Abstract
Background/Objectives: Microorganisms such as bacteria, viruses, and fungi are frequently the cause of infections. Antimicrobial agents, such as antibiotics, antivirals, and antifungals, are used to target and eliminate these infectious agents. On the other hand, inflammation is a natural response of the immune system to injury, infection, or irritation. Although herbal remedies have been used to treat these conditions for centuries and can be effective in certain situations, it is crucial to use them with caution. Not all herbal remedies are supported by scientific evidence, and their safety and efficacy can vary. Thus, we conducted this review to determine the potential health benefits of agarwood as an antimicrobial and anti-inflammatory agent. Methods: Three databases (PubMed, Scopus, and Google Scholar) were used to search for original papers submitted between 2013 and 2023, using the Medical Subject Heading (MeSH) terms “agar-wood” crossed with the terms “antimicrobial” and/or “anti-inflammatory”. Synonyms and relevant search terms were also searched. Results: The most-studied agarwood for antimicrobial and anti-inflammatory agents is Aquilaria sinensis. Some studies have shown its potential application as a potent inhibitor of fungi, including Lasiodiplodia theobromae, Fusarium oxysporum, and Candida albicans. Moreover, it is capable of inhibiting Bacillus subtilis and Staphylococcus aureus activities. Several chromones detected in agarwood have been shown to inhibit NF-κB activation, LPS-induced NO production, and superoxide anion generation. In conclusion, more research is needed, particularly regarding future intervention studies, to enhance our knowledge and understanding of agarwood and its isolates. Conclusions: This review reveals that despite the absence of clinical trials, agarwood exhibits antimicrobial and anti-inflammatory properties.
Keywords: Aquilaria spp., antimicrobial, anti-inflammatory
1. Introduction
The primary source of agarwood, also known as oud, is Aquilaria trees. These trees are native to several Asian nations, mainly located in Southeast Asia. In some countries, Aquilaria trees grow in natural forests and in plantations [1]. In countries such as Thailand, Cambodia, Vietnam, Malaysia, Indonesia, Laos, India, Bangladesh, and parts of China, Aquilaria trees that produce agarwood are often found in natural forests [2]. Specific locations where agarwood is found within these countries can vary due to climate and soil conditions [3]. Certain ecological conditions, such as hilly or mountainous regions with particular soil types and sufficient rainfall, are more conducive to the growth of these plants. In recent years, some countries have tried cultivating Aquilaria trees in plantations. These plantations aim to sustainably produce agarwood by stimulating the resinous formation in the trees. Plantations can be found in Southeast Asian countries such as Malaysia and Indonesia [4].
While some preliminary research has suggested the presence of antimicrobial properties in agarwood, concrete scientific evidence supporting this claim is limited, and most studies were in the early research stages. It has been demonstrated that agarwood leaf ethanol extract exhibits antibacterial action against both bacteria and fungi. The extract contains bioactive compounds like flavonoids and tannins which contribute to this antimicrobial activity [5]. Agarwood produces oud oil, which is rich in a variety of volatile compounds that include sesquiterpenes such as α- and β-guaiene, agarospirol, agarol, and various other sesquiterpenoids and phenylethyl chromones [6]. These bioactive substances found in agarwood have been shown to possess antimicrobial properties. The general potential mechanisms through which agarwood might exert antimicrobial effects could include (a) the disruption of cell membranes, by which agarwood’s compounds have the potential to cause the microorganisms’ cell membranes to rupture, allowing internal organelles to seep out, eventually causing cell death [7]; (b) interference with microbial enzymes, whereby the components of agarwood may block or obstruct vital microbial enzymes, impairing the metabolic activities that are necessary for microbial viability [8]; (c) antioxidant effects that may help fight microbial infections by modifying microbial viability and lowering oxidative stress [9]; (d) the modulation of gene expression by compounds found in agarwood that may affect the pathogenicity, survival, or replication of the microbes [10]; (e) impact on the biofilm formation of bacterial colonies that are frequently resistant to antimicrobial treatments [11].
Additionally, agarwood’s potential anti-inflammatory properties have also been investigated. Agarwood extracts have shown potential in inhibiting various inflammatory mediators such as cytokines, prostaglandins, and leukotrienes [12]. These mediators play critical roles in the inflammatory response, and the ability of agarwood to inhibit their release or activity suggests anti-inflammatory potential. Some studies indicate that agarwood extracts may inhibit enzymes like cyclooxygenase (COX) and lipoxygenase (LOX) involved in the generation of inflammatory mediators including prostaglandins and leukotrienes [7]. By inhibiting these enzymes, agarwood could potentially suppress the inflammatory cascade. Moreover, agarwood may modulate the immune response, potentially influencing the activities of immune cells involved in inflammation. Its immunomodulatory effects could help regulate inflammatory responses. Additionally, agarwood might interfere with specific cellular signaling pathways involved in inflammation, such as NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAPKs (mitogen-activated protein kinases), which are associated with the regulation of inflammatory responses. Inflammation often involves oxidative stress, and the antioxidant activity of agarwood may help neutralize free radicals and reduce inflammation induced by oxidative stress. Thus, this scoping review was conducted to determine the effect of agarwood as a potential antimicrobial and anti-inflammatory agent.
2. Results
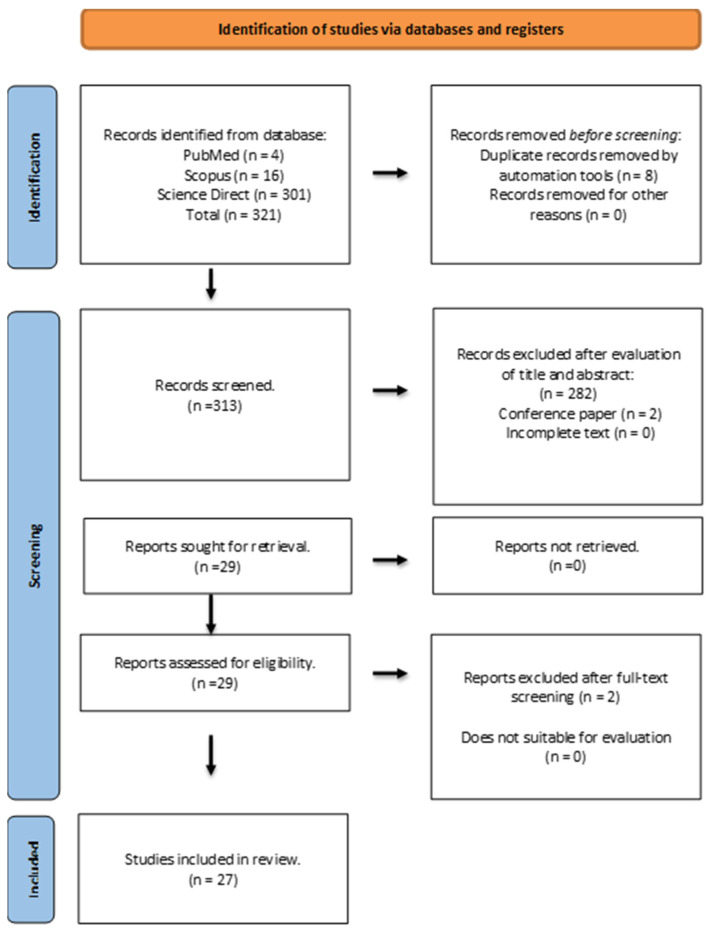
We examined 321 publications in the initial stage before screening. Following screening, 282 out of 313 articles were excluded according to established inclusion and exclusion criteria. Articles that have met the requirements of being written in English or Malay, having available full text, and being peer-reviewed were included, ensuring the selection of high-quality and relevant research. In contrast, review papers, letters to the editor, or duplicate articles were excluded, as these did not contribute original research data or were redundant (Figure 1). We also limited the publication period to 10 years for the following reasons:
-
(a)
Relevant and recent publications are required to ensure that the review reflects the most current research and knowledge.
-
(b)
Limiting the timeframe helps to maintain the focus on recent developments and trends within a manageable scope.
-
(c)
Limiting the publication period makes the reviewing process more feasible in terms of time and effort.
Figure 1.
Flowchart of the search strategy.
A more refined search, taking into account the availability of peer-reviewed publications, full text papers, and library collection access, yielded a total of 29 articles. Only 27 full-text publications were deemed relevant after additional evaluation, and these were included for final review (Table 1). For a more thorough evaluation of the evidence supporting the efficacy of agarwood as a possible antibacterial and anti-inflammatory agent, all relevant publications were printed out. While the ten-year limit is standard, it can vary depending on the field, topic, and specific requirements of the review paper. Ultimately, the timeframe chosen should balance comprehensiveness with relevance to provide the most insightful and valuable literature synthesis.
Table 1.
Tabulated summary of articles included in this review.
| Reference | Objective | Method | Findings | Conclusion/Recommendation | |
|---|---|---|---|---|---|
| 1 | [13] |
|
|
|
|
| 2 | [14] |
|
|
|
|
| 3 | [15] |
|
|
|
|
| 4 | [16] |
|
|
|
|
| 5 | [17] |
|
|
|
|
| 6 | [18] |
|
|
|
|
| 7 | [19] |
|
|
|
|
| 8 | [20] |
|
|
|
|
| 9 | [21] |
|
|
|
|
| 10 | [22] |
|
|
|
|
| 11 | [23] |
|
|
|
|
| 12 | [24] |
|
|
|
|
| 13 | [25] |
|
|
|
|
| 14 | [26] |
|
|
|
|
| 15 | [27] |
|
|
|
|
| 16 | [28] |
|
|
|
|
| 17 | [29] |
|
|
|
|
| 18 | [30] |
|
|
|
|
| 19 | [31] |
|
|
|
|
| 20 | [32] |
|
|
|
|
| 21 | [33] |
|
|
|
|
| 22 | [34] |
|
|
|
|
| 23 | [35] |
|
|
|
|
| 24 | [36] |
|
|
|
|
| 25 | [37] |
|
|
|
|
| 26 | [38] |
|
|
|
|
| 27 | [39] |
|
|
|
|
3. Discussion
Most studies on agarwood have been related to its aromatic properties, its traditional applications in perfumes, and its potential medicinal benefits when used topically or inhaled through aromatherapy. While agarwood is not commonly consumed as a food or part of a regular diet, it has been utilized in some cultures for its purported medicinal properties. In certain traditional practices, agarwood has been used in minimal quantities or as an ingredient in herbal remedies for the treatment of various health conditions such as digestive issues, asthma, pain relief, and it has even been used as an aphrodisiac [7]. Agarwood may also be found in traditional medicines such as tea or extracts. As with any herbal or natural remedy, it is essential to be aware of potential allergic reactions, interactions with medications, and the lack of scientific evidence supporting its effectiveness and safety for consumption. Therefore, the safety and efficacy of consuming agarwood for these purposes must be extensively studied in clinical trials. It is necessary to highlight that the consumption of agarwood or its derivatives should be approached with caution and under the guidance of a healthcare professional due to limited scientific evidence regarding its safety and potential side effects. As of our knowledge cutoff date of January 2023, more scientific research explicitly focusing on agarwood consumption was still needed.
At the initial stage, we tried to separate antimicrobial and anti-inflammatory activities. However, during the write-up process, we found that combining antimicrobial and anti-inflammatory activities offers several advantages and synergistic effects. Thus, it is suitable to incorporate them because many infections are accompanied by inflammation, and vice versa. Treatment can provide a more comprehensive therapeutic approach by targeting antimicrobial activities to combat the disease and anti-inflammatory activity to reduce inflammation and its associated symptoms. Moreover, research regarding compounds or formulations that exhibit antimicrobial and anti-inflammatory properties can develop novel therapeutic agents with unique mechanisms of action and improved therapeutic profiles. Overall, combining antimicrobial and anti-inflammatory activities represents a promising medical and pharmaceutical research approach, aiming to provide more effective, targeted, and holistic treatments for infectious and inflammatory diseases.
In this scoping review, we found that a group of researchers had investigated the chemical composition of agarwood from different Asian countries, and they discovered that the main volatile components were oleic acid 3-(octadecyloxy) propyl ester, 3-ethyl-5-(2-ethylbutyl)-octadecane, and docosanoic acid 1,2,3-propanetriyl ester [13]. Interestingly, the main active ingredients of A. sinensis were aromatic compounds, sesquiterpenoids, and chromone compounds. They also recorded that agarwood displayed more significant antibacterial effects against Gram-positive than against Gram-negative bacteria. This effect may be due to the LPS layer on the Gram-negative bacteria cell wall, which prevents hydrophobic compounds from entering the cells and reduces the bacteriostatic impact [40]. The inhibition rates they obtained were in the following order: S. aureus > B. subtilis > E. coli [13].
Canli et al. conducted the first study to screen the antimicrobial properties of the ethanolic extraction of A. agallocha roots in vitro [14]. A. agallocha is considered to be synonymous with A. malaccensis. By performing a disk diffusion method on 17 bacteria and one fungus (Escherichia, Bacillus, Enterococcus, Salmonella, Candida, Enterobacter, Klebsiella, Staphylococcus genera, Listeria, and Pseudomonas), they found that ethanol extracts were active against most of the strains, especially against E. faecium, L. monocytogenes ATCC 7644, B. subtilis DSMZ 1971, C. albicans DSMZ 1386, S. epidermidis DSMZ 20044, and S. aureus ATCC 25923. Unfortunately, they did not identify the active substances elucidating the mechanism of action involved in this antimicrobial activity.
Another group of researchers also reported the antimicrobial effect of agarwood on Proteus mirabilis and S. aureus [15]. Using the Kirby–Bauer disc diffusion assay, they discovered that the average diameter zones blocked by the ethanol extract of agarwood leaves in S. aureus measured 12.50 mm (300 mg/mL), 13.51 mm (400 mg/mL), and 15.80 mm (500 mg/mL). The study also reported that the average diameter zone blocked by the ethanol extract of agarwood leaves in P. mirabilis concentrations of 300 mg/mL, 400 mg/mL, and 500 mg/mL were 12.10 mm, 13.26 mm, and 15.19 mm, respectively. As previously reported by Wang et al. [13], this study also confirmed that ethanol extracts of agarwood leaves have antimicrobial properties against selected Gram-positive and Gram-negative bacteria.
In another study, an ethanol extract of A. malaccensis leaf was also tested for its antimicrobial activity against selected fungi and bacteria that grow on the skin [16]. It was found that the antibacterial activity of the extract (1.25–20% concentration) showed an auspicious result against S. aureus, where it was categorized as susceptible at a 5% concentration. However, S. epidermis and Propionibacterium acnes at 20% concentrationd are categorized as intermediate, whereas all other tested concentrations are categorized as resistant. The study also reported the anti-fungal activity of C. albicans, which was classified as intermediate at 20%, whereas the other tested concentrations were classified as resistant; however, other fungi of Trichophyton sp. showed the inhibitory zone as resistant for all concentrations. This suggests that flavonoids, tannins, and triterpenoids are the active compound groups that contributed to its antimicrobial activity. The antimicrobial efficacy of A. crassna leaf aqueous extract against S. epidermidis has also been found [17]. The extract suppressed S. epidermidis growth in 2 mg (12.0 ±1.0 mm), 4 mg (15.0 ± 0.4 mm), and 6 mg (18.0 ± 1.0 mm) concentrations, as determined by disc diffusion assay. S. epidermidis was disposed by the extract with the MIC and MBC of 6 and 12 mg/mL, respectively. This action is due to the disruption of the biofilm and ruptured cell walls, which ultimately changed the shape of the bacteria.
Sometimes, an ingredient has a better effect when mixed with other active ingredients. Jihadi et al. [18] reported the first work performed using a combination of polymyxin B and A. malacensis extract via an in vitro study targeting Acinetobacter baumannii and Klebsiella pneumonia. Polymyxin B is an antibiotic that belongs to the polymyxin group of antibiotics. In this study, polymyxin B was utilized at a clinically relevant dose of 1 μg/mL, with susceptibility breakpoints at MIC of ≤2 μg/mL [41]. The performance of this combination extract was assessed using in vitro time-kill tests and analysis of GC-MS at 4 and 24 h. The researchers discovered that crude extract, either combined with polymyxin B or used alone, significantly decreased and inhibited bacteria over a 24 h period. However, the combination yields a considerably higher bactericidal effect exceeding ≥3 log10 CFU/mL at the end of the 24 h research period, notably for the extract at 64 mg/mL, compared to the results for polymyxin B. alone, which were only about ≥1 log10 CFU/mL. The GC-MS investigation of A. malaccensis ethanolic crude leaf extract revealed over sixty compounds, including a large amount of phytol and 9,12-octadecadienal. Compounds believed to contribute to the extract’s antimicrobial activity include phytol, 9,12-octadecadienal, oleic acid, n-decanoic acid, n-hexadecanoic acid, and squalene.
In another intriguing investigation, A. malaccensis was employed as a biogenic medium to produce CuO NPs with antimicrobial properties [19]. The boiled leaf extract reacted with 5 mM CuSO4.5H2O at pH 6 and was incubated at 70 °C without shaking, resulting in a high rate of CuO NPs production and exhibiting a UV absorbance peak of 430 nm. Field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) indicated that the nanoparticles are primarily spherical, with sizes ranging from 6 to 32 nm. Antimicrobial investigations revealed that 20 μL and 40 μL of 70 μg/μL CuO NPs effectively inhibited the Gram-positive bacteria B. subtilis, with average zones of inhibition measuring 24.43 ± 0.10 mm and 27.31 ± 0.13 mm, respectively.
In Vietnam, effects of essential oils derived from the trunk of Vietnam-originated agarwood of A. banaensis P.H.Hô against B. subtilis, S. aureus, E. coli, L. fermentum, P. aeruginosa, S. enteric, and C. albicans were explored. The researchers discovered that the essential oil from the trunk had a more promising antimicrobial impact than the leaf extract [20]. The study examined the chemical composition of the leaf extract, which included β-selinene, β-caryophyllene, β-elemene, α-humulene, α-selinene, and β-guaiol. In contrast, the trunk contained oleic acid, tetradecanoic acid, and hexadecanoic acid, which could explain the discrepancies in the results for these two extracts as possible antimicrobial agents.
During the COVID-19 pandemic, agarwood was also explored for its effectiveness using an in silico approach. The potential antiviral activity of oleanin triperpenoids in agarwood against coronavirus 2 (SARS-CoV-2) has been investigated using Lipinski’s rule of five and the prediction of absorption, distribution, metabolism, and excretion (ADME) [21]. The study found that four oleanin triperpenoids, 11-oxo-β-amyrin (ΔG = −9.8 kcal/mol), hederagenin-an (ΔG = −9.6 kcal/mol), 3β-acetoxyfriedelane (ΔG = −9.4 kcal/mol), and ursolic acid (ΔG = −9.5 kcal/mol), displayed a higher affinity to coronavirus 2 (SARS-CoV-2) than did lopinavir (ΔG = −6.2 kcal/mol) and remdesivir (ΔG = −7.2 kcal/mol) when molecularly docked in the main protease (Mpro) receptor. The prediction of ADME contributed to the development of hederagenin, a potential oral medication in which several primary amino acids, including methionine 49 and 165, glutamine 189, proline 168, threonine 25, and arginine 188, were engaged in the interactions.
Based on the study of the phytochemical properties of distilled water and various parts of the agarwood plant (A. malaccensis Lamk), the secondary metabolites of the glycosides were found to be responsible for the antimicrobial activity derived from several agarwood parts, including the leaf, trunk, skinned stem, and bark [22]. The distilled water from A. malaccensis Lamk exhibits antimicrobial activity against Streptococcus mutants and is effective in resisting the bacteria.
Conversely, Dahham et al. [23] isolated β-caryophyllene from A. crassna essential oil, a chemical classified as a terpene. β-caryophyllene was identified as the largest component at 8.1%, followed by 1-phenanthrenecarboxylic acid at 7.1% and 2-naphthalene-methanol at 6.2%. The antimicrobial action of β-caryophyllene was evaluated against human pathogenic bacteria and specific strains of fungi. β-caryophyllene showed substantial antibacterial effect against all examined microbial strains, including B. subtilis, S. aureus, B. cereus, P. aeruginosa, A. niger, E. coli, P. citrinum, R. oryzae, K. pneumoniae, and T. reesei, as well as significant anti-fungal activity compared to that of kanamycin.
In recent years, utilizing plant extracts as biopesticides for nanoparticle synthesis has gained popularity due to its low cost, eco-friendliness, and single biosynthesis approach. In Indonesia, Prasetya [24] explored the antibacterial activity of A. agallocha oil nanoemulsion against multidrug-resistant bacteria (MDR) and antibiotic-nonresistant bacteria inlcuding S. aureus (ATCC 43300), E. coli (ATCC 35218), E. coli (ATCC 25922), K. pneumoniae (ATCC 700603), K. pneuomoniae (ATCC 8724. T), and S. aureus (ATCC 25923). The study reported that with a 99.35% transmittance, the 1% agarwood oil nanoemulsion concentration exhibited the lowest size, measuring 17.7 nm. The inhibition zones for S. aureus ATCC 25923 and K. pneumoniae ATCC 8724 were 2.6 mm and 3.3 mm, respectively. The non-resistant E. coli ATCC 25922 showed an inhibitory zone of 13.3 mm. Based on their findings, the researchers suggested that a higher concentration of the oil nanoemulsion may be required to boost its inhibitory action against MDR than that required for non-resistant antibiotic bacteria. In another meticulous study on nanoparticles, Ga’al et al. [25] thoroughly investigated Pogostemon cablin essential oil (PcEO) and biosynthesized silver nanoparticles (AgNPs) against the larvae and pupae of the dengue and zika virus vector Aedes (Ae) albopictus. Their research, which included formation and biophysical characterization, was conducted with utmost care and attention to detail. The study found that biofabricated AgNPs were the most hazardous to Ae. Albopictus when compared to the results for the evaluated essential oils. The LC50 values of AsEO ranged from 44.23 (I) to 166 (pupae), PcEO ranged from 32.49 (I) to 90.05 (IV), AsEO-AgNPs ranged from 0.81 (I) to 1.12 (IV), and PcEO-AgPNs ranged from 0.85 (I) to 1.19 (IV). Their research also revealed that the synthetic AgNPs had a significantly greater impact on the epithelial cells and brush borders of both the control and treated larvae when compared to that of the essential oils (AsEO and PcEO).
Meanwhile, Chen et al. [26] reported that the chemical method improved the quality of the agarwood derived from A. sinensis (S1) compared with that obtained from wild agarwood (S2) and healthy trees (S3). They also evaluated the antimicrobial activities of that particular essential oil. From GC-MS chromatograms, they determined the similarity of essential oils of S1 and S2, with a high concentration of sesquiterpenes and aromatics. However, S3’s essential oil included a significant concentration of fatty acids and alkanes. The essential oils of S1 and S2 were more effective at inhibiting S. aureus and B. subtilis. Even at the maximum experimental dose of 2 mg/mL, the three extracts showed little activity against E. coli.
Lasiodiplodia theobromae (F) was utilized in a work by another researcher to stimulate A. sinensis (Lour.) Gilg to produce agarwood [27]. The chemical composition of F was determined using GC-MS. Their findings revealed that essential oils derived from A. sinensis generated by L. theobromae were chemically and antimicrobial similar to those derived from wild agarwood (W). F’s essential oil was identical to W’s, with a high concentration of sesquiterpenes and aromatic components. However, the essential oil of uninoculated healthy trees (H) contained a high concentration of alkanes. Regarding their anti-fungal activity, F’s and W’s essential oils were more potent inhibitors of F. oxysporum, L. theobromae, and C. albicans than were H’s essential oil.
In the same family as Aquilaria, G. versteegii fruit extract was assessed for antimicrobial activity against S. aureus and E. coli by Hidayati et al. [28]. G. versteegii is a tree from the Thymelaeaceae family, known to produce agarwood. The researchers used n-hexane, dichloromethane, and methanol for the extraction process and employed agar well diffusion and GC-MS methods for analysis. The dichloromethane extract, particularly at a 40% concentration, exhibited the most potent antimicrobial activity, with a 13.17 mm inhibition zone against S. aureus, compared to 7 mm for E. coli. The extracts demonstrated total and partial inhibition against S. aureus and E. coli. Several compounds were identified by GC-MS analysis, including oleic, stearic acid, palmitic, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, squalene, bis-(2-ethylhexyl) phthalate, methyl octadec-9-enoate, and 2-monopalmitin derivatives. The study revealed a more potent antimicrobial effect against S. aureus, particularly with the 40% concentration of dichloromethane extract, and identified various compounds in the G. versteegii fruit extracts.
For studies that assess agarwood as an anti-inflammatory agent, Yu et al. [29] investigated the anti-inflammatory properties of 5,6,7,8-tetrahydro-2-(2-phenylethyl) chromones derived from A. sinensis by utilizing the RAW 264.7 cell inhibition effect on lipopolysaccharide (LPS)-induced nitric oxide (NO) release. Among the novel compounds, Compound 2 [(5S,6R,7S,8S)-8-chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone] displayed the highest IC50 value of 3.46 μM, indicating considerable anti-inflammatory action.
Like many other terpenes and natural compounds, sesquiterpenes have sparked widespread interest due to their involvement in biological systems and their potential medicinal use. In 2019, Yu et al. [30] recorded the existence of eleven sesquiterpenes in A. sinensis. Compound baimuxinol (1) was among the new natural products found. They recorded that compounds 1,4 and 9 displayed anti-inflammatory activity, with IC50 values of (2.5 ± 0. 35), (3.2 ± 0.2), and (4.3 ± 0.56) μmol/L, respectively.
Flavonoids are abundant in medicinal plants and fruits and exhibit a variety of pharmacological actions. A flavonoid compound, pilloin, isolated from A. sinensis, was tested for anti-inflammatory action in vitro and in vivo [31]. This compound’s ability to downregulate pro-inflammatory cytokines (e.g., TNF-α and IL-6), as well as enzymes (e.g., iNOS and COX-2), to inhibit NF-κB and MAPK signaling pathways in LPS-activated macrophages, and to suppress the phenotypes and functions of activated macrophages (i.e., ROS production and phagocytic activity) holds great promise for clinical applications. Pilloin’s reduction of LPS-induced cytokine production (e.g., TNF-α and IL-6) in the serum and tissues of septic mice could potentially lead to novel therapeutic strategies.
Wang et al. [33] conducted an extensive study on the anti-inflammatory properties of several A. sinensis compounds. The methanolic extract from resinous A. sinensis has shown remarkable potential in inhibiting NF-κB activation in RAW 264 LPS-stimulated macrophages. The relative luciferase activity values ranged from 0.31 ± 0.05 to 0.55 ± 0.09, which were significantly lower than the vehicle control value of 1.03 ± 0.02. This finding indicates a compelling effectiveness that merits further exploration. Moreover, some compounds might decrease LPS-induced NO generation in RAW 264.7 cells, without cytotoxicity, after 24 h of treatment [33]. In addition, several chromone-related compounds exhibited more than an 80% inhibition towards superoxide anion production by human neutrophils at 50 μM of formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP) [35]. Moreover, the methanolic extract of several compounds of A. sinensis’ stem bark inhibited (IC50 ≤ 12.51 μM) superoxide anion production in human neutrophils’ response to fMLP/cytochalasin B. Additionally, 7-Hydroxy-6-methoxy-2-(2-phenylethyl)chromone, velutin, 3′-hydroxygenkwanin, 6,7-di-methoxy-2-(2-phenylethyl)chromone, and ergosta-4,6,8(14),22-tetraen-3-one demonstrated the most remarkable effectiveness among the isolates (IC50 values ≤ 15.25 μM) by inhibiting elastase release induced by fMLP/CB [36].
Anti-inflammatory effects of 2-(2-phenylethyl) chromone derivatives using ethanolic extract of resinous A. sinensis have also been reported by Huo et al. [37]. Their preliminary analysis of the structure–activity relationship documented that the presence of a chlorine substituent and an epoxy group on the A-ring were associated with the anti-inflammatory activity of 5,6,7,8-tetrahydro-2-(2-phenylethyl)-chromones. Compounds 2–4, 11, 12, and 15 significantly inhibited NO generation in LPS-stimulated RAW 264.7 cells, with IC50 values of 1.6–7.3 μM. Additionally, these compounds showed no significant cytotoxicity (up to 100 μM) after 24 h of LPS treatment, as measured using the MTT technique. In another study utilizing the same ethanolic extraction method, several compounds displayed a considerable reduction of NO generation in LPS-stimulated RAW 264.7 cells, with IC50 values ranging from 7.0 to 12.0 μM and no cytotoxicity effect (up to 80 μM) after 24 h of LPS treatment [34]. Huo et al. [32] used LC–MS-guided fractionation to isolate fifteen previously undescribed 2-(2-phenylmethyl)chromone dimers from A. sinensis and two known analogues. As might be expected, these isolated compounds effectively inhibited NO generation in LPS-stimulated RAW 264.7 cells, with IC50 values ranging from 0.6–37.1 μM, indicating their potential as anti-inflammatory agents. These findings highlight the possible anti-inflammatory capabilities of these chromone derivatives, emphasizing their therapeutic potential, while not inducing cytotoxic effects at relevant concentrations.
A. sinensis leaves have previously been found to exhibit analgesic and anti-inflammatory properties [42]. A. sinensis leaves also contain eight α-glucosidase inhibitors [43], which may be utilized as a traditional treatment for diabetes. However, based on an in vivo study by Sattayasai et al. [38] using the rat paw assay at 800 mg/kg, methanolic A. crassna leaf extract showed no anti-inflammatory activity. Nevertheless, the extract possesses antipyretic, analgesic, and anti-oxidative properties.
The results of a study published by Peng et al. [39] investigating incense smoke from agarwood revealed low amounts of TNF-α and IL-1α derived from normal inactivated RAW 264.7 cells; however, with LPS exposure, higher amounts were obtained after 24 h of incubation. Indomethacin treatment resulted in concentration-dependent AAW, BCDA, and AWIT decreases at 20, 40, and 80 μg/mL TNF-α, and the IL-1α levels were substantially more significant than those in the normal group (p < 0.05 or p < 0.01), indicating improved anti-inflammatory effects.
4. Materials and Methods
Three databases (PubMed, Scopus, and Google Scholar) were used to search for original papers published from 2013 to 2023 using the Medical Subject Heading (MeSH) terms “agarwood” crossed with the terms “antimicrobial” and/or “anti-inflammatory”. Publications with available full papers were assessed, and only studies published in English and Malay were considered for evaluation. Papers including human and clinical studies of agarwood were included. Letters to the editor and reviews, however, were not included. Duplicate articles were eliminated.
5. Limitations of the Study
This review paper has several limitations because some information cannot be fully obtained from the original article. The use of various tested materials, such as the type of solvent used in the extraction of either ethanol or methanol, the type of essential oil, and the type of nanoemulsion, also makes it difficult to draw more accurate conclusions. The small number of studies also limits the ability to identify the effectiveness of agarwood.
6. Conclusions
We examined in depth twenty-seven relevant studies worldwide related to agarwood as a potential anti-inflammatory and antimicrobial, consisting of experiments involving chemical composition, in silico, in vitro, in vivo, and combined in vitro and in vivo methods. We found that the active compounds in agarwood positively affect its effectiveness. More research is needed, particularly regarding future intervention studies, to enhance our knowledge and understanding of agarwood and its isolates. These promising compounds are ripe for biomedical exploration and could become powerful tools in treating and preventing microbial and inflammatory diseases. Investing in this research could lead to groundbreaking advancements in healthcare.
7. Future Perspective
The future perspective of agarwood as an antimicrobial and anti-inflammatory agent is promising, based on findings driven by ongoing research. In addition to its possible potential applications in various fields, agarwood could potentially be used in pharmaceuticals, cosmetics, and food preservation due to its antimicrobial effects. It can also potentially be used as an alternative to synthetic agents. With increasing concerns over antibiotic resistance, natural products like agarwood could serve as alternatives to synthetic antimicrobial agents. Agarwood has also been traditionally used in various cultures for its anti-inflammatory properties; hence, it has the potential to treat inflammatory conditions such as arthritis, skin inflammation, and gastrointestinal disorders.
Acknowledgments
The authors express gratitude to the Director-General of Health, Malaysia and the Director of the Institute for Medical Research (IMR), Malaysia, for granting permission to publish this article.
Author Contributions
A.A.R., M.A.J., S.A.R., F.D.A.N. and M.N.M.N. wrote the original draft, conducted the literature review, and revised the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors affirm that they have no financial interests or personal relationships that could potentially bias the findings presented in this paper. This commitment to transparency underlines the integrity of their research.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jensen A. Domestication of Aquilaria spp. and rural poverty—Socio-economic and genetic aspects of the planting boom in the; Proceedings of the Wood of the Gods, in Poverty Reduction and Shifting Cultivation Stabilization in the Uplands of Lao PDR: Technologies, Approaches and Methods for Improving Upland Livelihoods; Luang Prabang, Laos. 27–30 January 2004; pp. 233–239. [Google Scholar]
- 2.Oldfield S., Lusty C., MacKinven A. The World List of Threatened Trees. IUCN; Gland, Switzerland: 1998. [Google Scholar]
- 3.de Alwis W.N.H., Subasinghe S.M.C.U.P., Hettiarachchi D.S. Characterisation and variation of agarwood resins from Gyrinops walla. J. Trop. For. Sci. JTFS. 2019;31:222–229. doi: 10.26525/jtfs2019.31.2.222229. [DOI] [Google Scholar]
- 4.Wyn L.T., Anak N.A. Wood for Trees: A Review of the Agarwood (Gaharu) Trade in Malaysia. Petaling Jaya; Selangor, Malaysia: 2010. [Google Scholar]
- 5.Batubara R., Hanum T.I., Surjanto Phytochemical and tannin content in two species of agarwood leaves from Mandailing Natal Regency North Sumatera Province. AIP Conf. Proc. 2018;2049:030009. doi: 10.1063/1.5082510. [DOI] [Google Scholar]
- 6.Tajuddin S.N., Yusoff M.M. Chemical composition of volatile oils of Aquilaria malaccensis (Thymelaeaceae) from Malaysia. Nat. Prod. Commun. 2010;5:1965–1968. doi: 10.1177/1934578X1000501229. [DOI] [PubMed] [Google Scholar]
- 7.Alamil J.M.R., Paudel K.R., Chan Y., Xenaki D., Panneerselvam J., Singh S.K., Gulati M., Jha N.K., Kumar D., Prasher P., et al. Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases. Molecules. 2022;27:3038. doi: 10.3390/molecules27093038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S., Yu Z., Wang C., Wu C., Guo P., Wei J. Chemical Constituents and Pharmacological Activity of Agarwood and Aquilaria Plants. Molecules. 2018;23:342. doi: 10.3390/molecules23020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurmiati N., Periadnadiz P., Rosadi Z.F. Antimicrobial and Antioxidant Potential of Some Agarwood Plant Extracts (Aquilaria malaccensis Lamk.) Against escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213 and Candida albicans (C.P Robin) Berkhout 1923. Int. J. Progress. Sci. Technol. 2024;43:314–325. [Google Scholar]
- 10.Xu Y., Zhang Z., Wang M., Wei J., Chen H., Gao Z., Sui C., Luo H., Zhang X., Yang Y., et al. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013;14:227. doi: 10.1186/1471-2164-14-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S., Mohler J., Mahajan S.D., Schwartz S.A., Bruggemann L., Aalinkeel R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms. 2023;11:1614. doi: 10.3390/microorganisms11061614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rubis G., Paudel K.R., Manandhar B., Singh S.K., Gupta G., Malik R., Shen J., Chami A., MacLoughlin R., Chellappan D.K., et al. Agarwood Oil Nanoemulsion Attenuates Cigarette Smoke-Induced Inflammation and Oxidative Stress Markers in BCi-NS1.1 Airway Epithelial Cells. Nutrients. 2023;15:1019. doi: 10.3390/nu15041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M.R., Li W., Luo S., Zhao X., Ma C.H., Liu S.X. GC-MS Study of the Chemical Components of Different Aquilaria sinensis (Lour.) Gilgorgans and Agarwood from Different Asian Countries. Molecules. 2018;23:2168. doi: 10.3390/molecules23092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canlı K., Yetgin A., Akata I., Altuner E.M. In vitro antimicrobial screening of aquilaria agallocha roots. Afr. J. Tradit. Complement. Altern. Med. 2016;13:178–181. doi: 10.21010/ajtcam.v13i5.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sari R., Kristian F., Fajriaty I. Uji aktivitas antibakteri ekstrak etanol daun gaharu (Aquilaria microcarpa Baill.) terhadap bakteri Salmonella typhi dan Bacillus subtilis secara in vitro. J. Komunitas Farm. Nas. 2021;1:139–154. [Google Scholar]
- 16.Batubara R., Wirjosentono B., Siregar A.H., Harahap U., Tamrin T. Bioactive compounds of ethanol extract from agarwood leaves (Aquilaria malaccensis) and antimicrobial activity against bacteria and fungi growing on the skin. Biodiversitas J. Biol. Divers. 2021;22:d220553. doi: 10.13057/biodiv/d220553. [DOI] [Google Scholar]
- 17.Kamonwannasit S., Nantapong N., Kumkrai P., Luecha P., Kupittayanant S., Chudapongse N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013;12:20. doi: 10.1186/1476-0711-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jihadi N.I.M., Maifiah M.H.M., Rahim N.A., Sani M.S.A., Hashim Y.Z.H.-Y., Kamal K.M. Combination of polymyxin B and Aquilaria malaccensis extract enhanced the killing and inhibited the growth of Acinetobacter baumannii and Klebsiella pneumoniae. Malays. J. Microbiol. 2022;18:27–36. [Google Scholar]
- 19.Valan S.L., Cruz A., De Jacob P., Djearamane S. Sustainable synthesis of copper oxide nanoparticles using Aquilaria malaccensis (Agarwood) leaf extract as reducing agent. Int. J. Technol. 2022;13:1115–1125. doi: 10.14716/ijtech.v13i5.5845. [DOI] [Google Scholar]
- 20.Van Y.T., Dinh D., Tran D.M., Tran T.N., Nguyen H., Duong T.T., Doan T.Q., Nguyen H.T., Ogunwande I.A. The antimicrobial activity and essential oil constituents of the leaves and trunks of Aquilaria banaensis P.H.Hô (Thymelaeceae) from Vietnam. Nat. Prod. Res. 2024;38:744–752. doi: 10.1080/14786419.2023.2196624. [DOI] [PubMed] [Google Scholar]
- 21.Anugrah R., Mumtaz R.K., Suryasaputra D. Study In-Silico Oleanane Triterpenoids in Aquilaria spp. as a COVID-19 Antiviral. IOP Conf. Ser. Earth Environ. Sci. 2022;1104:012027. doi: 10.1088/1755-1315/1104/1/012027. [DOI] [Google Scholar]
- 22.Nurminah M., Batubara R., Ismanelly T., Albert Taking essential oil by water distillation and antibacterial activity test of refined water from agarwood plant parts (Aquilaria malaccensis Lamk) IOP Conf. Ser. Earth Environ. Sci. 2021;782:032076. doi: 10.1088/1755-1315/782/3/032076. [DOI] [Google Scholar]
- 23.Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B., Ezzat M.O., Majid A.S., Majid A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules. 2015;20:11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasetya Y.A. Aktivitas nanoemulsi minyak agarwood bouya (Aquilaria agallocha) terhadap bakteri multidrug resistant (MDR) Ber. Biol. 2022;21:79–89. [Google Scholar]
- 25.Ga’al H., Fouad H., Mao G., Tian J., Jianchu M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2018;46:1171–1179. doi: 10.1080/21691401.2017.1365723. [DOI] [PubMed] [Google Scholar]
- 26.Chen H., Yang Y., Xue J., Wei J., Zhang Z., Chen H. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) gilg trees. Molecules. 2011;16:4884–4896. doi: 10.3390/molecules16064884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z., Han X.-m., Wei J.-h., Xue J., Yang Y., Liang L., Li X.-j., Guo Q.-m., Xu Y.-h., Gao Z.-h. Compositions and antifungal activities of essential oils from agarwood of Aquilaria sinensis (Lour.) Gilg induced by Lasiodiplodia theobromae (Pat.) Griffon. & Maubl. J. Braz. Chem. Soc. 2014;25 doi: 10.5935/0103-5053.20130263. [DOI] [Google Scholar]
- 28.Hidayati E., Handayani Y., Sudarma I.M. Antibacterial Activity of Gyrinops versteegii Fruit Extracts against Staphylococcus aureus and Escherichia coli and GC-MS Analysis. J. Math. Fundam. Sci. 2022;54:249–260. doi: 10.5614/j.math.fund.sci.2022.54.2.3. [DOI] [Google Scholar]
- 29.Yu Z., Wang C., Zheng W., Chen D., Liu Y., Yang Y., Wei J. Anti-inflammatory 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones from agarwood of Aquilaria sinensis. Bioorg. Chem. 2020;99:103789. doi: 10.1016/j.bioorg.2020.103789. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z.X., Wang C.H., Chen D.L., Liu Y.Y., Wei J.H. Anti-inflammatory sesquiterpenes from agarwood produced via whole-tree agarwood-inducing technique of Aquilaria sinensis. Zhongguo Zhong Yao Za Zhi. 2019;44:4196–4202. doi: 10.19540/j.cnki.cjcmm.20190723.201. [DOI] [PubMed] [Google Scholar]
- 31.Tsai Y.C., Wang S.L., Wu M.Y., Liao C.H., Lin C.H., Chen J.J., Fu S.L. Pilloin, A Flavonoid Isolated from Aquilaria sinensis, Exhibits Anti-Inflammatory Activity In Vitro and In Vivo. Molecules. 2018;23:3177. doi: 10.3390/molecules23123177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huo H.X., Gu Y.F., Zhu Z.X., Zhang Y.F., Chen X.N., Guan P.W., Shi S.P., Song Y.L., Zhao Y.F., Tu P.F., et al. LC-MS-guided isolation of anti-inflammatory 2-(2-phenylethyl)chromone dimers from Chinese agarwood (Aquilaria sinensis) Phytochemistry. 2019;158:46–55. doi: 10.1016/j.phytochem.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang S.L., Tsai Y.C., Fu S.L., Cheng M.J., Chung M.I., Chen J.J. 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis with Anti-Inflammatory Effects in LPS-Induced Macrophages. Molecules. 2018;23:289. doi: 10.3390/molecules23020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo H.X., Zhu Z.X., Song Y.L., Shi S.P., Sun J., Sun H., Zhao Y.F., Zheng J., Ferreira D., Zjawiony J.K., et al. Anti-inflammatory Dimeric 2-(2-Phenylethyl)chromones from the Resinous Wood of Aquilaria sinensis. J. Nat. Prod. 2018;81:543–553. doi: 10.1021/acs.jnatprod.7b00919. [DOI] [PubMed] [Google Scholar]
- 35.Wang S.L., Liao H.R., Cheng M.J., Shu C.W., Chen C.L., Chung M.I., Chen J.J. Four New 2-(2-Phenylethyl)-4H-chromen-4-one Derivatives from the Resinous Wood of Aquilaria sinensis and Their Inhibitory Activities on Neutrophil Pro-Inflammatory Responses. Planta Med. 2018;84:1340–1347. doi: 10.1055/a-0645-1437. [DOI] [PubMed] [Google Scholar]
- 36.Wang S.L., Hwang T.L., Chung M.I., Sung P.J., Shu C.W., Cheng M.J., Chen J.J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis. Molecules. 2015;20:20912–20925. doi: 10.3390/molecules201119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo H.X., Gu Y.F., Sun H., Zhang Y.F., Liu W.J., Zhu Z.X., Shi S.P., Song Y.L., Jin H.W., Zhao Y.F., et al. Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia. 2017;118:49–55. doi: 10.1016/j.fitote.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Sattayasai J., Bantadkit J., Aromdee C., Lattmann E., Airarat W. Antipyretic, analgesic and anti-oxidative activities of Aquilaria crassna leaves extract in rodents. J. Ayurveda Integr. Med. 2012;3:175–179. doi: 10.4103/0975-9476.104427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng D.-Q., Yu Z.-X., Wang C.-H., Gong B., Liu Y.-Y., Wei J.-H. Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS. Int. J. Anal. Chem. 2020;2020:4575030. doi: 10.1155/2020/4575030. [DOI] [Google Scholar]
- 40.Romano K.P., Hung D.T. Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2023;1870:119407. doi: 10.1016/j.bbamcr.2022.119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behera B., Mathur P., Das A., Kapil A., Gupta B., Bhoi S., Farooque K., Sharma V., Misra M.C. Evaluation of susceptibility testing methods for polymyxin. Int. J. Infect. Dis. 2010;14:e596–e601. doi: 10.1016/j.ijid.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhou M., Wang H., Suolangjiba, Kou J., Yu B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. Leaves extract. J. Ethnopharmacol. 2008;117:345–350. doi: 10.1016/j.jep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Feng J., Yang X.W., Wang R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry. 2011;72:242–247. doi: 10.1016/j.phytochem.2010.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.