Abstract
Introduction
Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are associated with weight loss, diverse cardiorenal benefits and improved glycaemic control. However, the effects of SGLT2i on physical function and fitness are uncertain. The Dapagliflozin, Exercise Training and physicAl function trial investigates whether the SGLT2i dapagliflozin, alone or in combination with structured exercise training, improves physical function compared with diet-induced weight loss in adults with type 2 diabetes mellitus (T2DM), overweight/obesity and impaired physical function.
Methods and analysis
This single-centre randomised controlled trial will assign 1:1:1, 135 adults with T2DM and low physical function to receive one of three treatments: (1) dapagliflozin (10 mg once-daily) alone, (2) dapagliflozin (10 mg once-daily) plus structured exercise training or (3) diet control (where participants are supported to achieve 3% weight loss, equivalent to estimated weight loss with dapagliflozin treatment). Primary and secondary outcomes will be assessed at baseline, 12 and 24 weeks. The primary outcome is the difference in physical function, assessed using the modified Physical Performance Test, between the treatment groups and diet control at 24 weeks. Secondary outcomes include MRI-measured cardiac structure and function, maximal aerobic capacity, resting metabolic rate, device-measured physical activity and sleep, body composition, haemoglobin A1c and cardiovascular risk markers.
Ethics and dissemination
The Heath Research Authority (HRA) and the Medicines and Healthcare Products Regulatory Authority (MHRA) Research Ethics Committee have approved the study. The findings of the study will be published in peer-reviewed journals.
Trial registration number
ISRCTN11459997.
EudraCT number
2019-004586-41.
Keywords: Frailty, DIABETES & ENDOCRINOLOGY, CLINICAL PHARMACOLOGY, Randomized Controlled Trial
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The impact of dapagliflozin alone and in combination with exercise training on physical function is under-researched.
This is the first dedicated randomised controlled trial investigating the impact of dapagliflozin on physical function as a primary outcome.
The design of the study is robust and will be conducted by an expert multidisciplinary research team.
Self-selection/participation bias when recruiting volunteers to randomised controlled trials might limit the generalisability of the findings to the broader population of people with type 2 diabetes mellitus.
Introduction
Type 2 diabetes mellitus (T2DM) constitutes a state of accelerated metabolic ageing and physical deconditioning, increasing the risk of early-onset frailty or functional impairment.1,4 Given frailty increases the individual and economic burden of T2DM,5,7 frailty status has been proposed as a key component of treatment decision-making and goal setting for people with T2DM.6 8 Adding complexity to patient management planning, frailty in T2DM often occurs within the context of obesity.9 Weight loss through energy restriction ameliorates physical frailty in older adults with obesity10 but can lead to a proportionate loss of lean mass (LM) and relative aerobic capacity, thereby limiting the magnitude of benefits achieved with weight loss through energy restriction alone.11,13
Over the last decade, several new classes of glucose-lowering therapy have been licensed for use in T2DM that also have weight-lowering effects. Sodium-glucose co-transporter 2 inhibitors (SGLT2i), of which Dapagliflozin is the most widely prescribed in Europe,14 act via increased urinary glucose excretion.15 The DECLARE-TIMI 58 (Dapagliflozin Effect on CardiovascuLAR Events trial led by the TIMI group) trial (n >17 000) reported a significantly reduced risk of cardiovascular (−17%) and renal outcomes (−24%) following dapagliflozin therapy in people with T2DM. The cardiorenal benefits elicited following SGLT2i therapy are related to several pleiotropic effects including reduced inflammation and oxidative stress, improved cellular metabolism and vascular function and increased erythropoietin levels.16 The beneficial effects of SGLT2 inhibition are likely to be exerted on multiple physiological systems, potentially augmenting cardiac and skeletal muscle function. Ultimately, this might translate to improvements in physical function that are independent of weight loss and glycaemic control.
Exercise training can improve physical function in the absence of weight loss, driven by beneficial effects on LM and relative aerobic capacity.10 11 13 Quantifying the impact of exercise training alongside existing T2DM management therapies on physical function is a priority for advancing T2DM research.17 The precise interaction between exercise and SGLT2i therapy is poorly understood.18 19 In a small study of people with obesity, adiposity decreased after 12 weeks of aerobic exercise training and dapagliflozin.20 However, compared with exercise with placebo, dapagliflozin therapy elevated lactate and fasting glucose levels during and after exercise training, respectively, while a post-exercise improvement in insulin sensitivity was reported in the placebo group only.20 This study involved people with normal glycaemic control, and it is unclear whether similar responses would occur in those with T2DM.
A 24-week trial investigated the effect of dapagliflozin with or without unsupervised resistance exercise, in adults with T2DM.21 Skeletal muscle index and fat-free mass significantly decreased in both the exercise and control groups, suggesting that exercise interventions may not consistently preserve LM during pharmacologically induced weight loss.21 Supervised exercise programmes elicit greater improvements in physical function22 and are more effective at maintaining LM in older adults.23 Therefore, optimising the benefits of exercise in combination with SGLT2i therapy will likely require adequate supervision of participants undergoing exercise training. To our knowledge, there are no dedicated randomised controlled trials (RCTs) investigating the impact of dapagliflozin, with structured exercise training, on physical function as the primary outcome measure.
Aims and objectives
This study aims to investigate the effect of dapagliflozin, with and without structured exercise training, on physical function compared with matched diet-induced weight loss in adults with T2DM, overweight/obesity and impaired physical function. Secondary objectives are to examine the intervention effect on cardiac function and structure and other markers of physiology underpinning changes in physical function.
Hypotheses
Dapagliflozin, alone and in combination with exercise training, will improve physical function compared with matched diet-induced weight loss, with greater improvements in the combined therapy group.
Methods and analysis
Study design and setting
This is an open-label, single-centre, RCT (EudraCT Number: 2019-004586-41) conducted at the Leicester Diabetes Centre (LDC) and Glenfield Hospital, UK.
Using a validated online system (SealedEnvelope.com), 135 participants will be randomised (1:1:1) to receive one of three treatments over 24 weeks:
Diet control (DIET-CON).
Dapagliflozin (10 mg) once-daily (DAPA).
Dapagliflozin (10 mg) once-daily plus structured aerobic and resistance exercise (DAPA+EX).
Randomisation is stratified by sex, ethnicity and previous use of glucose-lowering therapies.
Recruitment and study population
The study is recruiting participants between 20 May 2021 and 30 March 2025. Participants are identified from primary and secondary care, research volunteer databases and community advertisement. Eligibility criteria are listed in table 1. Briefly, participants are aged 40–75 years, with overweight or obesity, diagnosed T2DM and show evidence of functional limitation or frailty.
Table 1. Eligibility criteria.
| Inclusion criteria | |
| Age | 40–75 years |
| Sex | Male and female |
| Type 2 diabetes mellitus (T2DM) | Diagnosed T2DM, treated by lifestyle management alone or in combination with monotherapy or combination oral glucose-lowering pharmacotherapies (with the exception of predefined exclusion criteria; see below) |
| Haemoglobin A1c | 6.5%–10% (47–86 mmol/mol) inclusive.Amended 8 October 2021 after 23 participants screened to:≤10.5% (≤91 mmol/mol) |
| Frailty or functional limitation | At least one of:
|
| Body mass index | ≥25 kg/m2 (≥22.5 kg/m2 for south Asian ethnicity). |
| Weight stable | <3.kg weight change in preceding 3 months. |
| Treatment stable | No significant change to glucose-lowering regimen in the preceding 3 months. |
| Capacity/willingness | Able and willing to give informed consent.Understands spoken English. |
| Exercise ability | In the opinion of the investigator is able to take part in structured exercise training. |
| Exclusion criteria | |
| Other diabetes | Individuals with type 1, gestational, monogenic diabetes or latent autoimmune diabetes in adults. |
| Other diabetes therapies | Currently taking sodium-glucose co-transporter-2 inhibitors (SGLT2i), glucagon-like peptide-1 agonists, or basal-bolus or premixed insulin therapies. |
| Severe frailty | Scoring 0 on the SPPB, or otherwise presenting with severe functional limitations. |
| Dietary practices | Adherence to a severely energy-restricted diet (<800 kilocalories per day). |
| Renal impairment | Estimated glomerular filtration rate (eGFR)<60 mL/min/1.73 m2 or as per licencing at the point of prescription.Amended 4 October 2023 after 141 participants screened to: eGFR<15 mL/min/1.73 m2 or as per licensing at the point of prescription.Individuals with familial renal glycosuria. |
| Liver and pancreatic disorders | Documented or self-reported cirrhosis.History of chronic pancreatitis. |
| Cardiac disease | Established heart failure. |
| Dietary intolerances | Hereditary galactose intolerance, total lactase deficiency or glucose-galactose malabsorption. |
| Genitourinary infections | History of recurrent balanitis, vaginal or urinary tract infections. |
| Conditions impacting weight and/or safety | Active malignancy (eligibility at discretion of study clinician).Serious illness with life expectancy <1 year or other significant illness which, in the opinion of a study clinician, precludes involvement. |
| Alcohol intake | History of excessive alcohol consumption. |
| Contraindications to interventions | Contraindications to exercise or SGLT2i therapy. |
| Pregnancy and lactation | Current or planned pregnancy, or breast feeding.Females of childbearing potential, unwilling to use adequate contraceptive methods during the study period. |
| Participation in other trials | Current participation in another research study with investigational medical product. |
Patient and public involvement
During protocol development, patient focus groups provided feedback on perceived acceptability of the intervention and number and length of study visits. At the end of the study, participants will be sent a summary of the findings.
Summary of main study visits and contacts
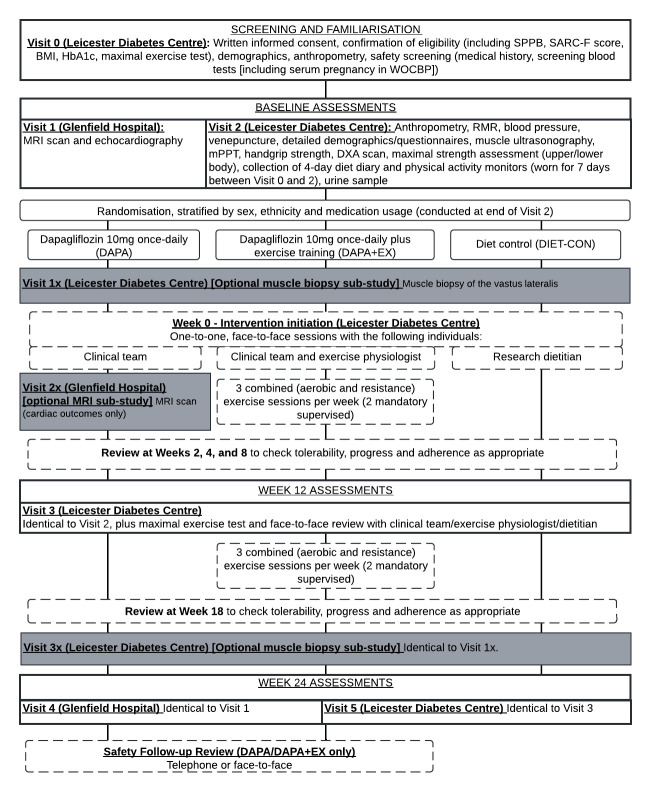
Participants attend a consent and screening visit (visit 0), five main study visits (visits 1–5), a treatment initiation visit (week 0) and five face-to-face/telephone reviews (figure 1). Those participating in optional substudies may attend up to three further visits (visits 1×, 2×, 3×).
Figure 1. Schedule of study visits. BMI, body mass index; DXA, dual-energy X-ray absorptiometry; HBA1c, haemoglobin A1c; mPPT, modified physical performance test; MRI, magnetic resonance imaging; RMR, resting metabolic rate; SPPB, Short Physical Performance Battery; WOCBP, women of childbearing potential.
Visit 0: consent and screening
After providing written informed consent, participants provide a blood sample, undergo anthropometric assessment and complete the strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F) questionnaire, Short Physical Performance Battery (SPPB) and a maximal treadmill exercise test. Eligibility is confirmed once all results are obtained. Participants wear a GENEActiv wrist-worn accelerometer (Activinsights, Kimbolton, UK) for 7 days after visit 0.
Visits 1 and 4: cardiac MRI and echocardiography
Participants undergo cardiac MRI and echocardiography at the beginning (visit 1) and end (visit 4) of the trial.
Visits 2, 3 and 5: physical function and secondary outcomes
Visit 2 assessments include the modified physical performance test (mPPT), fasted blood and urine samples, anthropometry, indirect calorimetry, muscle ultrasonography, dual-energy X-ray absorptiometry (DXA), upper and lower body muscle strength, and questionnaires. Visits 3 and 5 are identical to visit 2 with the addition of the SPPB and maximal exercise test. Food diary (3 weekdays and 1 weekend day) and accelerometer data (7 days) are collected prior to visits 2, 3 and 5.
Weeks 0, 2, 4, 8 and 18: randomisation, intervention initiation and ongoing review
On completion of all baseline measures, participants are randomised and invited for a face-to-face intervention initiation visit (week 0) with the following trial team members: dietitian (DIET-CON); study clinician (DAPA); study clinician and exercise physiologist (DAPA+EX). Face-to-face or telephone review of intervention progress, tolerability and adherence is conducted at week 2, 4, 8 and 18. Participants in the DAPA and DAPA+EX groups have a further safety follow-up review after drug cessation.
Summary of optional substudy visits
For eligible participants, there are two optional substudies.
Visit 1× and 3×: Skeletal muscle biopsy substudy
We aim to consent at least 10 participants from each group for a fasted skeletal muscle biopsy from the vastus lateralis (VL) at visits 1× (before intervention initiation) and 3x (end of trial period).
Visit 2×: cardiac MRI substudy
We aim to consent twenty participants from the DAPA group to have an additional MRI scan at visit 2× (2 weeks after initiating dapagliflozin).
Outcomes
Primary and secondary outcomes are displayed in table 2.
Table 2. Outcomes and measurement time points.
| Outcome | Assessment method | Visit |
| Primary outcome | ||
| Physical function | mPPT | 2, 3, 5 |
| Key secondary outcomes | ||
| Cardiac function and structureLiver, pancreas and kidney structure(MRI and echocardiogram) | Systolic and diastolic strain and strain ratesLV volumeLV massLV mass/volume ratioAortic distensibilityResting/stress-induced myocardial perfusionMyocardial perfusion reserveLiver, pancreas and kidney multiparametric assessment | 1, 4 |
| Additional physical function measures | SPPBWHODAS 2.0mMRC dyspnoea Scale | 0, 3, 52, 3, 52, 3, 5 |
| Aerobic capacity (maximal treadmill exercise test) | Absolute V̇O2peak (L/min) V̇O2peak relative to LM (mL/kg/LM/min) and body weight (mL/kg/BW/min) | 0, 3, 5 |
| Muscle strength | Handgrip dynamometryIsometric and isokinetic quadriceps strength (Biodex)Bicep curl strength | 2, 3, 52, 3, 52, 3, 5 |
| Anthropometry | HeightWeightBMIWaist circumference | 0, 2, 3, 5 |
| Body composition (DXA scan and BIA) | Whole-body and regional FM, LM, FFM, BMD | 2, 3, 5 |
| Skeletal muscle ultrasonography | Quadriceps muscle diameter, volume and quality | 2, 3, 5 |
| Resting metabolic rate | Indirect calorimetry | 2, 3, 5 |
| Physical activity and sleep(GENEactiv accelerometers) | 7-day physical activity monitor | 2, 3, 5 |
| Subjectively measured physical activity | GPPAQ | 2, 3, 5 |
| Glycaemic control | HbA1c | 0, 3, 5 |
| Cardiovascular risk factors | Blood pressureHeart rateLipid profileInflammatory markers | 0, 2, 3, 52, 3, 52, 3, 5 |
| Additional secondary outcomes | ||
| Indices of kidney function | eGFRUACR | 0, 3, 52, 3, 5 |
| Dietary intake and appetite | 4 day food diaryCoEQ | 2, 3, 5 |
| Mental well-being and quality of life | HADSDDS-17EQ-5D-5L | 2, 3, 52, 3, 52, 3, 5 |
| Skeletal muscle anabolic/catabolic and inflammatory signalling pathways | Skeletal muscle biopsy | 1×, 3× |
| Cardiac function (MRI) in the DAPA-only group | As described for visits 1 and 4 | 2× |
BIA, bioelectrical impedance analysis; BMD, bone mineral density; BMI, body mass index; BW, body weight; CoEQ, Control of Eating Questionnaire; DDS-17, Diabetes Distress Scale-17; DXA, dual-energy X-ray absorptiometry; eGFR, estimated glomerular filtration rate; EQ-5D-5L, European QoL-5 Dimensions-5 Level; FFM, fat-free mass; FM, fat mass; GPPAQ, General Practitioner Physical Activity Questionnaire; HADS, Hospital Anxiety and Depression Score; HbA1c, haemoglobin A1c; LM, lean mass; LV, left ventricle; mMRC Dyspnea Scalemodified Medical Research Council Dyspnea ScalemPPT, modified physical performance test; SPPB, short physical performance battery; UACR, urine albumin to creatinine ratio; V̇O2peak, peak oxygen consumption; WHODAS, WHO Disability Assessment Schedule
Assessments
mPPT (primary outcome)
The mPPT assesses an individual’s ability to complete nine essential tasks for functional independence: (1) book lift, (2) putting on and removing a coat, (3) retrieving a penny from the floor, (4) repeated sit-to-stands, (5) 360° turn, (6) walking, (7 and 8) stair climbs (one and four flights) and (9) balance ability. Each task is scored 0–4 based on speed and ability (36 points available in total). Baseline mPPT score independently predicts death and nursing home admission (β=−0.13, p<0.05) in older adults24 and is sensitive to change following structured exercise interventions.10 25
Additional physical function assessment
The SPPB is a composite test assessing (1) the time taken to complete five sit-to-stand repetitions, (2) 4 m walking speed and (3) balance ability.26 The WHO Disability Assessment Schedule 2.0 and mMRC Dyspnoea Scale are short, established measures of functional health and disability27 and of dyspnoea on exertion,28 respectively.
Cardiorespiratory fitness
Following a 3 min warm-up (3 km/hour, 0% gradient), participants undergo a graded maximal treadmill test at a fixed speed (based on individual walking speed), with increasing gradient of 1% per minute. The test ends when the participant reaches voluntary exhaustion, their age-predicted maximum heart rate or at the discretion of the supervising clinician. Heart rate and rate of perceived exertion are recorded throughout. Expired air is collected and processed through an automated breath-by-breath analyser (CORTEX Metalyser 3B, Cranlea, UK). A 10-breath rolling average is calculated for V̇O2. Peak oxygen uptake (V̇O2peak), defined as the highest recorded average V̇O2 value, is reported as an absolute value (L of oxygen), and relative to LM (mL of oxygen per kg LM) and body mass (mL of oxygen per kg body mass).
MRI and echocardiography
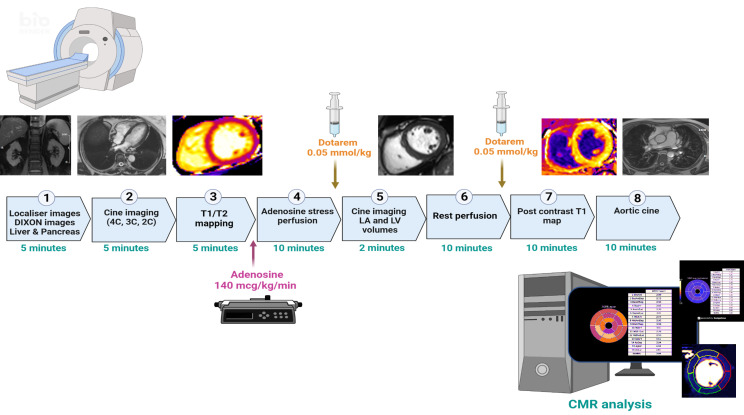
Cardiac MRI is carried out on a 3-Tesla platform (Siemens Skyra or VIDA, Erlangen Germany) with an 18-channel phased-array cardiac receiver coil (figure 2). Body composition (T-1 weighted Dixon VIBE sequence acquired from T9 to the top of femoral condyles), liver, pancreas and kidneys multiparametric assessment (organ composition with proton density fat fraction map, liver iron content (T2*) a MOLLI-T1 for inflammation) are conducted using the COVERSCAN acquisition protocol (Perspectum, Oxford, UK).29
Figure 2. 3-Tesla CMR protocol for the baseline and week 24 CMR covering thoracic and abdominal subcutaneous fat (DIXON) left ventricular function (cine), arterial stiffness and late gadolinium enhancement for assessment of fibrosis. CMR, cardiovascular MR; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle, 2, 3, 4c: 2, 3, 4 chamber.
Following localisers, balanced steady state free precession (bSSFP) left ventricular (LV) cine images are acquired in two-chamber, three-chamber and four-chamber views (retrospective electrocardiographic gating, 8 mm slice thickness; 2 mm gap; temporal resolution <50 ms; reconstructed to 30 phases), followed by myocardial tissue characterisation. A modified look locker inversion recovery sequence at the basal/mid segments for T1 is acquired and a mid-T2 map (Myomap). Unless contraindicated, participants undergo adenosine stress and rest perfusion. Myocardial blood flow is quantified using a dual pulse sequence acquisition with fully automatic inline calculations.30 A short axis stack image is acquired (bSSFP) covering the entire LV with aortic cine (at level of pulmonary artery) with simultaneous measurement of pulse pressure for calculation of aortic distensibility.31 Late gadolinium-enhanced images, postcontrast T1 maps determine focal and diffuse myocardial scar, respectively.
Visceral, subcutaneous and intraorgan fat (including liver and pancreas) and associated indices are determined from the resulting images as previously described.32,34
Left and right ventricular volumes will be analysed by Perspectum (CVI42, Cardiovascular Imaging, Canada).35 Assessment of peak diastolic filling rate, systolic/diastolic global longitudinal and circumferential strain rates are performed in the CMR lab at the University of Leicester (CVI42 software).36 Rest/stress myocardial blood flows, and perfusion reserve, are collated based on the automated analyses and in the presence of artefacts or failed segmentation are drawn manually.37 Native and postcontrast T1 time are quantified and corrected for haematocrit for calculation of the extracellular volume.38
Transthoracic echocardiography is performed using an iE33 system with S5-1 transducer (Philips Medical Systems, Best, the Netherlands) to assess diastolic transmitral flow velocities, E/A ratio and early diastolic mitral annular velocities (e’) to estimate LV filling pressures.39
Body composition
Height, weight, waist circumference and neck circumference are collected. Whole-body and regional fat mass, LM, fat-free mass and bone mineral density assessment via DXA scan use standardised protocols (GE Lunar iDXA). Supplementary body composition estimation is performed using bioelectric impedance (Tanita Corporation, Tokyo, Japan).
Resting blood pressure
Arterial blood pressure is measured in the seated position using an automated sphygmomanometer. The average of the last two measurements out of three is used. Heart rate is recorded.
Indirect calorimetry
Fasting resting metabolic rate is assessed using a ventilated hood system (GEMNutrition). Participants are supine, remaining awake and still. The hood is placed over the participant’s head to enable the measurement of all expired gases over ~40 min via indirect calorimetry, according to local operating procedures.
Muscle ultrasonography
Muscle ultrasound of the rectus femoris (RF) and VL is performed using a portable ultrasound system. With the participants supine and their knee fully extended, landmarks of the upper and lower RF are identified by palpation. The distance between the two sites is measured. Parameters of interest (assessed at 50% of the RF length) include muscle cross-sectional area, muscle and subcutaneous fat thickness, fibre angle pennation and echo intensity (acquired postscan using frame capture software ‘Image j’). Muscle and subcutaneous thickness of the VL are assessed laterally at the RF measurement site.
Muscle strength
Quadriceps strength of the right leg is measured using a fixed dynamometer (BIODEX, Medical Systems, Shirley, New York, USA). Maximal isometric strength is assessed at 90º knee flexion and defined as the highest peak torque (newton-metre, Nm) obtained over three attempts. Isokinetic strength testing requires repeated maximal concentric reciprocal contractions (60°, 90° and 180º per second) for five repetitions. Sets are separated by a 60 s rest.
Upper body strength is assessed using the ‘arm curl’ test in both arms, as previously described.40 Participants perform as many dumbbell bicep curls as possible in 30 s. The dumbbell weight (2–4 kg) selected at visit 2 will remain consistent for subsequent visits.
Handgrip strength is measured using a hand-held dynamometer in a seated position with the elbow flexed at a right angle and the forearm neutral. Participants grip the device as hard as possible, for three alternating repetitions on each side. The peak force output (kg) from the three attempts from both hands is recorded for analysis.41
Physical activity and sleep
A wrist-worn accelerometer is worn for 7 days (24 hours per day; within 28 days prior to visit 2, and 14±7 days prior to visits 3 and 5) to assess physical activity and sleep. Triaxial acceleration data are captured at a frequency of 100 Hz and processed using an open-source R programme (GGIR).42 Participants self-report their sleeping and waking times, and periods of non-wear. Outcomes include sleep duration and quality, daily physical activity volume and intensity profile, along with time spent sedentary and in light-intensity, moderate-intensity and vigorous-intensity physical activity. Physical activity and walking pace are self-reported using the general practitioner Physical Activity Questionnaire.43
Mental well-being and quality of life
Anxiety and depression are assessed by the Hospital Anxiety and Depression Scale Questionnaire.44 Diabetes-related emotional distress and quality of life are assessed using the 17-item Diabetes Distress Scale45 and the European QoL-5 Dimensions,46 respectively.
Dietary habits
Participants complete a standardised 4-day food diary prior to visits 2, 3 and 5. Anonymised data are entered into nutritional analysis software (Nutritics, Dublin, Ireland) to estimate daily energy intake, macronutrient and micronutrient composition. Severity and type of food cravings are assessed using the Control of Eating Questionnaire.47
Circulatory and urinary biomarkers
Blood tests for lipid profile (total cholesterol, high-density and low-density lipoprotein cholesterol, and triglycerides), fasting insulin and glucose, haemoglobin A1c (HbA1c), inflammation (C reactive protein), cardiac function (N-terminal pro-B-type natriuretic peptide [NTproBNP]), creatinine and estimated glomerular filtration rate (eGFR) are collected via venepuncture. Lipid profile, glucose, HbA1c, creatinine and eGFR are analysed at the University Hospitals of Leicester pathology unit. The remaining pseudoanonymised samples are stored at −80°C until analysis. Urine albumin-to-creatinine ratio is also assessed.
Optional substudy procedures
Visit 1× and 3×: muscle biopsy
Fasted skeletal muscle biopsies collected at baseline (visit 1×) and week 24 (visit 3×) are performed under local anaesthetic. Approximately 100 mg of muscle tissue is taken from the VL using the biopsy needle technique.
Biopsy samples are analysed for markers of inflammation (interleukin-6, interleukin-10, tumour necrosis factor-alpha), catabolism (MuRF-1, MAFbx, ubiquitin conjugates), mitochondrial abundance (porin, mtDNA copy number, mitochondrial complex abundance) and biogenesis (PGC1-alpha, NRF-1, Tfam) and insulin signalling pathway activation (P-Akt and P-P70S6K). Protein and mRNA expression are quantified using western blotting and quantitative reverse transcription PCR, respectively.48 A venous blood sample is taken concurrently for analysis of circulating cardiometabolic and inflammatory biomarkers, to contextualise the molecular findings.
Visit 2×: optional MRI substudy
An additional cardiac MRI scan is performed 2 weeks after intervention initiation (visit 2×). Only non-contrast LV and aortic cine imaging is acquired, as described above.
Study interventions
DAPA group
Participants in the DAPA group are prescribed dapagliflozin (10 mg once-daily). Medication is dispensed through the hospital pharmacy at weeks 0, 2 and 12 visits. Clinical review of tolerance, adherence and adverse events (AEs) occurs within study visits at weeks 2, 12, 24 and during weeks 4, 8 and 18 telephone review appointments. At the discretion of the study clinician, the dose of dapagliflozin may be reduced to 5 mg once-daily or ceased temporarily or permanently. The start or coprescription of pharmacotherapies (excluding medications defined a priori as exclusion criteria; table 1) is permitted to optimise glycaemic control, as deemed necessary by the prescribing physician.
DAPA+EX group
The DAPA+EX intervention is as described for DAPA, plus progressive, combined exercise (three times per week; ~30 min each of aerobic and resistance exercise per session). Moderate-intensity (HRmax~70%–80%) aerobic exercise is performed using a treadmill, cycle ergometer, cross-trainer or static rower depending on participant preference. Resistance exercises are individualised. One session per week prioritises resistance machines (eg, leg press, leg extension, chest press) to increase strength and mass of the major muscle groups (‘resistance-strength’), for 10–15 repetitions per set to ‘near-failure’ at ~70% of predicted one repetition maximum. Two sessions per week involve body weight and banded exercises based on functional movements, balance and flexibility (‘resistance-function’).
Initially (weeks 1–12), a minimum of two sessions per week are supervised at the LDC. One session may be unsupervised in a free-living environment; exercise equipment and instructions will be provided. Thereafter (week 13 onwards), a minimum of one session per week should be supervised, and up to two sessions weekly unsupervised. To monitor adherence when unsupervised, participants will keep an exercise log and wear a heart rate monitor if willing (polar or equivalent).
DIET-CON group
The control group follows a person-centred hypocaloric dietary plan that aims for ~3% wt loss equivalent to that anticipated in the DAPA group.49 50 The dietary plan is individualised based on participants dietary habits and preferences. Previous diet and weight history is explored, including previous dietetic or nutritional input, weight loss attempts, successes and barriers. Social history, current lifestyle and health behaviours are discussed, and current weight loss motivations and goals are identified. An action plan is then agreed with the participant, based on their needs and preferences, and the dietitian’s clinical judgement, considering which dietary strategy is likely to be most successful. Subsequent dietary reviews aim to discuss and highlight any successes or challenges and adapt the plan accordingly. The participants meet with a dietitian, one-to-one (face to face where possible), at week 0, visits 3 and 5, for regular dietetic review of their progress and adherence to their targets (figure 1).
AE reporting
All AEs are documented, reviewed at each visit and followed until resolution or until the event is considered stable. Serious AEs are reported to the sponsor and funder within 24 hours of discovery or notification of the event. Causality related to any study interventions is noted.
Statistical methods and analysis
Sample size
Based on published data,10 to detect a clinically meaningful two-unit difference in the mPPT, with an SD of 2.4 units, 90% power and an alpha error rate of 0.025, requires 38 participants per group to complete the study. Anticipating a 15% drop-out per group, we will recruit a total of 135 participants (n=45 per group).
Statistical analysis
A full statistical analysis plan will be finalised prior to database lock. The primary outcome (mPPT at 24 weeks) will be assessed by comparing (1) DAPA+EX and DIET-CON and (2) DAPA and DIET-CON, using generalised linear modelling adjusted for baseline mPPT score, variables used in stratification of randomisation and age. Data distribution will be checked for normality and appropriate distributions and transformed to achieve the best model fit. A p<0.025 will be considered significant to account for multiple tests. Should either comparison against DIET-CON be significant, further comparison will be conducted between the DAPA and DAPA+EX groups. The intervention effect will be reported as mean change (97.5% CI). To assess the treatment response over time, we will undertake a generalised estimating equation model with an exchangeable correlation matrix to account for repeated measures (12 and 24 weeks); data distribution checks and adjustments will be applied as above.
The primary outcome results will be stratified by age (threshold at 65 years) and sex. Generalised linear models will include interaction terms for age and sex by group to determine the impact of these variables on the intervention effect.
Secondary outcomes will be analysed and reported as above, without individual p values. Exploratory analyses will be conducted to determine the association between the primary outcome and key secondary outcomes.
The primary and secondary outcomes will be analysed using a complete case approach. Two sensitivity analyses will then be applied for the primary outcome only: (1) full intention-to-treat analysis, with missing data imputed using multiple imputation and (2) per-protocol analysis, restricting inclusion to those who have adhered to at least 75% of prescribed exercise sessions, where there is no evidence that under 75% of prescribed medication has been taken, and those who achieve at least 3% wt loss at 24 weeks, for exercise, dapagliflozin and dietary interventions, respectively.
Ethics and dissemination
Ethics approval
Ethical approval was granted by the Research Ethics Committee of Coventry and Warwickshire, West Midlands (20/WM/0117) and the Medicines and Healthcare Products Regulatory Authority. The study is conducted in line with the Declaration of Helsinki 2013 and adheres to the regulations for Good Clinical Practice.
Trial oversight and governance
The trial is sponsored by the University of Leicester. A trial steering committee (TSC), comprising an independent chair, two independent expert clinicians, the chief investigator (CI), key coinvestigators and the trial statistician, is responsible for the overall management and oversight of the trial. Approximately every 6 months, the TSC reviews and approves protocol amendments, evaluates recruitment rates, protocol adherence, retention, compliance, safety issues and planned analyses and acts on recommendations from the Data and Safety Monitoring Committee (DSMC). Participant safety, data integrity and analysis plans are overseen by the DSMC, comprising an independent clinician, chair and statistician. The DSMC meets approximately every 6 months, with input from the CI and/or trial statistician.
Dissemination
Findings will be disseminated through publication in peer-reviewed journals, scientific conferences, educational presentations and the media (including social media). At the end of the study, participants will be sent a summary of the findings.
Discussion
Interventions to prevent the onset or progression of poor physical function and frailty in people with T2DM are needed. People with T2DM report reduced physical function to be a major challenge and a priority target for T2DM management plans.51 In analyses from the DAPA-HF and DELIVER trials, dapagliflozin treatment showed a comparable risk/benefit profile for cardiovascular outcomes, irrespective of presence or severity of frailty.52 53 Despite this, people with T2DM, cardiovascular disease and frailty are less commonly prescribed SGLT2i’s than their non-frail counterparts.54 The paucity of empirical data demonstrating the effect of SGLT2i’s on physical function has likely contributed to the underprescription of these therapies to frail cohorts. A recent systematic review and meta-analysis summarised 11 studies investigating the effect of glucose-lowering therapies on physical function in people with T2DM.55 Only one trial using an SGLT2i included self-reported physical function as a secondary outcome measure and reported no change in physical function in the treatment, compared with placebo, group.56
Given SGLT2i therapy leads to weight loss and significantly improved cardiorenal health, it is hypothesised that these health benefits will support meaningful improvements in physical function for people with T2DM. The addition of structured exercise training is hypothesised to augment improvements in physical function. However, should changes to body composition and physical function be different in the intervention versus dietary control groups, these findings should be considered within the context of an anticipated shift in substrate utilisation from predominant glucose to lipid in those receiving SGLT2i therapy.57 This RCT will test our hypotheses while seeking to elucidate the physiology underpinning changes in physical function through assessment of several secondary outcome measures. Increasingly, frailty screening should be embedded into usual care for diabetes management.6 8 Findings will inform effective treatment for people with coexisting T2DM and frailty.
Strengths and limitations
To our knowledge, this is the first dedicated RCT investigating the impact of dapagliflozin with and without structured exercise training on physical function as a primary outcome. The design of the study is robust and conducted by an expert multidisciplinary research team. As it has been well established that structured exercise training can improve physical function and help preserve LM during weight loss induced by energy restriction,1025 58,61 a fourth ‘diet plus exercise arm’ and supporting hypothesis was not included in this trial. The study includes a high-risk cohort of older adults with frailty or functional limitations, which may limit the generalisability of the findings to a wider population of people with T2DM. Finally, participants are recruited from Leicester, Leicestershire and Rutland, UK. Although a single-centre study, Leicester and the surrounding areas are ethnically, culturally and socioeconomically diverse representing a microcosm of modern Britain, increasing the relevance of our findings to the wider population.
Acknowledgements
We thank the members of the Trial Steering Committee, Data Safety Monitoring Committee and the exercise lab, clinical and dietetics teams at the LDC.
Footnotes
Funding: This work was supported by AstraZeneca; grant number ESR-19-14431.
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084482).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Jack A Sargeant, Email: jack.sargeant@uhl-tr.nhs.uk.
Ehtasham Ahmad, Email: ehtasham999@hotmail.com.
Emily James, Email: e.james@leicester.ac.uk.
Luke Baker, Email: lab69@leicester.ac.uk.
Joanna M Bilak, Email: jmb99@leicester.ac.uk.
Nicole A Coull, Email: nicole.coull@uhl-tr.nhs.uk.
Gaurav Singh Gulsin, Email: gg149@leicester.ac.uk.
James A King, Email: J.A.King@lboro.ac.uk.
Kamlesh Khunti, Email: kk22@leicester.ac.uk.
Emma Redman, Email: Emma.redman@uhl-tr.nhs.uk.
Alex Rowlands, Email: alex.rowlands@leicester.ac.uk.
Emma Watson, Email: emma.watson@leicester.ac.uk.
Joanne V Wormleighton, Email: joanne.wormleighton@uhl-tr.nhs.uk.
Gerry P McCann, Email: gpm12@leicester.ac.uk.
Thomas Yates, Email: ty20@leicester.ac.uk.
Melanie J Davies, Email: melanie.davies@uhl-tr.nhs.uk.
References
- 1.Reusch JEB, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14:77–86. doi: 10.1007/s11154-012-9234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayer AA, Dennison EM, Syddall HE, et al. Type 2 Diabetes, Muscle Strength, and Impaired Physical Function. Diabetes Care. 2005;28:2541–2. doi: 10.2337/diacare.28.10.2541. [DOI] [PubMed] [Google Scholar]
- 3.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased Muscle Strength and Quality in Older Adults With Type 2 Diabetes. Diabetes. 2006;55:1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 4.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–7. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro-Rodríguez M, Carnicero JA, Garcia-Garcia FJ, et al. Frailty as a Major Factor in the Increased Risk of Death and Disability in Older People With Diabetes. J Am Med Dir Assoc. 2016;17:949–55. doi: 10.1016/j.jamda.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Strain WD, Down S, Brown P, et al. Diabetes and Frailty: An Expert Consensus Statement on the Management of Older Adults with Type 2 Diabetes. Diabetes Ther. 2021;12:1227–47. doi: 10.1007/s13300-021-01035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao C-T, Wang J, Chien K-L, et al. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17:130. doi: 10.1186/s12933-018-0772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair AJ, Abdelhafiz A, Dunning T, et al. AN INTERNATIONAL POSITION STATEMENT ON THE MANAGEMENT OF FRAILTY IN DIABETES MELLITUS: SUMMARY OF RECOMMENDATIONS 2017. J Frailty Aging . 2018;7:1–11. doi: 10.14283/jfa.2017.39. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad E, Sargeant JA, Yates T, et al. Type 2 Diabetes and Impaired Physical Function: A Growing Problem. Diabetol. 2022;3:30–45. doi: 10.3390/diabetology3010003. [DOI] [Google Scholar]
- 10.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss EP, Jordan RC, Frese EM, et al. Effects of Weight Loss on Lean Mass, Strength, Bone, and Aerobic Capacity. Med Sci Sports Exerc. 2017;49:206–17. doi: 10.1249/MSS.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou N, Scoubeau C, Forton K, et al. Lean Mass Loss and Altered Muscular Aerobic Capacity after Bariatric Surgery. Obes Facts. 2022;15:248–56. doi: 10.1159/000521242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985) 2007;102:634–40. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosiborod M, Cavender MA, Fu AZ, et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilding JPH. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metab Clin Exp. 2014;63:1228–37. doi: 10.1016/j.metabol.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632–44. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris A, Bright C, Cocks M, et al. Recommendations from Diabetes UK’s 2022 diabetes and physical activity workshop. Diabet Med. 2023;40:e15169. doi: 10.1111/dme.15169. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein ML, Williams DM, O’Neil LK, et al. Physical exercise and non-insulin glucose-lowering therapies in the management of Type 2 diabetes mellitus: a clinical review. Diabet Med. 2019;36:349–58. doi: 10.1111/dme.13865. [DOI] [PubMed] [Google Scholar]
- 19.England CY, Andrews RC. James Lind Alliance research priorities: should diet and exercise be used as an alternative to drugs for the management of type 2 diabetes or alongside them? Diabet Med. 2020;37:564–72. doi: 10.1111/dme.14217. [DOI] [PubMed] [Google Scholar]
- 20.Newman AA, Grimm NC, Wilburn JR, et al. Influence of Sodium Glucose Cotransporter 2 Inhibition on Physiological Adaptation to Endurance Exercise Training. J Clin Endocrinol Metab. 2019;104:1953–66. doi: 10.1210/jc.2018-01741. [DOI] [PubMed] [Google Scholar]
- 21.Bouchi R, Sonoda N, Itoh J, et al. Effects of intensive exercise combined with dapagliflozin on body composition in patients with type 2 diabetes: a randomized controlled trial. Endocr J. 2021;68:329–43. doi: 10.1507/endocrj.EJ20-0599. [DOI] [PubMed] [Google Scholar]
- 22.Lacroix A, Hortobágyi T, Beurskens R, et al. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med . 2017;47:2341–61. doi: 10.1007/s40279-017-0747-6. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix A, Kressig RW, Muehlbauer T, et al. Effects of a Supervised versus an Unsupervised Combined Balance and Strength Training Program on Balance and Muscle Power in Healthy Older Adults: A Randomized Controlled Trial. Gerontology. 2016;62:275–88. doi: 10.1159/000442087. [DOI] [PubMed] [Google Scholar]
- 24.Reuben DB, Siu AL, Kimpau S. The predictive validity of self-report and performance-based measures of function and health. J Gerontol. 1992;47:M106–10. doi: 10.1093/geronj/47.4.m106. [DOI] [PubMed] [Google Scholar]
- 25.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376:1943–55. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Measuring health and disability: manual for who disability assessment schedule. 2021. https://www.who.int/publications/i/item/measuring-health-and-disability-manual-for-who-disability-assessment-schedule-(-whodas-2.0) Available.
- 28.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–6. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 29.Waddell T, Bagur A, Cunha D, et al. Greater ectopic fat deposition and liver fibroinflammation and lower skeletal muscle mass in people with type 2 diabetes. Obesity (Silver Spring) 2022;30:1231–8. doi: 10.1002/oby.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellman P, Hansen MS, Nielles-Vallespin S, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19:43. doi: 10.1186/s12968-017-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulsin GS, Swarbrick DJ, Hunt WH, et al. Relation of Aortic Stiffness to Left Ventricular Remodeling in Younger Adults With Type 2 Diabetes. Diabetes. 2018;67:1395–400. doi: 10.2337/db18-0112. [DOI] [PubMed] [Google Scholar]
- 32.Demerath EW, Shen W, Lee M, et al. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85:362–8. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 34.Schweitzer L, Geisler C, Pourhassan M, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102:58–65. doi: 10.3945/ajcn.115.111203. [DOI] [PubMed] [Google Scholar]
- 35.Petersen SE, Matthews PM, Francis JM, et al. UK Biobank’s cardiovascular magnetic resonance protocol. J Cardiovasc Magn Reson. 2016;18:8. doi: 10.1186/s12968-016-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulsin GS, Brady EM, Swarbrick DJ, et al. Rationale, design and study protocol of the randomised controlled trial: Diabetes Interventional Assessment of Slimming or Training tO Lessen Inconspicuous Cardiovascular Dysfunction (the DIASTOLIC study) BMJ Open. 2019;9:e023207. doi: 10.1136/bmjopen-2018-023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown LAE, Gulsin GS, Onciul SC, et al. Sex- and age-specific normal values for automated quantitative pixel-wise myocardial perfusion cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2023;24:426–34. doi: 10.1093/ehjci/jeac231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everett RJ, Treibel TA, Fukui M, et al. Extracellular Myocardial Volume in Patients With Aortic Stenosis. J Am Coll Cardiol. 2020;75:304–16. doi: 10.1016/j.jacc.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson S, Rana B, Oxborough D, et al. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Res Pract . 2020;7:G59–93. doi: 10.1530/ERP-20-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rikli RE, Jones CJ. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J Aging Phys Act. 1999;7:129–61. doi: 10.1123/japa.7.2.129. [DOI] [Google Scholar]
- 41.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 42.Migueles JH, Rowlands AV, Huber F, et al. GGIR: A Research Community–Driven Open Source R Package for Generating Physical Activity and Sleep Outcomes From Multi-Day Raw Accelerometer Data. J Meas Phys Behav. 2019;2:188–96. doi: 10.1123/jmpb.2018-0063. [DOI] [Google Scholar]
- 43.Ahmad S, Harris T, Limb E, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60-74 year old primary care patients. BMC Fam Pract. 2015;16:113. doi: 10.1186/s12875-015-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjelland I, Dahl AA, Haug TT, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 45.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626–31. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 46.Feng Y-S, Kohlmann T, Janssen MF, et al. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647–73. doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalton M, Finlayson G, Hill A, et al. Preliminary validation and principal components analysis of the Control of Eating Questionnaire (CoEQ) for the experience of food craving. Eur J Clin Nutr. 2015;69:1313–7. doi: 10.1038/ejcn.2015.57. [DOI] [PubMed] [Google Scholar]
- 48.Watson EL, Viana JL, Wimbury D, et al. The Effect of Resistance Exercise on Inflammatory and Myogenic Markers in Patients with Chronic Kidney Disease. Front Physiol. 2017;8:541. doi: 10.3389/fphys.2017.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matthaei S, Bowering K, Rohwedder K, et al. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial. Diabetes Care. 2015;38:365–72. doi: 10.2337/dc14-0666. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Rahman N, Manor O, Valinsky L, et al. What is important for people with type 2 diabetes? A focus group study to identify relevant aspects for Patient-Reported Outcome Measures in diabetes care. PLoS One. 2022;17:e0277424. doi: 10.1371/journal.pone.0277424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients With Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation. 2022;146:1210–24. doi: 10.1161/CIRCULATIONAHA.122.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butt JH, Dewan P, Merkely B, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure With Reduced Ejection Fraction : A Post Hoc Analysis of the DAPA-HF Trial. Ann Intern Med. 2022;175:820–30. doi: 10.7326/M21-4776. [DOI] [PubMed] [Google Scholar]
- 54.Malik ME, Butt JH, Strange JE, et al. Initiation of SGLT2 inhibitors and GLP-1 receptor agonists according to level of frailty in people with type 2 diabetes and cardiovascular disease in Denmark: a cross-sectional, nationwide study. Lancet Healthy Longev. 2023;4:e552–60. doi: 10.1016/S2666-7568(23)00164-2. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad E, Arsenyadis F, Almaqhawi A, et al. Impact of novel glucose-lowering therapies on physical function in people with type 2 diabetes: A systematic review and meta-analysis of randomised placebo-controlled trials. Diabet Med. 2023;40:e15083. doi: 10.1111/dme.15083. [DOI] [PubMed] [Google Scholar]
- 56.Grandy S, Sternhufvud C, Ryden A, et al. Patient-reported outcomes among patients with type 2 diabetes mellitus treated with dapagliflozin in a triple-therapy regimen for 52 weeks. Diabetes Obes Metab. 2016;18:306–9. doi: 10.1111/dom.12604. [DOI] [PubMed] [Google Scholar]
- 57.Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508.:72227. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eglseer D, Traxler M, Embacher S, et al. Nutrition and Exercise Interventions to Improve Body Composition for Persons with Overweight or Obesity Near Retirement Age: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Adv Nutr. 2023;14:516–38. doi: 10.1016/j.advnut.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez P, Taaffe DR, Galvão DA, et al. Resistance training effectiveness on body composition and body weight outcomes in individuals with overweight and obesity across the lifespan: A systematic review and meta-analysis. Obes Rev. 2022;23:e13428. doi: 10.1111/obr.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller CT, Fraser SF, Levinger I, et al. The effects of exercise training in addition to energy restriction on functional capacities and body composition in obese adults during weight loss: a systematic review. PLoS One. 2013;8:e81692. doi: 10.1371/journal.pone.0081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sardeli AV, Komatsu TR, Mori MA, et al. Resistance Training Prevents Muscle Loss Induced by Caloric Restriction in Obese Elderly Individuals: A Systematic Review and Meta-Analysis. Nutrients. 2018;10:423. doi: 10.3390/nu10040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–60. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]