any personal information about potential and enrolled participants will be coded and handled confidentially before, during and after the trial. The study data will be archived at Hokkaido University for 5 years after study completion or 3 years after disclosure of the study results. The participants will be able to obtain the final results of the study. The jRCT databases will contain detailed information on this study. The study will be evaluated by a monitor, independent of the investigators. No audits of the study sites are scheduled. According to the Clinical Trials Act in Japan, adverse events and other information, including modifications of the trial, will be reported and disclosed publicly. The findings obtained from this study will be presented at academic meetings and in peer-reviewed academic journals.
Availability of data and materials
There is no plan for sharing data from this study. However, on completion of the data set, data may be made available on reasonable request and after appropriate approvals.
Study background and expected outcomes
In this study, we hypothesise that SGLT2 inhibitors improve cardiovascular outcomes in patients with diabetes undergoing PD and assess the change in plasma BNP levels in PD patients with T2D treated with SGLT2 inhibitors. To the best of our knowledge, this is the initial prospective clinical trial, comparing the efficacy of SGLT2 inhibitors to a control group in terms of cardiac outcomes in patients with diabetes undergoing PD.
Diabetes mellitus and CKD are commonly linked to major CVD risk factors. Notably, in patients with ESRD, cardiovascular events represent the primary cause of mortality.13 For individuals undergoing dialysis, optimal fluid volume management constitutes a crucial facet of dialysis adequacy. However, this approach is occasionally challenging due to multiple factors, including inadequate self-management and dialysis procedures. Excessive fluid retention can result in hypertension, fluid overload and subsequent myocardial hypertrophy, ultimately contributing to the cardiac events.14,16
SGLT2 inhibitors, a class of oral drugs that lower blood glucose levels by facilitating urinary glucose excretion, have been documented to not only improve renal function, but also to influence the development of CVD. These inhibitors increase sodium excretion in the urine and urine volume, potentially leading to appropriate fluid volume management and subsequently lowering blood pressure. In addition to their diuretic effects, SGLT2 inhibitors promote the enhancement of tubular oxygenation, proper tubuloglomerular feedback and the regulation of renal inflammation, thereby resulting in renoprotection.5 These effects have been demonstrated even in patients with diabetes with severe renal dysfunction.8 17 Moreover, these reno-protective effects are believed to contribute to the improvement of CVD. In addition to SGLT2 inhibition, SGLT1 inhibition can potentially mitigate CVD risk by involving the reduction of myocardial oxidative stress, inhibition of excessive inflammation, improvement in vascular endothelial function and reduction of vessel wall abnormalities.5 Notably, among SGLT2 inhibitors, canagliflozin has a moderate effect to inhibit SGLT1.6 Therefore, we employed canagliflozin in this study, expecting cardiac protection through both direct and indirect effects.
Furthermore, considering that SGLT2 is expressed on the human peritoneum and its inhibition has shown to reduce glucose uptake and enhance ultrafiltration through the peritoneum in animal models,18 19 it is speculated that SGLT inhibitions in PD patients might maintain an osmotic pressure gradient, contributing to better management of fluid volume and preventing CVD. In this study, we accessed BNP levels as a marker of CVD. BNP is rapidly produced and secreted, primarily in the ventricle, in response to stretching stress. It serves as a useful marker of left ventricular systolic function and cardiac dysfunction. Moreover, in dialysis patients, BNP is associated with left ventricular mass and function, predicting mortality. Furthermore, SGLT2 inhibitors have reduced BNP levels in patients with heart failures and diabetes, suggesting that BNP could be an optimal marker in this study.
Acknowledgements
We thank study participants, their families and investigators in this study.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Prepub: Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084846).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Naoko Matsuoka, Email: naokomatsuoka815@huhp.hokudai.ac.jp.
Daigo Nakazawa, Email: daigo-na@med.hokudai.ac.jp.
Saori Nishio, Email: saorin@med.hokudai.ac.jp.
Kyu Yong Cho, Email: kyuyong-cho@med.hokudai.ac.jp.
Tomochika Maoka, Email: t.maoka@east.ntt.co.jp.
Nobuharu Kaneshima, Email: nobuharu.kaneshima.ht@east.ntt.co.jp.
Rie Yamamoto, Email: rie.yamamoto@east.ntt.co.jp.
Junya Yamamoto, Email: ysgbs814@yahoo.co.jp.
Mamiko Shimamoto, Email: mamiko.shimamoto@doc.city.sapporo.jp.
Minoru Makita, Email: makita@huhp.hokudai.ac.jp.
Satoko Iriuda, Email: IZS02567@nifty.ne.jp.
Kento Igarashi, Email: chincusus@gmail.com.
Yosuke Ito, Email: itohyo@nirenomori.jp.
Akiko Kato, Email: akakumijp@yahoo.co.jp.
Junpei Yoshikawa, Email: sp7n3cz9@star.ocn.ne.jp.
Takashi Kudo, Email: nattu1231@yahoo.co.jp.
Takahiro Nagashima, Email: nagashima_takahiro@kitamirch.jp.
Yoichi M Ito, Email: ito-ym@med.hokudai.ac.jp.
Tatsuya Atsumi, Email: at3tat@med.hokudai.ac.jp.
References
- 1.de Boer IH, Bakris GL. Diabetic Kidney Disease: A Determinant of Cardiovascular Risk in Type 1 Diabetes. Diabetes Care. 2018;41:662–3. doi: 10.2337/dci17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhandari SK, Zhou H, Shaw SF, et al. Causes of Death in End-Stage Kidney Disease: Comparison between the United States Renal Data System and a Large Integrated Health Care System. Am J Nephrol. 2022;53:32–40. doi: 10.1159/000520466. [DOI] [PubMed] [Google Scholar]
- 3.Yanai H, Hakoshima M, Adachi H, et al. Multi-Organ Protective Effects of Sodium Glucose Cotransporter 2 Inhibitors. Int J Mol Sci. 2021;22:4416. doi: 10.3390/ijms22094416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Costanzo A, Esposito G, Indolfi C, et al. SGLT2 Inhibitors: A New Therapeutical Strategy to Improve Clinical Outcomes in Patients with Chronic Kidney Diseases. Int J Mol Sci. 2023;24:8732. doi: 10.3390/ijms24108732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey CJ, Day C, Bellary S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr Diab Rep. 2022;22:39–52. doi: 10.1007/s11892-021-01442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolov V, Yakovleva T, Chu L, et al. Differentiating the Sodium-Glucose Cotransporter 1 Inhibition Capacity of Canagliflozin vs. Dapagliflozin and Empagliflozin Using Quantitative Systems Pharmacology Modeling. CPT Pharmacometrics Syst Pharmacol. 2020;9:222–9. doi: 10.1002/psp4.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakris G, Oshima M, Mahaffey KW, et al. Effects of Canagliflozin in Patients with Baseline eGFR <30 ml/min per 1.73 m(2): Subgroup Analysis of the Randomized CREDENCE Trial. Clin J Am Soc Nephrol. 2020;15:1705–14. doi: 10.2215/CJN.10140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow GM, Vart P, Jongs N, et al. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol. 2021;32:2352–61. doi: 10.1681/ASN.2021020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrón B, Remón C, Pérez-Fontán M, et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int Suppl. 2008;2008:S42–51. doi: 10.1038/sj.ki.5002600. [DOI] [PubMed] [Google Scholar]
- 10.Flythe JE, Chang TI, Gallagher MP, et al. Blood pressure and volume management in dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:861–76. doi: 10.1016/j.kint.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes A 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43:S37–47. doi: 10.2337/dc20-S004. [DOI] [PubMed] [Google Scholar]
- 12.Sezai A, Sekino H, Unosawa S, et al. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. doi: 10.1186/s12933-019-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoccali C, Mark PB, Sarafidis P, et al. Diagnosis of cardiovascular disease in patients with chronic kidney disease. Nat Rev Nephrol. 2023;19:733–46. doi: 10.1038/s41581-023-00747-4. [DOI] [PubMed] [Google Scholar]
- 14.Koc M, Toprak A, Tezcan H, et al. Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol Dial Transplant. 2002;17:1661–6. doi: 10.1093/ndt/17.9.1661. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Axelsson J, Lindholm B, et al. Volume status and blood pressure in continuous ambulatory peritoneal dialysis patients. Blood Purif. 2005;23:373–8. doi: 10.1159/000087194. [DOI] [PubMed] [Google Scholar]
- 16.Tian N, Guo Q, Zhou Q, et al. The Impact of Fluid Overload and Variation on Residual Renal Function in Peritoneal Dialysis Patient. PLoS One. 2016;11:e0153115. doi: 10.1371/journal.pone.0153115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and Renal Outcomes With Canagliflozin According to Baseline Kidney Function. Circulation. 2018;138:1537–50. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schricker S, Oberacker T, Fritz P, et al. Peritoneal Expression of SGLT-2, GLUT1, and GLUT3 in Peritoneal Dialysis Patients. Kidney Blood Press Res. 2022;47:125–34. doi: 10.1159/000520894. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Fan J, Zheng C, et al. SGLT-2 inhibitors reduce glucose absorption from peritoneal dialysis solution by suppressing the activity of SGLT-2. Biomedicine & Pharmacotherapy. 2019;109:1327–38. doi: 10.1016/j.biopha.2018.10.106. [DOI] [PubMed] [Google Scholar]