Abstract
Moringa oleifera is renowned for its high antioxidant activity. However, few studies have been conducted on its effects on aquatic animals. The aim of this experiment was to investigate the optimal fermentation process of M. oleifera leaves and to evaluate the effects of fermented M. oleifera leaves on crayfish (9.11 ± 0.3 g) in terms of growth performance, antioxidant capacity, and gut microbiological parameters. By optimizing the fermenting material/water ratio, fermentation time, temperature, and strain, the optimal fermentation conditions of a 10% water ratio + 48 h + 30 °C + inoculation with 2% B. amyloliquefaciens (107 CFU mL−1) were obtained. These conditions resulted in notable increases in the contents of the total protein, total phenols, flavonoids, and amino acids (p < 0.05) while also leading to a notable decrease in the content of tannins in contrast to those of unfermented M. oleifera leaves (p < 0.05). The fermented M. oleifera (FMO) leaves were incorporated at five concentrations, including 0% (control (CT)), 0.25% (0.25FMO), 0.5% (0.5FMO), 1% (1FMO), and 2% (2FMO). The results showed that the 1FMO group performed better in terms of the final body weight (FBW), weight gain rate (WGR), and specific weight gain rate (SGR) compared with the CT group (p < 0.05). In addition, amylase and lipase activities were significantly higher in the 1FMO and 2FMO groups compared with the other groups (p < 0.05). The fermented M. oleifera leaves significantly increased the catalase (CAT) activity in the crayfish (p < 0.05). The superoxide dismutase (SOD) activity was significantly increased in the 0.25FMO, 1FMO, and 2FMO groups, and the malondialdehyde (MDA) content was significantly decreased while the glutathione peroxidase (GSH-Px) content was significantly increased in the 0.5FMO, 1FMO, and 2FMO groups (p < 0.05). Furthermore, the 1FMO group was observed to significantly increase the abundance of Firmicutes while simultaneously reducing the abundance of Aeromonas (p < 0.05) and adjusting the structure of the intestinal microbiome. In conclusion, this study established the optimal fermentation conditions for M. oleifera and obtained a product with high nutrient and low tannin contents. Furthermore, the incorporation of 1% FMO was demonstrated to facilitate growth, enhance the antioxidant capacity, and optimize the gut microbiology in crayfish.
Keywords: fermentation process, growth performance, antioxidant capacity, intestinal microbiome, M. oleifera, crayfish
1. Introduction
Moringa oleifera is a plant of the genus Moringa in the Moringaceae family, which, because of its high nutritional value, such as proteins, fatty acids, and vitamins, is of interest to many experts and scientists [1]. M. oleifera leaves have been demonstrated to provide humans with nutrients when consumed directly or incorporated into foodstuffs [2,3,4]. For animals, the search for a cheap and nutritious alternative feed has become more problematic as feed prices continue to rise. As M. oleifera is rich in nutrients, the use of M. oleifera as a feed substitute for soybean meal, fish meal, and other feeds has become a concern for scholars at present [5,6]. Vargas-Sánchez et al. (2019) made M. oleifera leaves into silage for dairy cows and found that the cows had a stronger immune system than those fed normal silage [7]. At present, a lot of M. oleifera feed is used for ruminant and livestock animals for achieving better breeding results [8,9], but we have also found that M. oleifera leaves contain anti-nutritional factors, such as tannins, which can reduce the digestibility of nutrients and impair intestinal health if consumed in excess [10,11]. It is, therefore, crucial to identify an effective method for increasing the nutrient contents and reducing the anti-nutritional factors present in M. oleifera leaves, as this will be of great significance for the future utilization of M. oleifera leaves as animal feed. Numerous studies have shown that the protein contents of plant products and palatability of feeds can be improved through fermentation techniques [12,13,14]. Typically, plants are fermented to reduce the carbohydrate content of indigestible oligosaccharides and polysaccharides, which improves vitamin B availability and amino acid synthesis [15]. Zhang et al. (2017) have found that solid-state fermentation using Bacillus subtilis CICC 10440 mixed with M. oleifera leaf powder at a ratio of 1:60 produces the maximum amount of soluble protein [16].
Procambarus clarkii, also known as crayfish, is preferred by a wide range of consumers for its nutritious and tasty meat [17]. However, with the rapid development of aquaculture, there are more diseases and a shortage of protein-source feed resources, such as fishmeal and soybean meal, and feed prices continue to rise, affecting the development of the entire feed industry. Siddik et al. (2020) demonstrated that the replacement of fishmeal with fermented animal-source protein improved the gut microbiology, immune performance, and resistance to Vibrio mimicus in crayfish [18]. Jerimoth et al. (2023) showed that the addition of 1% M. oleifera leaves increased the growth performance of Clarias gariepinus [19]. Kaleo et al. (2019) showed that the addition of 0.5% M. oleifera leaf extracts improved the growth performance, increased immunity, and alleviated the stress caused by high ammonia levels in Macrobrachium rosenbergii [20]. Kamble et al. (2019) demonstrated that the addition of 0.5% M. oleifera leaves was able to improve the growth and immunity of Nile tilapia [21].
However, the use of fermented M. oleifera leaves as a functional feed for crayfish is still less researched. Therefore, these experiments were conducted to obtain the optimal fermentation conditions of M. oleifera leaves by liquid fermentation and to study the effects of fermented M. oleifera leaves as feed additives on the growth performance and antioxidant capacity of crayfish as an experimental object, thus laying the foundation for the application of fermented M. oleifera leaf feed in the aquaculture process.
2. Materials and Methods
2.1. M. oleifera Leaves, Probiotics, and Crayfish
M. oleifera leaves were obtained from Xi’an Xihai Biotechnology Co., Ltd., Xi’an, China. B. subtilis S1X-15, B. subtilis JD, B. subtilis ZSP, B. subtilis IIIA-2, and B. amyloliquefaciens JY-24 were obtained from Jiangsu Su Wei Microbiology Research Co., Ltd., Wuxi, China and kept at the Freshwater Fisheries Research Centre (FFRC). The crayfish were obtained from Yangzhong Experimental Base at the Jiangsu Institute of Freshwater Aquatic Research (Zhenjiang, China).
2.2. Exploration of the Optimal Fermentation Conditions of M. oleifera Leaves
2.2.1. Preparation of Bacterial Suspension and Fermented M. oleifera Leaves
The fermentation strains were inoculated in a Luria–Bertani slant medium and cultured at 37 °C for 24 h. The strains were inoculated in 3 Erlenmeyer flasks containing 15 mL of sterilized distilled water, and the concentration of the bacterial suspension was adjusted to 107 CFU mL−1.
The dried M. oleifera leaves were crushed and sieved through an 80-mesh sieve to obtain powder, which was thoroughly mixed according to a material/water ratio of 2.5–12.5% to obtain pulp. The bacterial suspension was added to the fermentation tank containing the pulp at 2%, and the M. oleifera leaf pulp was used for the next single-factor and orthogonal experiments.
2.2.2. Single-Factor Fermentation Test
The Optimal Material/Water Ratio of the Fermented M. oleifera Leaves
To determine the appropriate fermentation material/water ratio, the M. oleifera leaf powder was combined with water at concentrations of 2.5%, 5%, 7.5%, 10%, and 12.5% (m/v); adjusted to pH 8.6; sterilized in an autoclave at 121 °C for 30 min; and inoculated with 2% B. subtilis S1X-15 at a temperature of 35 °C and a shaker speed of 180 rpm. After fermentation, the sample was centrifuged for 10 min at 6000 rpm and 4 °C, and the supernatant was used to assay the total protein and total phenolic contents. Three replicates were performed for each experiment.
The Optimal Fermentation Temperature of the Fermented M. oleifera Leaves
To explore the appropriate fermentation temperatures, the fermentation temperature was set at 25 °C, 30 °C, 35 °C, 40 °C, and 45 °C. M. oleifera leaf powder and water were mixed at 10%, autoclaved, and then inoculated with 2% B. subtilis S1X-15, maintaining the shaker speed at 180 rpm for 48 h. Samples were processed and screened under the same conditions as those described in “The Optimal Material/Water Ratio of the Fermented M. oleifera Leaves” of Section 2.2.2. Three replicates were performed for each experiment.
The Optimal Fermentation Strains of the Fermented M. oleifera Leaves
To investigate the appropriate fermentation strains, a temperature of 35 °C, a fermentation time of 48 h, and a material/water ratio of 10% were maintained; fermentation broth samples were inoculated with B. subtilis S1X-15, B. subtilis JD, B. subtilis ZSP, B. subtilis IIIA-2, and B. amyloliquefaciens JY-24 at an inoculum amount of 2%; and a shaking speed of 180 rpm was maintained. Samples were processed and screened under the same conditions as those described in “The Optimal Material/Water Ratio of the Fermented M. oleifera Leaves” of Section 2.2.2. Three replicates were performed for each experiment.
The Optimal Fermentation Time of the Fermented M. oleifera Leaves
To obtain the appropriate fermentation time, fermentation times of 12 h, 24 h, 38 h, 72 h, and 96 h were set; the temperature was maintained at 35 °C; the material/water ratio was 10%; the samples were inoculated with 2% B. subtilis S1X-15; and the shaking speed was 180 rpm. Samples were processed and screened under the same conditions as those described in “The Optimal Material/Water Ratio of the Fermented M. oleifera Leaves” of Section 2.2.2. Three replicates were performed for each experiment.
2.2.3. Orthogonal Experiment of Fermented M. oleifera Leaves
The orthogonal test was performed based on the one-way test according to the L9(34) orthogonal table [22]. The total protein content was used as a screening indicator to determine the optimal conditions for M. oleifera leaves. The factor level and orthogonal test table are shown in Table 1. There were 3 replications for each experiment.
Table 1.
Orthogonal test of fermented M. oleifera leaves.
| Number | Factors | |||
|---|---|---|---|---|
| Strain A |
Material/Water Ratio (%) B |
Temperature (°C) C |
Time (h) D |
|
| 1 | 1 (S1X-15) | 1 (7.5%) | 1 (30 °C) | 1 (24 h) |
| 2 | 1 (S1X-15) | 2 (10.0%) | 2 (35 °C) | 2 (48 h) |
| 3 | 1 (S1X-15) | 3 (12.5%) | 3 (40 °C) | 3 (72 h) |
| 4 | 2 (IIIA-2) | 1 (7.5%) | 2 (35 °C) | 3 (72 h) |
| 5 | 2 (IIIA-2) | 2 (10.0%) | 3 (40 °C) | 1 (24 h) |
| 6 | 2 (IIIA-2) | 3 (12.5%) | 1 (30 °C) | 2 (48 h) |
| 7 | 3 (JY-24) | 1 (7.5%) | 3 (40 °C) | 2 (48 h) |
| 8 | 3 (JY-24) | 2 (10.0%) | 1 (30 °C) | 3 (72 h) |
| 9 | 3 (JY-24) | 3 (12.5%) | 2 (35 °C) | 1 (24 h) |
2.2.4. Determination of the Total Protein Content
The total protein content was determined according to the experimental method proposed by Loffler [23]. A bovine serum albumin protein standard (the protein was obtained from Beijing Solarbio Technology Co., Ltd., Beijing, China; kit code: A8020) was diluted twofold, and the enzyme assay was carried out at a wavelength of 595 nm. A standard curve was constructed, and the concentration of the sample protein was calculated according to the protein standard curve.
2.2.5. Determination of the Flavonoid Content
The methodology was followed according to the description of the plant flavonoid content test kit from Beijing Solarbio Technology Co., Ltd. (kit code: BC1335). Samples were extracted by ultrasonic extraction with 60% ethanol at 60 °C for 30 min. The tannic acid standard was diluted twice, and the enzyme standard was measured at 470 nm to construct a standard curve according to the tannic acid standard curve to calculate the content of flavonoids in the sample.
2.2.6. Determination of the Total Phenol Content
The test kit for the total phenol content from Beijing Solarbio Technology Co., Ltd. (kit code: BC1345) was employed. The standard solution of tannic acid was diluted twofold, and the enzyme assay was performed at a wavelength of 760 nm to construct a standard curve. The content of the total phenol in the samples was then calculated according to the standard curve.
2.2.7. Determination of the Tannin Content
The tannin content was checked using the test kit of Beijing Solarbio Technology Co., Ltd. (kit code: BC1395). Briefly, the samples were extracted with the extract solution and then subjected to a water bath at 70 °C for 30 min. The tannin standard solution was diluted twice with the extract solution and then subjected to an enzymatic assay at 275 nm to construct a standard curve, which was then used to calculate the tannin content in the samples.
2.2.8. Determination of the Total Amino Acid Content
In accordance with the specifications set forth by Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China (kit code: A026-1-1), the total amino acid determination kit is to be utilized. A volume of 500 µL of the supernatant was transferred to a 2 mL centrifuge tube, followed by the addition of 1 mL of the amino acid reaction solution. Subsequently, the amino acid developing solution was added, and the mixture was mixed. The enzyme assay was then conducted at a wavelength of 650 nm, according to the standard curve, in order to calculate the content of the total amino acids in the samples.
2.3. Experimental Culture of Crayfish
2.3.1. Experimental Design and Diet
M. oleifera leaves were fermented under the optimal conditions, and the liquid was pre-cooled at −80 °C for 2 h and freeze dried at −50 °C for 48 h to obtain the fermented M. oleifera leaf powder. The culture experiment was designed as a completely randomized experimental design, with 450 healthy and similarly sized crayfish (9.11 ± 0.3 g), which were then divided into five groups with 0% (CT), 0.25% (0.25FMO), 0.5% (0.5FMO), 1% (1FMO), and 2% (2FMO) doses of fermented M. oleifera leaves. Each group consisted of 30 crayfish with three replicates and was cultured in fifteen cement ponds (600 L; 1 m × 1m × 0.6 m) for 60 days.
The formulations and approximate compositions of the experimental diets are shown in Table 2. Various raw materials were crushed through a 60-mesh sieve and gradually mixed and then oil and an appropriate amount of water were added. A twin-screw extruder, manufactured by Guangzhou Huagong Optical, Mechanical & Electrical Technology Co., Ltd. in Guangzhou, China, was used for granulating the sinking pellets.
Table 2.
Ingredients and approximate compositions of the basal diets.
| Ingredient | Diet | ||||
|---|---|---|---|---|---|
| CT (Basal Diet) |
0.25FMO (Basal Diet + 0.25FMO) |
0.5FMO (Basal Diet + 0.5FMO) |
0.5FMO (Basal Diet + 1FMO) |
2FMO (Basal Diet + 2FMO) |
|
| Domestic fish meal | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Soybean meal | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Rapeseed meal | 18.0 | 18.0 | 18.0 | 18.0 | 18.0 |
| Shrimp meal | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Peanut meal | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Spray-dried blood cell powder | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Domestic DDGS | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| α-starch | 18.79 | 18.54 | 18.29 | 17.79 | 16.79 |
| Rice bran | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Soybean oil | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| MCP | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Premix | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Carboxymethyl cellulose | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Salt | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Ecdysone | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Squid paste | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Calcium bicarbonate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Methionine | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| M. oleifera | 0 | 0.25 | 0.5 | 1 | 2 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Nutrient composition | |||||
| Crude protein | 29.94% | 29.94% | 29.94% | 29.94% | 29.94% |
| Crude lipid | 9.05% | 9.05% | 9.05% | 9.05% | 9.05% |
| Moisture | 12.43% | 12.43% | 12.43% | 12.43% | 12.43% |
Notes: (1) Fish oil and (2) soybean oil were obtained from Wuxi Tongwei Feedstuffs Co., Ltd. (Wuxi, China). (3) Vitamin and mineral premix (IU, g, or mg kg−1 of the diet): vitamin A, 25,000 IU; vitamin D3, 20,000 IU; vitamin E, 200 mg; vitamin K3, 20 mg; thiamin, 40 mg; riboflavin, 50 mg; calcium pantothenate, 100 mg; pyridoxine HCl, 40 mg; cyanocobalamin, 0.2 mg; biotin, 6 mg; folic acid, 20 mg; niacin, 200 mg; inositol, 1000 mg; vitamin C, 2000 mg; choline, 2000 mg; calcium biphosphate, 20 g; sodium chloride, 2.6 g; potassium chloride, 5 g; magnesium sulfate, 2 g; ferrous sulfate, 0.9 g; zinc sulfate, 0.06 g; cupric sulfate, 0.02 g; manganese sulfate, 0.03 g; sodium selenate, 0.02 g; cobalt chloride, 0.05 g; potassium iodide, 0.004 g.
2.3.2. Feeding Management
In order to provide an optimal environment for the crayfish, tubes and tiles were introduced to each pond. Following a three-day cultivation period, the crayfish were provided with sustenance on three occasions per day, at 7:00, 12:00, and 19:00. Adaptations were made according to the dietary requirements of the crayfish. The aquaculture process was maintained at a depth of 30 cm, with waterflow maintained at 1 L min−1 within the recirculation system. Rearing conditions included a temperature of 29–30 °C, a pH range of 7.0–7.5, and dissolved oxygen levels above 5 mg L−1, with ammonia, nitrogen, and nitrite levels below 0.1 mg L−1.
2.3.3. Sample Collection and Growth Indicator Determination
Following an extensive 60-day breeding period, the crayfish underwent a 24-h fasting phase. The total weight and dietary intake were measured, yielding data on the weight-gain rate and feed conversion rate. Hepatopancreatic and intestinal tissues (6 per tank, 18 per group) were preserved at −20 °C for antioxidant and digestive enzyme assessments. Another crayfish from the same tank had their intestines promptly harvested and stored at −80 °C for subsequent gut microbiome analyses.
The growth rate (WGR), specific weight-gain rate (SGR), and feed conversion ratio (FCR) for the crayfish were calculated as follows:
| WGR (%) = (G2 − G1)/G1 × 100 |
| SGR (%/d) = (lnG2 − lnG1)/D × 100 |
| FCR = G3/G′ |
Notes: G1 is the average initial weight of the crayfish (g), G2 is the average final weight of the crayfish (g), D is the duration of the culture trial (days), G3 is the total food intake (g), G′ is the total weight gain.
2.3.4. Determination of Digestive Enzymatic Activities
The intestines of the crayfish were used for the determination of the α-amylase, lipase, and trypsin activities. Digestive enzyme kits were purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (α-amylase kit code: C016-1-1, lipase kit code: A054-1-1, and trypsin kit code: A080-2-2).
2.3.5. Determination of the Antioxidant Enzymatic Activities
For the determination of the antioxidant capacities, the hepatopancreases of the crayfish and the total antioxidant capacity (kit code: A015-1-2), total superoxide dismutase (kit code: A001-1), malondialdehyde (kit code: A003-1), glutathione peroxidase (kit code: A005-1-2), and catalase (kit code: A007-1-1) test kits from Nanjing Jiancheng Biotechnology Co., Ltd. were used.
The methods for the determination of the glutamic transaminase (AST) and glutamic pyruvic transaminase (ALT) activities were based on the kits supplied by Nanjing Jiancheng Biotechnology Co., Ltd. The kits used were the AST kit (code: C010-1-1) and the ALT kit (code: C009-1-1).
2.3.6. Microbiome Analysis
The intestines of the CT, 1FMO, and 2FMO groups were transported via dry ice to Nanjing Genepioneer Biotechnologies Co., Ltd., Nanjing, China, with 3 replicates in each group. DNA was extracted with an E.Z.N.A.® Soil Kit (Omega Bio-Tek, Norcross, GA, USA), and primers were designed to amplify the V3-V4 region to yield a 420 bp fragment. The Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) was used to sequence the data. The Silva 16S rRNA database was selected for the 16S rRNA motif. By comparing amplicon sequence variant (ASV) sequences to those in the Silva bacterial database, the species classification information corresponding to each ASV was obtained, and the most abundant sequences were selected. The QIIME2 consensus research algorithm was used for classifying each ASV feature sequence, and species annotation was performed using default parameters in QIIME2 software (qiime2-2021.11).
2.3.7. Bioinformatic Analysis
The annotated species were employed for microbiological analyses, including the diversity index, cluster analysis, differential analysis, KEGG pathway prediction, and correlational analysis. The diversity index was compared between the groups by analyzing the observed species, the Chao1 index, and the Shannon and Simpson indices (p < 0.05 indicated a significant difference). The magnitude of the abundance was observed by clustering species at the genus and phylum levels (p < 0.05 indicated a statistically significant difference in the abundance). The differential species for each group were obtained by LEfSe analysis (p = 0.05; LDA = 4). The KEGG pathways in each group were obtained by comparing KEGG libraries, including KEGG primary and secondary pathways. Correlational analyses of phylum- and genus-level species with antioxidant indicators were performed in order to observe the levels of the associations between gut microbes and antioxidant indicators [24].
2.4. Statistical Analysis
The data were analyzed by analysis of variance (ANOVA) and multiple comparisons (Duncan) using SPSS 20.0 software. An independent sample t-test was performed to test the differences before and after the fermentation of the M. oleifera leaves. The results are expressed as means ± SDs. p < 0.05 was considered to indicate a significant difference. Bioinformatic analysis was performed using OmicStudio tools at https://www.omicstudio.cn/tool (accessed on 1 April 2024).
3. Results
3.1. Exploration of the Optimal Fermentation Conditions of M. oleifera Leaves
3.1.1. The Optimal Material/Water Ratio of the Fermented M. oleifera Leaves
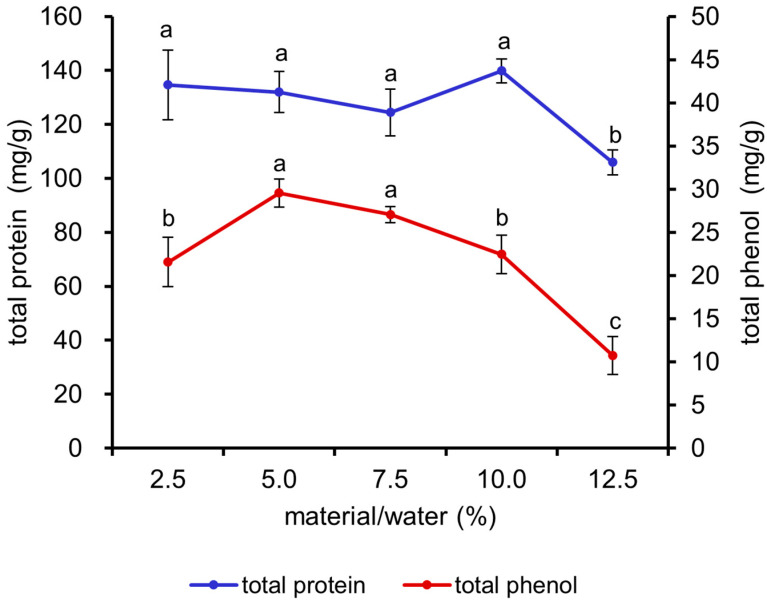
As illustrated in Figure 1, the total protein content exhibited a significant decrease at a 12.5% material/water ratio (p < 0.05). The total phenol content demonstrated an initial increase, followed by a decline, with increasing material/water ratio, and the maximum value was attained at ratios of 5% and 7.5% (p < 0.05). Consequently, the 5%, 7.5%, and 10% ratios were selected for the orthogonal experiment.
Figure 1.
Effects of different material/water ratios on the total protein and total phenol contents of fermented M. oleifera leaves. Note: different letters in the figure indicate significant differences, p < 0.05.
3.1.2. The Optimal Fermentation Temperature of the Fermented M. oleifera Leaves
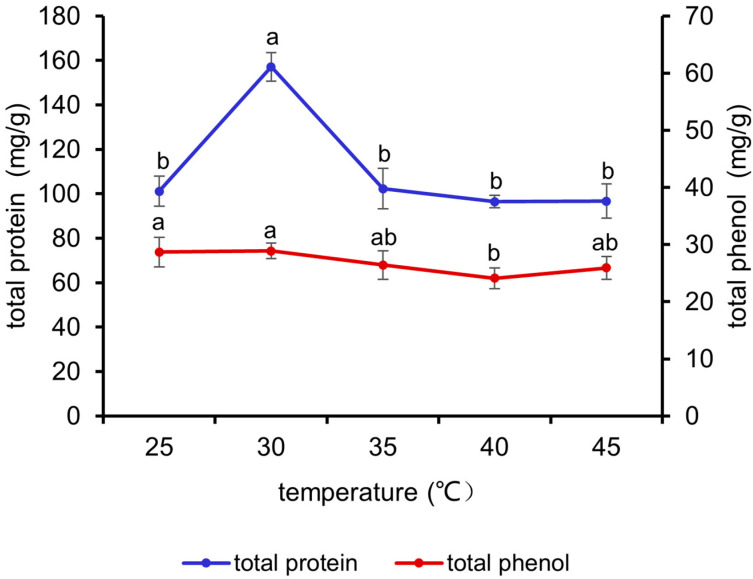
It can be seen from Figure 2 that the total protein content exhibited an initial increase, followed by a decline, with increasing fermentation temperature and reached the maximum value at 30 °C (p < 0.05). The total phenol content demonstrated a decline with increasing temperature. Accordingly, 25 °C, 30 °C, and 35 °C were selected for the orthogonal experiment.
Figure 2.
Effects of different temperatures on the total protein and total phenol contents of fermented M. oleifera leaves. Note: different letters in the figure indicate significant differences, p < 0.05.
3.1.3. The Optimal Fermentation Strains of the Fermented M. oleifera Leaves
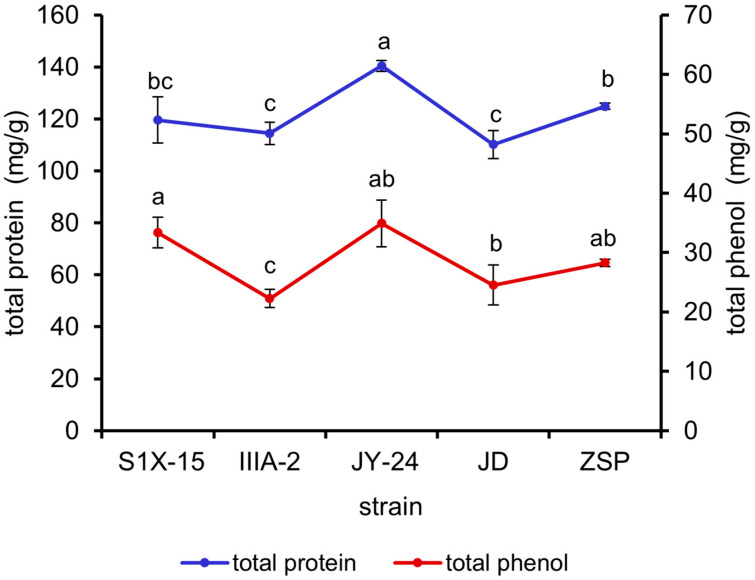
The results in Figure 3 reveal that the total protein content of the M. oleifera leaves fermented by B. amyloliquefaciens JY-24 was significantly higher than those of the M. oleifera leaves fermented by the other strains (p < 0.05). The total phenol contents of the B. subtilis-S1X-15- and B. amyloliquefaciens-JY-24-fermented M. oleifera leaves were found to be higher than those of the M. oleifera leaves fermented by the remaining strains. Therefore, B. subtilis S1X-15, B. subtilis IIIA-2, and B. amyloliquefaciens were selected for orthogonal experiments.
Figure 3.
Effects of different strains on the total protein and total phenol contents of fermented M. oleifera leaves. Note: different letters in the figure indicate significant differences, p < 0.05.
3.1.4. The Optimal Fermentation Time of the Fermented M. oleifera Leaves
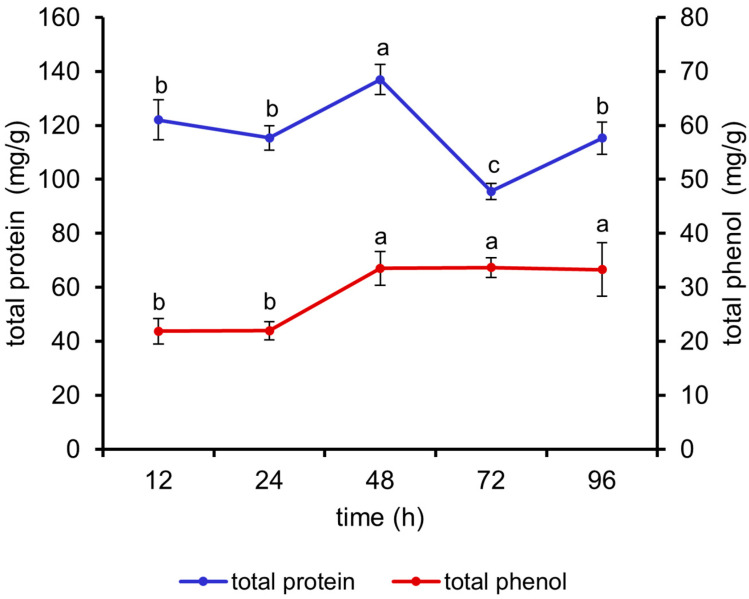
As can be seen in Figure 4, the total protein content of the fermented M. oleifera leaves reached the maximum value at 48 h (p < 0.05). The total phenol content increased with increasing fermentation time and started to increase significantly at 48 h (p < 0.05), with no significant difference between 48 and 96 h. Therefore, 24 h, 48 h, and 72 h were selected for orthogonal experiments.
Figure 4.
Effects of different fermentation times on the total protein and total phenol contents of fermented M. oleifera leaves. Note: different letters in the figure indicate significant differences, p < 0.05.
3.1.5. Orthogonal Experiment of Fermented M. oleifera Leaves
The results of the single-factor experiment were used to design the orthogonal tests, which were carried out to determine the most appropriate fermentation conditions. The results of the extreme difference analysis are presented in Table 3, with the total protein content used as an indicator. The primary relationship among the factors influencing the fermentation conditions was C > A > B > D. The optimal combination was identified as C1 A3 B2 D2, which corresponds to the fermentation strain B. amyloliquefaciens JY-24, a material/water ratio of 10%, a fermentation time of 48 hours, and a fermentation temperature of 30 °C.
Table 3.
Extreme variance analysis of fermented M. oleifera leaves.
| Strain A |
Material/Water Ratio (%) B |
Temperature (°C) C |
Time (h) D |
Protein Content (mg g−1) | |
|---|---|---|---|---|---|
| 1 | 1 (S1X-15) | 1 (7.5%) | 1 (30 °C) | 1 (24 h) | 257.6 ± 28.12 |
| 2 | 1 (S1X-15) | 2 (10%) | 2 (35 °C) | 2 (48 h) | 211.46 ± 18.59 |
| 3 | 1 (S1X-15) | 3 (12.5%) | 3 (40 °C) | 3 (72 h) | 128.63 ± 18.59 |
| 4 | 2 (IIIA-2) | 1 (7.5%) | 2 (35 °C) | 3 (72 h) | 77.04 ± 8.72 |
| 5 | 2 (IIIA-2) | 2 (10%) | 3 (40 °C) | 1 (24 h) | 192.32 ± 34.18 |
| 6 | 2 (IIIA-2) | 3 (12.5%) | 1 (30 °C) | 2 (48 h) | 205.16 ± 4.64 |
| 7 | 3 (JY-24) | 1 (7.5%) | 3 (40 °C) | 2 (48 h) | 213.84 ± 13.93 |
| 8 | 3 (JY-24) | 2 (10%) | 1 (30 °C) | 3 (72 h) | 325.48 ± 29.98 |
| 9 | 3 (JY-24) | 3 (12.5%) | 2 (35 °C) | 1 (24 h) | 171.43 ± 18.05 |
| k1 | 579.69 | 548.48 | 788.24 | 621.35 | |
| k2 | 474.96 | 729.26 | 459.93 | 630.46 | |
| k3 | 710.75 | 505.22 | 534.79 | 531.15 | |
| R | 235.79 | 224.04 | 328.31 | 99.31 | |
| Primary–secondary factors | C > A > B > D | ||||
| Optimal combination | C1 A3 B2 D2 | ||||
Note: A: strains; B: ratio of material/water; C: temperature; D: time; k1–3: the influence of each factor on the test index; R: the influence of each factor on the test index is reflected.
3.2. Changes in Nutrient Composition and Tannin Content of Fermented M. oleifera Leaves
The results before and after fermentation for M. oleifera leaves are illustrated in Table 4. Under the optimal conditions, the contents of the total protein, total phenolics, flavonoids, and amino acids before fermentation exhibited a marked increase (p < 0.01), while the content of tannins was significantly reduced in comparison to that observed after fermentation (p < 0.05).
Table 4.
Changes in nutrient composition and tannin content of fermented M. oleifera leaves.
| Before Fermentation | After Fermentation | p-Value | |
|---|---|---|---|
| Total protein (mg g−1) | 314.71 ± 28.41 | 416.53 ± 20.75 | 0.009 |
| Total phenols (mg g−1) | 6.93 ± 2.49 | 39.57 ± 4.12 | 0.001 |
| Flavonoids (mg g−1) | 0.32 ± 0.03 | 0.78 ± 0.09 | 0.007 |
| Amino acids (µmol g−1) | 235.58 ± 14.58 | 369.84 ± 4.23 | 0.002 |
| Tannins (nmol mg protein−1) | 0.22 ± 0.02 | 0.20 ± 0.005 | 0.012 |
3.3. Effect of Fermented M. oleifera Leaves on the Growth Performance of the Crayfish
The results of the growth performance of the crayfish are shown in Table 5. FBW, WGR, and SGR were significantly higher in the 1FMO group than those of the control and 0.25FMO groups (p < 0.05), and the difference in FCR among the groups was not significant (p > 0.05).
Table 5.
Effect of fermented M. oleifera leaves on growth performance of crayfish.
| CT | 0.25FMO | 0.5FMO | 1FMO | 2FMO | |
|---|---|---|---|---|---|
| IBW (g) | 9.14 ± 0.03 a | 9.07 ± 0.06 a | 9.12 ± 0.08 a | 9.13 ± 0.12 a | 9.10 ± 0.03 a |
| FBW (g) | 21.43 ± 1.85 bc | 19.62 ± 1.33 c | 23.10 ± 1.51 ab | 25.62 ± 1.67 a | 23.02 ± 1.32 ab |
| FCR | 1.73 ± 0.03 a | 1.55 ± 0.11 a | 1.53 ± 0.46 a | 1.63 ± 0.25 a | 1.97 ± 0.81 a |
| WGR (%) | 134.43 ± 19.48 bc | 116.15 ± 13.26 c | 153.44 ± 18.77 b | 180.41 ± 14.65 a | 152.94 ± 13.53 ab |
| SGR (%) | 1.70 ± 0.17 bc | 1.54 ± 0.13 c | 1.86 ± 0.15 ab | 2.06 ± 0.10 a | 1.86 ± 0.11 ab |
Note: Different letters in the different groups indicate significant differences, p < 0.05. WGR (%) = (G2 − G1)/G1 × 100; SGR (%/d) = (lnG2 − lnG1)/D × 100; FCR = G3/G’. G1 is the average initial weight of the crayfish (g), G2 is the average final weight of the crayfish (g), D is the duration of the culture trial (days), G3 is the total food intake (g), and G’ is the total weight gain.
3.4. Effect of Fermented M. oleifera Leaves on the Digestive Enzymes of the Crayfish
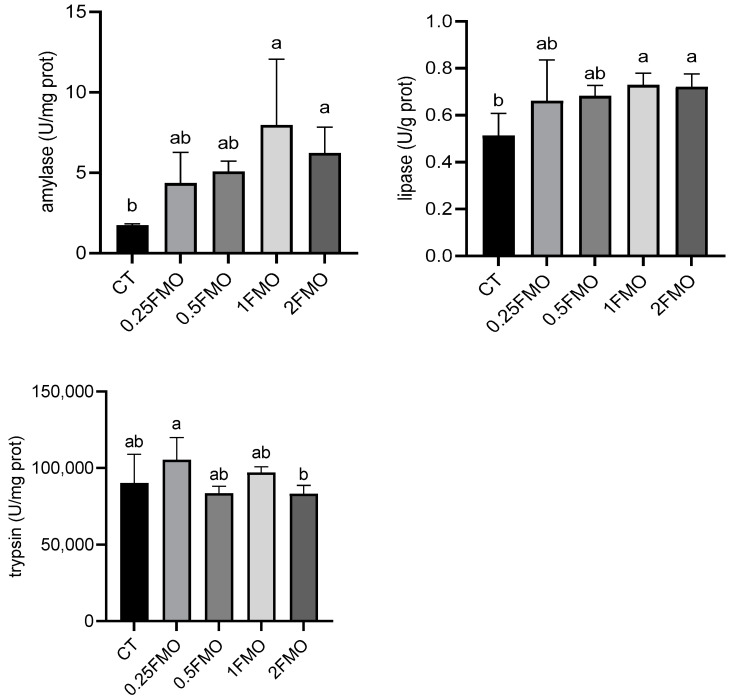
The results of digestive enzymatic activities are shown in Figure 5. The addition of fermented M. oleifera leaves significantly increased the α-amylase activity (p < 0.05). The 1FMO and 2FMO groups showed significantly increased lipase activity (p < 0.05), and the 2FMO group showed significantly decreased trypsin activity compared to the 0.25 group (p < 0.05), while the rest of the additive groups did not show any significant difference compared to the control group (p > 0.05).
Figure 5.
Effect of fermented M. oleifera leaves on the digestive enzymes of the crayfish. Note: different letters in the different groups indicate significant differences, p < 0.05.
3.5. Effect of Fermented M. oleifera Leaves on the Antioxidant Capacity of the Crayfish
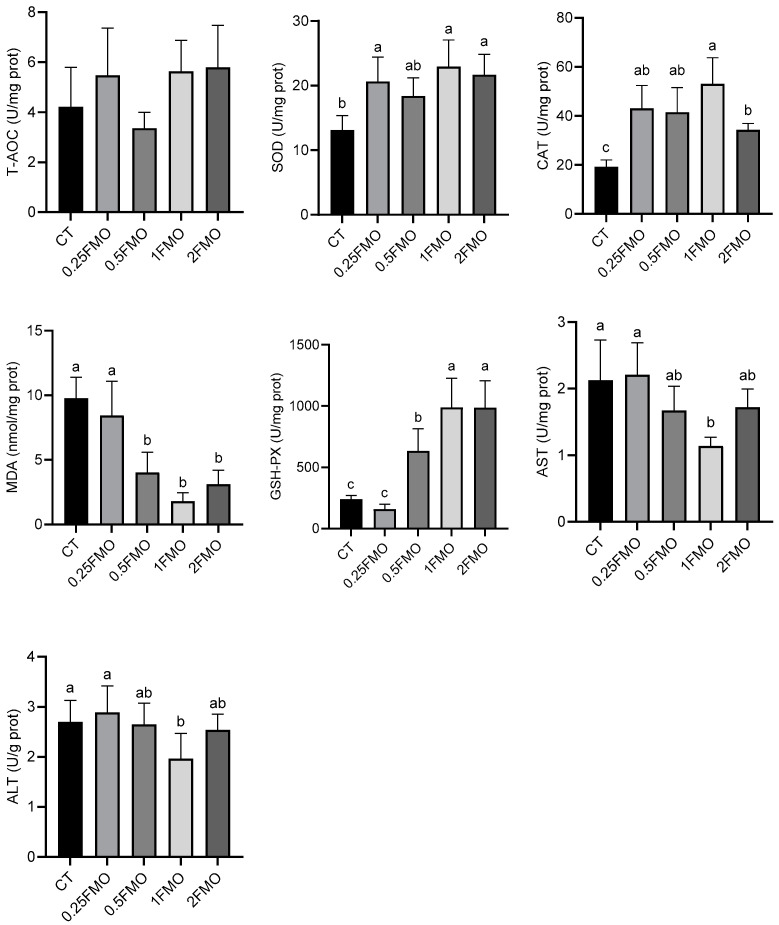
The results of the antioxidant enzymatic activities of the crayfish are shown in Figure 6. There was no significant difference in the total antioxidant capacity (T-AOC) among the groups (p > 0.05). The 0.25FMO, 1FMO, and 2FMO groups showed significantly increased SOD activity in contrast with the CT group (p < 0.05); the addition of the fermented M. oleifera leaves significantly increased the CAT activity compared to that of the CT group (p < 0.05). The 0.5FMO, 1FMO, and 2FMO groups showed significantly decreased MDA contents and increased GSH-Px activities compared to those of the CT group (p < 0.05), and the 1FMO group showed significantly reduced AST and ALT contents in contrast to those of the CT group (p < 0.05).
Figure 6.
Effect of fermented M. oleifera leaves on the antioxidant capacity of the crayfish. Note: different letters in the different groups indicate significant differences, p < 0.05.
3.6. Effect of Fermented M. oleifera Leaves on the Intestinal Microbiome of the Crayfish
3.6.1. Diversity Analysis of Intestinal Communities
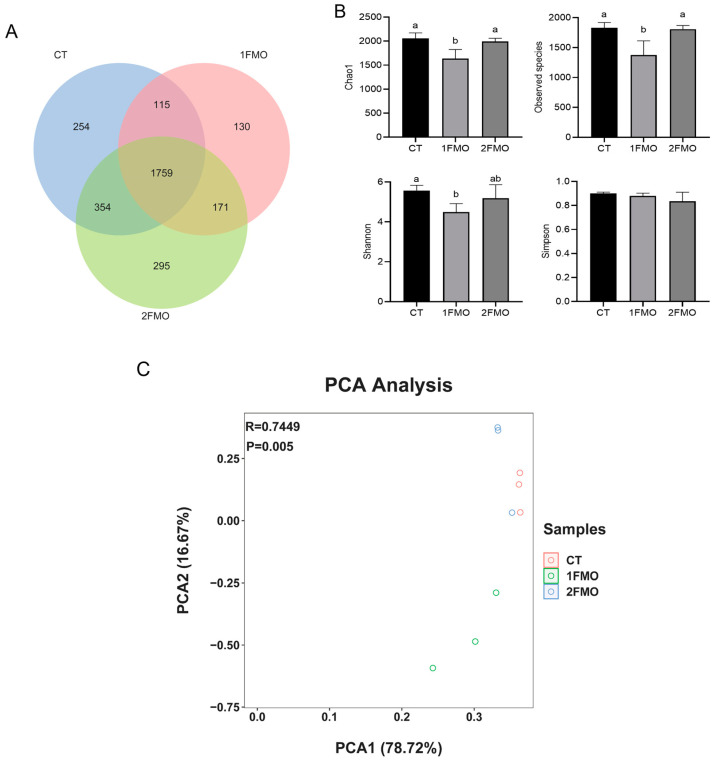
A Venn diagram was constructed based on the cluster analysis of the OTUs (Figure 7A). The number of OTUs shared by the CT, 1FMO, and 2FMO groups was 1759, of which the number of OTUs specific to the 2FMO group was 295; the number of OTUs specific to the CT group was 254, and the number of OTUs specific to the 1FMO group was 130.
Figure 7.
Effect of fermented M. oleifera leaves on the diversity analysis of the intestinal communities of the crayfish. Note: different letters in the different groups indicate significant differences, p < 0.05. (A): Venn diagram; (B): α-diversity; (C): PCA analysis.
After 60 days of culturing, the results of the crayfishes’ gut microbiome diversity are shown in Figure 7B. Chao1 and the observed species index were significantly lower in the 1FMO group than those of the CT and 2FMO groups (p < 0.05); the Shannon index of the 1FMO group was significantly lower than that of the CT group (p < 0.05), and there was no significant difference in the Simpson index between the groups (p > 0.05).
Analysis of the PCA results showed that the differences in the community structure between the CT and 2FMO groups were small, and the differences in the community structure between the 1FMO and CT groups were large (Figure 7C).
3.6.2. Analysis of the Structure of the Intestinal Microbiome of the Crayfish
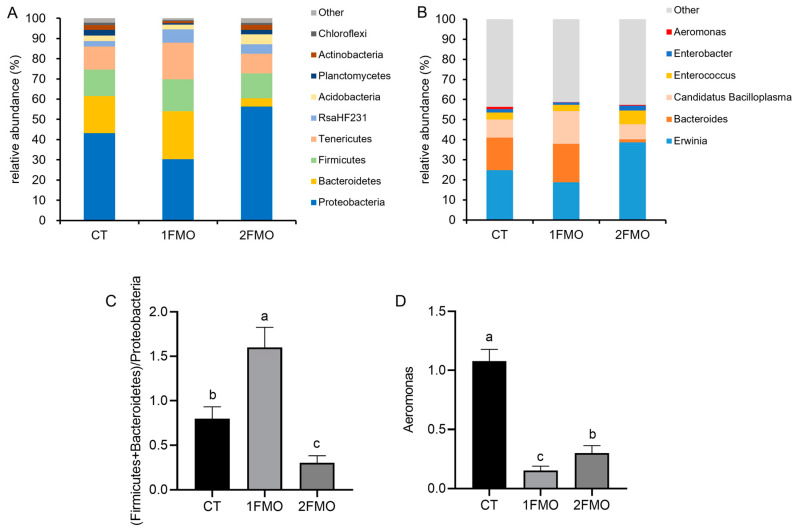
At the phylum level, Proteobacteria, Bacteroidetes, Firmicutes, and Tenericutes were the major phyla in the three groups (Figure 8A); at the genus level, Erwinia, Bacteroides, Candidatus Bacilloplasma, and Enterococcus were the major genera in the three groups (Figure 8B).
Figure 8.
Effect of fermented M. oleifera leaves on the intestinal microbiome of the crayfish. Note: different letters in the different groups indicate significant differences, p < 0.05. (A): phylum level; (B): genus level; (C): the ratio of (Firmicutes + Bacteroidetes)/Proteobacteria; (D): the abundance of Aeromonas.
The (Firmicutes + Bacteroidetes)/Proteobacteria ratio was significantly higher in the 1FMO group than in the CT group, while the ratio was significantly lower in the 2FMO group (p < 0.05, Figure 8C); the abundance of Aeromonas was significantly lower in the 1FMO group than in the CT and 2FMO groups (p < 0.05, Figure 8D).
3.6.3. Differential Analysis of the Intestinal Microbiome of the Crayfish
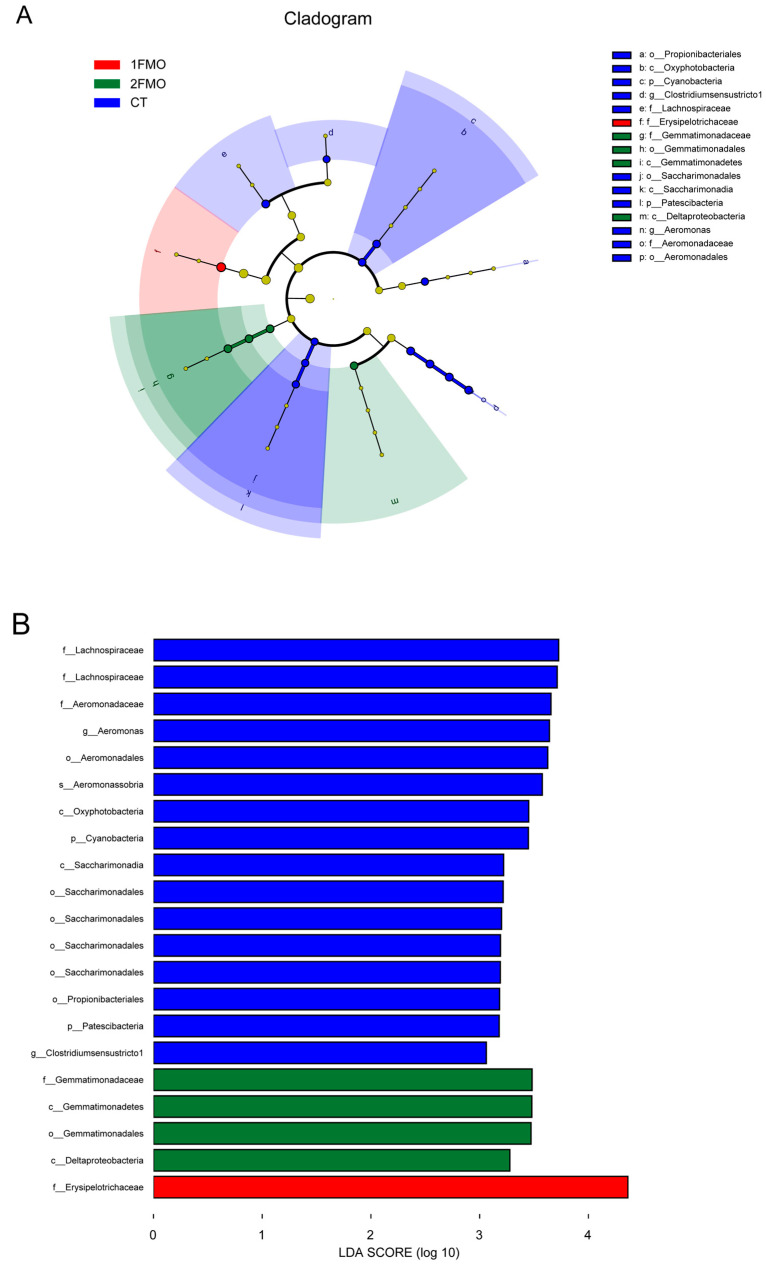
The LEfSe analysis results are shown in Figure 9 (p = 0.05, LDA = 3). The main differentiating species in the CT group were the species Aeromonas sobria of the phylum Proteobacteria, the order Saccharimonadales of the phylum Patescibacteria, the family Lachnospiraceae and genus Clostridium sensu stricto of the phylum Firmicutes, the class Oxyphotobacteria of the phylum Cyanobacteria, and the order Propionibacteriales of the phylum Actinobacteria. The main differential species in the 1FMO group was the family Erysipelotrichaceae of the phylum Firmicutes. The main differential species in the 2FMO group were the family Gemmatimonadaceae of the phylum Gemmatimonadetes and the class Deltaproteobacteria of the phylum Proteobacteria.
Figure 9.
Differential analysis of the effect of fermented M. oleifera leaves on the intestinal microbiome of the crayfish. Note: (A): species evolutionary branching diagram; (B): histogram of LDA distribution.
3.6.4. KEGG Analysis
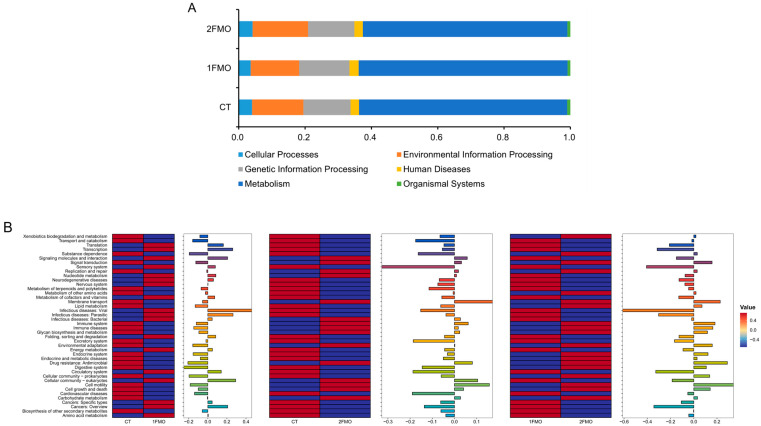
The results of the KEGG-enriched pathway analysis are shown in Figure 10. The CT group, 1FMO group, and 2FMO group pathways were mainly enriched in cellular processes, genetic information processes, metabolism processes, environmental information processes, human diseases, and organismal systems, especially metabolic systems.
Figure 10.
KEGG analysis of the effect of fermented M. oleifera leaves on the crayfish. Note: (A): KEGG Level-1 pathway; (B): KEGG Level-2 pathway.
The results of the KEGG secondary enrichment pathway showed that the 1FMO group increased the expressions of Signaling molecules and the interaction, Cancers: Overview, Transcription, Infectious diseases: Parasitic, Cellular community—eukaryotes and Infectious diseases: Viral pathways; and decreased the expression of Digestive system and Drug resistance: Antimicrobial pathways compared to the CT group (|log2FC| > 0.2). The 2FMO group decreased the expression of the Sensory system pathway compared to those of the CT group (|log2FC| > 0.2). Compared with the 2FMO group, the 1FMO group increased the expressions of Infectious diseases: Viral, Sensory system, Cancers: Overview, Circulatory system, Transcription, and Infectious diseases: Parasitic pathways; and decreased the expressions of the Membrane transport, Drug resistance: Antimicrobial and Cell motility pathways (|log2FC| > 0.2).
3.7. Correlational Analysis of Microbial Genera with Antioxidant Indices
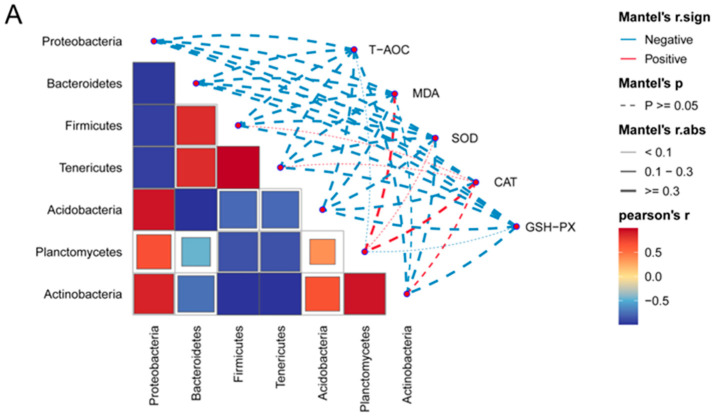
The crayfish gut phylum-level microorganisms and genus-level microorganisms were correlated with antioxidant indicators, and the results are shown in Figure 11. At the phylum level (Figure 11A), T-AOC and GSH-Px were all negatively correlated with Proteobacteria, Bacteroidetes, Firmicutes, Tenericutes, Acidobacteria, Planctomycetes, and Actinobacteria (p > 0.05). MDA and SOD were negatively correlated with Proteobacteria, Bacteroidetes, Firmicutes, Tenericutes, Acidobacteria, and Actinobacteria and positively correlated with Planctomycetes (p > 0.05). CAT was negatively correlated with Proteobacteria, Bacteroidetes, and Acidobacteria and positively correlated with Firmicutes, Tenericutes, Planctomycetes, and Actinobacteria (p > 0.05).
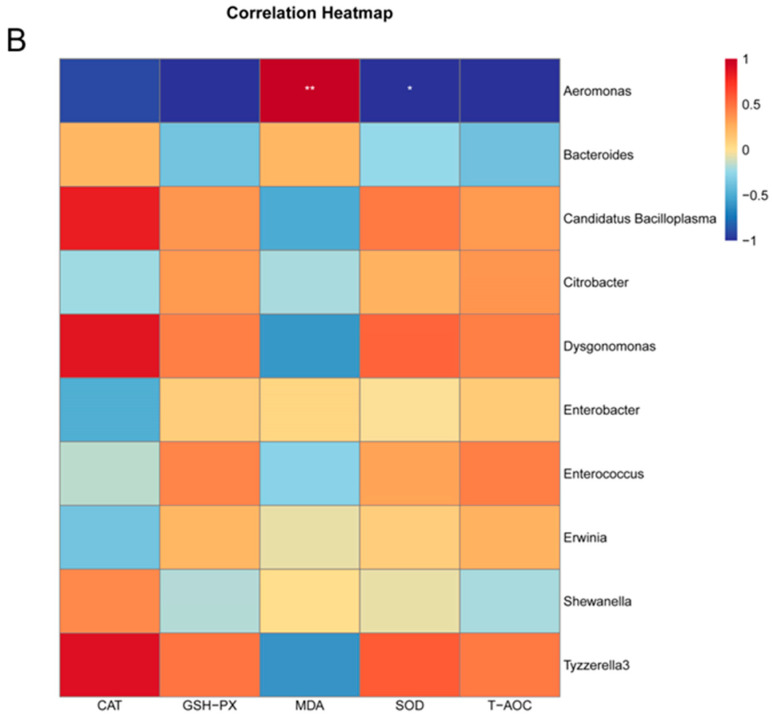
Figure 11.
Correlational analysis of microbial genera with antioxidant indices. Note: (A): phylum level; (B): genus level. “*” represents significant differences, and “**” represents extremely significant differences.
At the genus level (Figure 11B), T-AOC was negatively correlated with Aeromonas, Bacteroides, and Shewanella and positively correlated with Tyzzerella3, Enterococcus, Dysgonomonas, Citrobacter, Candidatus Bacilloplasma, Erwinia, and Enterobacter (p > 0.05). SOD was positively correlated with Tyzzerella3, Dysgonomonas, Candidatus Bacilloplasma, Enterococcus, Citrobacter, and Erwinia; negatively correlated with Bacteroides, Shewanella, and Enterobacter (p > 0.05); and significantly negatively correlated with Aeromonas (p < 0.05). MDA was negatively correlated with Tyzzerella3, Dysgonomonas, Candidatus Bacilloplasma, Enterococcus, Citrobacter, and Erwinia; positively correlated with Bacteroides, Shewanella, and Enterobacter (p > 0.05); and significantly positively correlated with Aeromonas (p < 0.05). GSH-Px was positively correlated with Tyzzerella3, Dysgonomonas, Candidatus Bacilloplasma, Enterococcus, Citrobacter, Erwinia, and Enterobacter and negatively correlated with Aeromonas, Bacteroides, and Shewanella (p > 0.05). CAT was positively correlated with Tyzzerella3, Dysgonomonas, Candidatus Bacilloplasma, Shewanella, and Bacteroides and negatively correlated with Aeromonas, Enterobacter, Erwinia, Citrobacter, and Enterococcus (p > 0.05).
4. Discussion
So far, we have found that fermentation can reduce anti-nutrient factors and improve the utilization of M. oleifera leaves. The main factors affecting fermentation are the strains, pH, material/water ratio, strain inoculation concentration, temperature, and time [13]. Protein and polyphenols are the main nutrients in M. oleifera leaves and can be a good source of antioxidants [25]; therefore, they were selected as screening indicators. Fermentation time is a key factor affecting the final fermentation product, and, typically, the rate of fermentation product production slows, or even stagnates, as the fermentation time increases. Too long or too short of a fermentation time can lead to increased costs or reduced fermentation quality [26]. In this experiment, the results showed that the protein and total phenolic contents were the highest when the fermentation time was 48 h. Ali et al. (2020) proved that the total amino acid and total isoflavone contents were the highest in the fermented M. oleifera leaves at 48 h, and it tended to decrease when the fermentation time reached 72 h and 96 h. These results proved that the nutrient content in the fermentation broth did not increase with increasing fermentation time [27]. Numerous studies have shown that fermentation strains have an important influence on fermentation products, and the selection of strains directly affects the quality of fermentation products [28,29]. In aquaculture, Bacillus is the most studied host-related bacteria, which is mainly because of the unique ability of Bacillus to grow in the intestinal tracts of aquatic animals. In addition, Bacillus can secrete a variety of digestive enzymes and keep the intestinal tract in an anaerobic environment, which can effectively inhibit the growth of pathogenic bacteria and improve the immunity of aquatic animals and their resistance to pathogenic bacteria [30]. In this study, the fermentation effect of B. amyloliquefaciens JY-24 was better than those of B. subtilis S1X-15, B. subtilis JD, and B. subtilis ZSP. Temperature is another important factor affecting the fermentation product, and microbial organisms can only deliver the maximum benefit at the optimal temperature. A fermentation temperature that is too high will lead to microbial organisms’ protein denaturation, reducing the rates of microbial growth and reproduction. On the contrary, if the fermentation temperature is too low, it will inhibit microbial organisms’ production of enzymes and reduce their enzymatic activities [31]. In this work, the total phenolic and protein contents appeared to increase and then decrease with increasing temperature, and the contents reached the highest at 30 °C. Therefore, 30 °C was selected as the optimal fermentation temperature. Water is the basis for the growth and reproduction of microorganisms and is necessary to assist microorganisms in their metabolism, absorption, and secretion of nutrients and in other life activities. The quality of the fermented feed is affected by the level of the water content, which, if too high or too low, will lead to contamination by stray bacteria or decreased nutrient contents [32]. In this study, the protein and total phenolic contents showed a tendency to increase and then decrease with increasing material/water ratio, and the appropriate range was between 5% and 10%.
Based on the single-factor results and L9(34) orthogonal test results, the optimal fermentation conditions of M. oleifera were the fermentation strain B. amyloliquefaciens JY-24, a fermentation material/water ratio of 10%, a fermentation time of 48 h, and a fermentation temperature of 30 °C. Large molecules were broken down into organic acids, soluble peptides, and other small molecules when plants were fermented, increasing the nutrient content and reducing the levels of harmful substances [33]. From the experimental results, it was observed that the protein content, amino acid content, total phenolic content, and flavonoid content of the fermented M. oleifera leaves were increased by 24.53%, 36.21%, 82.81%, and 58.58%, respectively, as compared to those of the unfermented leaves. The tannin content of the M. oleifera leaves was reduced by 8.4%. Tannins are the major anti-nutritional factor in M. oleifera leaves. Excessive levels of tannins in feeds affect palatability and reduce feed intake, and tannins are biodegraded in the gastrointestinal tract to produce low-molecular-weight phenolic compounds (which have a direct toxic effect on food animals), as well as metal ions, enzymes, sugars, and vitamin B12, and reduce feed utilization [34].
Studies have shown that during the fermentation process, probiotics ‘pre-digest’ the fermentation substrate by breaking down anti-nutritional factors and releasing nutrients [35,36]. Fermented feeds can form ‘biological, nutritional, and chemical barriers’ to promote growth, improve immunity, and protect gut health after feeding in aquatic animals [37,38]. Das et al. (2022) showed that solid-state fermentation of hemp oil cake by mixed strains (Saccharomyces cerevisiae and B. subtilis) increased its crude protein content and reduced crude fiber and anti-nutritional factors, such as tannins and saponins, and that replacing 20% of the soybean meal in the diet with fermented hemp oil cake significantly increased the growth performance, feed conversion ratio, and nutrient digestibility of Labeo rohita [39]. In this study, the addition of 1% fermented M. oleifera leaves to the feed improved the growth performance and feed utilization of the crayfish compared to the control group. The intestinal tract is the main place for the digestion and absorption of nutrients in aquatic animals, and the activities of digestive enzymes in crayfish highly reflect their ability to obtain nutrients from food, as well as their growth and development [40]. Dai et al. (2017) found that the digestive enzymatic activities of fast-growing shrimp were significantly higher than those of slow-growing shrimp and that the digestive enzymatic activities were positively correlated with the shrimps’ body weight and body length [41]. In the present study, the intestinal digestive enzymatic activities of the crayfish were significantly higher in the 1FMO and 2FMO groups than in the group without the addition of the fermented M. oleifera leaves. It was found that the addition of the L. acidophilus- and S. cerevisiae–solid-fermented seaweed (Enteromorpha prolifera) to the diet at 20–50 g kg−1 significantly increased the percentage of the weight gain and specific growth rate of red tilapia (Oreochromis mossambicus × Oreochromis niloticus), reduced the feed conversion ratio, increased hepatic and intestinal protease and amylase activities, and improved digestive performance [42].
The hepatopancreas is an important digestive organ in crayfish, with functions such as detoxification and the prevention of liver damage [43]. AST and ALT are amino–aminotransferases mainly found in liver cells, which are important for the metabolic activities of fish and shrimp, and the levels of AST and ALT become high only when there is damage to the liver [44,45]. In this experiment, it was found that the 1FMO group of the crayfish had the lowest activities of AST and ALT, proving that the addition of 1% fermented M. oleifera leaves to the crayfishes’ diets could better reduce liver damage. Crayfish have a poor immune system and usually rely only on non-specific immunity to fight pathogens, and the activities of SOD, CAT, GSH-Px, and MDA enzymes are important indicators to evaluate non-specific immunity in crayfish [46]. Jerimoth et al. (2023) demonstrated that adding 1% Moringa leaves to feed can enhance the serum immunity and improve the gut microbiota structure of catfish [19]. In this experiment, by measuring several classical non-specific immune indicators, the highest antioxidant enzymatic activity was found in the 1FMO group, indicating that the addition of 1% fermented M. oleifera leaves has a certain effect on improving the antioxidant capacity of crayfish. Some related studies have shown that the fermentation of feed can significantly increase the nutrient content and produce a variety of antioxidant substances, which are important for improving the growth performance and antioxidant capacity and enhancing the immune ability of farmed animals [47]. Similarly, the use of fermented M. oleifera leaves to replace 40–60% of the fishmeal in the diet significantly increased the serum’s immune enzyme (SOD, CAT, and lysozyme (LZM)) activities and C3 levels and reduced serum MDA levels in Carassius auratus [5].
Protecting the intestinal health of aquatic animals is an important function of fermented feeds. On one hand, after animals ingest fermented feeds, beneficial bacteria colonize the intestinal tract, compete with pathogenic bacteria for ecological sites, and secrete antimicrobial peptides, bacteriocins, etc. to inhibit the proliferation of pathogenic bacteria, thus achieving the goal of improving the structure of the animal’s intestinal microbiome [48]. On the other hand, metabolites secreted by probiotics after colonization of the gut, such as short-chain fatty acids (SCFAs) and lactic acid, have been shown to repair the host’s intestinal mucosa, build an intestinal barrier, and improve the host’s resistance to pathogenic bacteria [49,50]. In this experiment, the results of the LEfse analysis of variance showed that Aeromonas hydrophila was the main variant in the control group and that A. hydrophila is a major infectious agent that can cause septicemia in aquatic animals [51]. Compared with the control and 2FMO groups, the 1FMO group significantly decreased the abundance of Aeromonas and increased the ratio of (Firmicutes + Bacteroidetes)/Proteobacteria, and the PCA results showed that the 1FMO group improved the structure of the intestinal microbiome. The above results suggest that the addition of 1% fermented M. oleifera leaves has a promotive effect on the intestinal health of crayfish. Consistent with our results, Zhang et al. (2021) showed that fermented feeds increased the abundances of intestinal Firmicutes, Mycobacterium, Bacillus, and Lactobacillus and reduced the abundance of Vibrio in the intestinal tract of Penaeus vannamei [47]. The KEGG enrichment analysis showed that the three experimental groups were mainly focused on metabolic pathways, and the secondary pathway results showed that the addition of 1% fermented M. oleifera leaves to the diets increased the expression levels of signaling molecules and interactions—transcriptional, infectious disease: parasitic and infectious disease: viral pathways—compared to those of the control group. Antioxidant enzymes, such as SOD, scavenge ROSs in the cytoplasm and the SOD activity is an indicator of the body’s non-specific immune function. MDA is one of the end products of free-radical lipid peroxidation damage and is cytotoxic, reflecting the extent of peroxidative damage to the body [52]. Correlational analysis at genus level showed that T-AOC, SOD, GSH-Px, and CAT were negatively correlated with Aeromonas, but MDA was significantly positively correlated with Aeromonas. It was demonstrated that the accumulation of Aeromonas could lead to free-radical lipid peroxidation and reduce the antioxidant capacity of crayfish, resulting in oxidative damage to the crayfish organism and lowering its immunity.
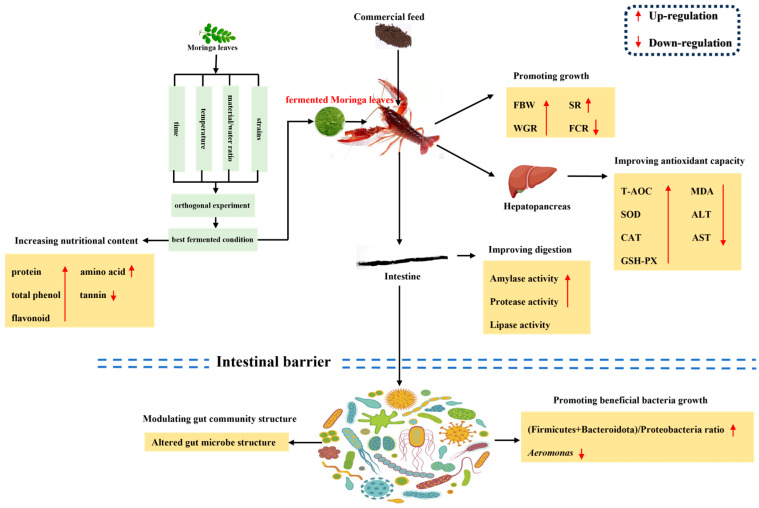
5. Conclusions
In conclusion, the optimal fermentation conditions for M. oleifera leaves were the fermentation strain of B. amyloliquefaciens JY-24 (107 CFU mL−1), a material/water ratio of 10%, a fermentation time of 48 h, and a fermentation temperature of 30 °C. These resulted in the production of a high-protein, high-total-phenol, high-amino-acid, and low-tannin product for M. oleifera leaves. According to the above experimental results, we summarize the positive effects of fermented M. oleifera leaves on crayfish (Figure 12). Briefly, the incorporation of 1% fermented M. oleifera leaves improves the growth of the crayfish by increasing the antioxidant capacity of the hepatopancreas, increasing the digestive capacity of the intestine, inhibiting the abundance of pathogenic bacteria, and promoting the growth of value-added beneficial intestinal bacteria. Nevertheless, this experiment was deficient in mechanistic studies on crayfish, and further research is required to explore the functional mechanism of the antioxidant capacity of fermented M. oleifera leaves.
Figure 12.
The possible health-promoting effects of fermented M. oleifera leaves on the crayfish in vivo.
Acknowledgments
The authors would like to thank the staff for their assistance during the experiments at the Key Laboratory of Aquatic Animal Nutrition and Health, Freshwater Fisheries Research Center, Chinese Academy of Fishery Science and Peng Shao (Yancheng Shangshui Environmental Biotechnology Engineering Co., Ltd., Yancheng, China).
Author Contributions
Conceptualization, Z.L., W.L. and K.M.; methodology, Z.L., W.L. and B.L.; software, Z.L. and W.L.; validation, C.S., X.Z. and Q.Z.; formal analysis, Z.L. and W.L.; resources, A.Z. and B.L.; data curation, Z.L. and W.L.; writing—original draft preparation, Z.L.; writing—review and editing, A.W. and B.L.; project administration, C.S. and B.L.; funding acquisition, Q.Z. and B.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The care and use of the animals followed the Animal Research Institute Committee guidelines of the Freshwater Fisheries Research Center at the Chinese Academy of Fishery Science, China. This study has been approved by the Committee of the Animal Research Institute of the Freshwater Fisheries Research Center at the Chinese Academy of Fishery Science, China (SYXK (Su) 2022-0031).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This work was supported by the National Key R&D Program of China (2023YFD2402000); the Central Public-Interest Scientific Institution Basal Research Fund of the Freshwater Fisheries Research Center, CAFS (2024JBFR06); Yancheng Fisheries’ High-Quality Development Topic (2022yc003); China Agriculture Research System of MOF and MARA (CARS-48); Jiangsu Province Agricultural Science and Technology’s Independent Innovation Fund (CX(23)2008); Project of the Key Laboratory of Genetic Breeding and Cultivation for Freshwater Crustaceans, Ministry of Agriculture and Rural Affairs, and Freshwater Fisheries Research Institute of Jiangsu Province (FC2023-11); and Natural Science Foundation of Jiangsu Province for Youths (BK20230178).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cao J.R., Shi T.T., Wang H.M., Zhu F., Wang J.H., Wang Y.S., Cao F.L., Su E.Z. Moringa oleifera leaf protein: Extraction, characteristics and applications. J. Food Compos. Anal. 2023;119:105234. doi: 10.1016/j.jfca.2023.105234. [DOI] [Google Scholar]
- 2.Adi A.C., Rachmah Q., Arimbi A.N. The Acceptance and Nutritional Value of Crispy Noodles Supplemented with Moringa oleifera as a Functional Snack for Children in a Food Insecure Area. Prev. Nutr. Food Sci. 2019;24:387–392. doi: 10.3746/pnf.2019.24.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shija A.E., Rumisha S.F., Oriyo N.M., Kilima S.P., Massaga J.J. Effect of Moringa oleifera leaf powder supplementation on reducing anemia in children below two years in Kisarawe District, Tanzania. Food Sci. Nutr. 2019;7:2584–2594. doi: 10.1002/fsn3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brar S., Haugh C., Robertson N., Owuor P.M., Waterman C., Fuchs G.J., Attia S.L. The impact of Moringa oleifera leaf supplementation on human and animal nutrition, growth, and milk production: A systematic review. Phytother. Res. 2022;36:1600–1615. doi: 10.1002/ptr.7415. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X.H., Sun Z.Y., Cai J.F., Wang J.H., Wang G.B., Zhu Z.L., Cao F.L. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III) Fish Shellfish Immunol. 2020;102:430–439. doi: 10.1016/j.fsi.2020.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Morsy T.A., Gouda G.A., Kholif A.E. In vitro fermentation and production of methane and carbon dioxide from rations containing Moringa oleifera leave silage as a replacement of soybean meal: In vitro assessment. Environ. Sci. Pollut. Res. 2022;29:69743–69752. doi: 10.1007/s11356-022-20622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas-Sánchez K., Garay-Jaramillo E., González-Reyes R.E. Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients. 2019;11:2907. doi: 10.3390/nu11122907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maurus G., Ho T.H., Lee P.-T. Effects of dietary Scutellaria baicalensis extract on growth performance, immune-related genes expression, and resistance against Vibrio parahaemolyticus in white shrimp (Litopenaeus vannamei) Res. Vet. Sci. 2023;159:160–170. doi: 10.1016/j.rvsc.2023.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Zeng B., Luo J.Y., Wang P., Yang L., Chen T., Sun J.J., Xie M.Y., Li M., Zhang H.J., He J.J., et al. The beneficial effects of Moringa oleifera leaf on reproductive performance in mice. Food Sci. Nutr. 2019;7:738–746. doi: 10.1002/fsn3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meziani S., Aissani A., Khemis I., Oomah B.D., Zaidi F. Physicochemical characterization and antibacterial activity of Moringa oleifera Lam leaf powder treated at different temperatures. Bioact. Carbohydr. Diet. Fibre. 2023;30:100389. doi: 10.1016/j.bcdf.2023.100389. [DOI] [Google Scholar]
- 11.Khalid S., Arshad M., Mahmood S., Ahmed W., Siddique F., Khalid W., Zarlasht M., Asar T.O., Hassan F.A.M. Nutritional and phytochemical screening of Moringa oleifera leaf powder in aqueous and ethanol extract. Int. J. Food Prop. 2023;26:2338–2348. doi: 10.1080/10942912.2023.2246685. [DOI] [Google Scholar]
- 12.Sun H.X., Kang X.Y., Tan H.Z., Cai H.Y., Chen D. Progress in Fermented Unconventional Feed Application in Monogastric Animal Production in China. Fermentation. 2023;9:947. doi: 10.3390/fermentation9110947. [DOI] [Google Scholar]
- 13.Siddik M.A.B., Julien B.B., Islam S.M.M., Francis D.S. Fermentation in aquafeed processing: Achieving sustainability in feeds for global aquaculture production. Rev. Aquacult. 2024;16:1244–1265. doi: 10.1111/raq.12894. [DOI] [Google Scholar]
- 14.Nkhata S.G., Ayua E., Kamau E.H., Shingiro J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018;6:2446–2458. doi: 10.1002/fsn3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C.Z., Li W.J. Optimization Technology of the LHS-1 Strain for Degrading Gallnut Water Extract and Appraisal of Benzene Ring Derivatives from Fermented Gallnut Water Extract Pyrolysis by Py-GC/MS. Molecules. 2017;22:2253. doi: 10.3390/molecules22122253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M.M., Huang Y.W., Zhao H.C., Wang T.F., Xie C.Q., Zhang D.Y., Wang X.J., Sheng J. Solid-state fermentation of Moringa oleifera leaf meal using Bacillus pumilus CICC 10440. J. Chem. Technol. Biotechnol. 2017;92:2083–2089. doi: 10.1002/jctb.5203. [DOI] [Google Scholar]
- 17.Wang J.Q., Ye J.W., Zhang Z.X., An Z.H., Wang T., Dong X.J. Comparison of the nutrient value, nonspecific immunity, and intestinal microflora of red swamp crayfish (Procambarus clarkii) in different culture modes. Aquacult. Rep. 2023;31:101683. doi: 10.1016/j.aqrep.2023.101683. [DOI] [Google Scholar]
- 18.Siddik M.A.B., Fotedar R., Chaklader M.R., Foysal M.J., Nahar A., Howieson J. Fermented Animal Source Protein as Substitution of Fishmeal on Intestinal Microbiota, Immune-Related Cytokines and Resistance to Vibrio mimicus in Freshwater Crayfish (Cherax cainii) Front. Physiol. 2020;1:1635. doi: 10.3389/fphys.2019.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerimoth K.E., Arnold E.I., Ruth E.A. Performance and gut microbiota of catfish (Clarias gariepinus) fed powdered Moringa oleifera leave as additive (Probiotics) Aquac. Fish. 2023 in press . [Google Scholar]
- 20.Kaleo I.V., Gao Q., Liu B., Sun C.X., Zhou Q.L., Zhang H.M., Shan F., Xiong Z., Liu B., Song C.Y. Effects of Moringa oleifera leaf extract on growth performance, physiological and immune response, and related immune gene expression of Macrobrachium rosenbergii with Vibrio anguillarum and ammonia stress. Fish Shellfish Immunol. 2019;89:603–613. doi: 10.1016/j.fsi.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Kamble M.T., Gallardo W., Salin K.R., Pumpuang S., Chavan R.B., Bhujel R.B., Medhe S.V., Kettewan A., Thompson K.D., Pirarat N. Effect of Moringa oleifera Leaf Extract on the Growth Performance, Hematology, Innate Immunity, and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2. Animals. 2024;14:953. doi: 10.3390/ani14060953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirisantimethakom L., Laopaiboon L., Sanchanda P., Chatleudmongkol J., Laopaiboon P. Improvement of butanol production from sweet sorghum juice by Clostridium beijerinckii using an orthogonal array design. Ind. Crops Prod. 2016;79:287–294. doi: 10.1016/j.indcrop.2015.11.012. [DOI] [Google Scholar]
- 23.Loffler B.M., Kunze H. Refinement of the coomassie brilliant blue-G assay for quantitative protein determination. Anal. Biochem. 1989;177:100–102. doi: 10.1016/0003-2697(89)90021-3. [DOI] [PubMed] [Google Scholar]
- 24.Xue M.Y., Jiang N., Fan Y.D., Yang T., Li M., Liu W.Z., Li Y.Q., Li B., Zeng L.B., Zhou Y. White spot syndrome virus (WSSV) infection alters gut histopathology and microbiota composition in crayfish (Procambarus clarkii) Aquacult. Rep. 2022;22:101006. doi: 10.1016/j.aqrep.2022.101006. [DOI] [Google Scholar]
- 25.Kashyap P., Kumar S., Riar C.S., Jindal N., Baniwal P., Guiné R.P.F., Correia P.M.R., Mehra R., Kumar H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants. 2022;11:402. doi: 10.3390/antiox11020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J.H., Shi L., Xu S.Y., Gu S.Y., Wen X., Xu D.Y., Luo J.Y., Huang Y.Y., Wang M. Optimal fermentation time for Nigrospora-fermented tea rich in bostrycin. J. Sci. Food Agric. 2021;101:2483–2490. doi: 10.1002/jsfa.10874. [DOI] [PubMed] [Google Scholar]
- 27.Ali M.W., Ilays M.Z., Saeed M.T., Shin D.H. Comparative assessment regarding antioxidative and nutrition potential of Moringa oleifera leaves by bacterial fermentation. J. Food Sci. Technol. 2020;57:1110–1118. doi: 10.1007/s13197-019-04146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heng X.Y., Chen H.Y., Lu C.X., Feng T., Li K.Y., Gao E.B. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases. LWT-Food Sci. Technol. 2022;154:112626. doi: 10.1016/j.lwt.2021.112626. [DOI] [Google Scholar]
- 29.Chen X.D., Song C., Zhao J., Xiong Z., Peng L.X., Zou L., Liu B.L., Li Q. Effect of a New Fermentation Strain Combination on the Fermentation Process and Quality of Highland Barley Yellow Wine. Foods. 2024;13:2193. doi: 10.3390/foods13142193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang X.L., Kang M.R., Yun L.L., Shen Y.H., Feng J.C., Yang G.K., Zhang J.X., Meng X.L. Sodium gluconate increases Bacillus velezensis R-71003 growth to improve the health of the intestinal tract and growth performance in the common carp (Cyprinus carpio L.) Aquaculture. 2023;563:738980. doi: 10.1016/j.aquaculture.2022.738980. [DOI] [Google Scholar]
- 31.Nor-Khaizura M.A.R., Flint S.H., McCarthy O.J., Palmer J.S., Golding M. Modelling the effect of fermentation temperature and time on starter culture growth, acidification and firmness in made-in-transit yoghurt. LWT-Food Sci. Technol. 2019;106:113–121. doi: 10.1016/j.lwt.2019.02.027. [DOI] [Google Scholar]
- 32.Hallsworth J.E. Water is a preservative of microbes. Microb. Biotechnol. 2022;15:191–214. doi: 10.1111/1751-7915.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y.T., Yu B., Lu Y.H., Wang J., Liang J.B., Tufarelli V., Laudadio V., Liao X.D. Optimization of the Fermentation Conditions to Reduce Anti-Nutritive Factors in Soybean Meal. J. Food Process. Preserv. 2017;41:e13114. doi: 10.1111/jfpp.13114. [DOI] [Google Scholar]
- 34.du Toit E.S., Sithole J., Vorster J. Leaf harvesting severity affects total phenolic and tannin content of fresh and dry leaves of Moringa oleifera Lam. trees growing in Gauteng, South Africa. S. Afr. J. Bot. 2020;129:336–340. doi: 10.1016/j.sajb.2019.08.035. [DOI] [Google Scholar]
- 35.Rahimnejad S., Zhang J.J., Wang L., Sun Y.Z., Zhang C.X. Evaluation of Bacillus pumillus SE5 fermented soybean meal as a fish meal replacer in spotted seabass (Lateolabrax maculatus) feed. Aquaculture. 2021;531:735975. doi: 10.1016/j.aquaculture.2020.735975. [DOI] [Google Scholar]
- 36.Flefil N.S., Ezzat A., Aboseif A.M., Negm El-Dein A. Lactobacillus-fermented wheat bran, as an economic fish feed ingredient, enhanced dephytinization, micronutrients bioavailability, and tilapia performance in a biofloc system. Biocatal. Agric. Biotechnol. 2022;45:102521. doi: 10.1016/j.bcab.2022.102521. [DOI] [Google Scholar]
- 37.Vo B.V., Siddiki M.A.B., Chaklader M.R., Fotedar R., Nahar A., Foysal M.J., Bui D.P., Nguyen H.Q. Growth and health of juvenile barramundi (Lates calcarifer) challenged with DO hypoxia after feeding various inclusions of germinated, fermented and untreated peanut meals. PLoS ONE. 2020;15:e0232278. doi: 10.1371/journal.pone.0232278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soltani M., Badzohreh G., Mirzargar S., Farhangi M., Shekarabi P.H., Lymbery A. Growth Behavior and Fatty Acid Production of Probiotics, Pediococcus acidilactici and Lactococcus lactis, at Different Concentrations of Fructooligosaccharide: Studies Validating Clinical Efficacy of Selected Synbiotics on Growth Performance of Caspian Roach (Rutilus frisii kutum) Fry. Probiot. Antimicrob. Proteins. 2019;11:765–773. doi: 10.1007/s12602-018-9462-3. [DOI] [PubMed] [Google Scholar]
- 39.Das K.C., Mohanty S., Sahoo P.K., Sahoo S., Prakash B., Swain P. Inclusion of different levels of solid-state fermented mahua oil cake on growth, digestibility and immunological parameters of rohu (Labeo rohita) Aquaculture. 2022;553:738049. doi: 10.1016/j.aquaculture.2022.738049. [DOI] [Google Scholar]
- 40.Mugwanya M., Dawood M.A.O., Kimera F., Sewilam H. Replacement of fish meal with fermented plant proteins in the aquafeed industry: A systematic review and meta-analysis. Rev. Aquacult. 2022;15:62–88. doi: 10.1111/raq.12701. [DOI] [Google Scholar]
- 41.Dai W.F., Yu W.N., Zhang J.J., Zhu J.Y., Tao Z., Xiong J.B. The gut eukaryotic microbiota influences the growth performance among cohabitating shrimp. Appl. Microbiol. Biotechnol. 2017;101:6447–6457. doi: 10.1007/s00253-017-8388-0. [DOI] [PubMed] [Google Scholar]
- 42.Yang H., Li Z.B., Chen Q., Li W.J., Sun Y.Z., Lu J. Effect of fermented Enteromopha prolifera on the growth performance, digestive enzyme activities and serum non-specific immunity of red tilapia (Oreochromis mossambicus × Oreochromis niloticus) Aquacult. Res. 2016;47:4024–4031. doi: 10.1111/are.12856. [DOI] [Google Scholar]
- 43.Cao X.T., Pan X.Y., Sun M., Liu Y., Lan J.F. Hepatopancreas-Specific Lectin Participates in the Antibacterial Immune Response by Regulating the Expression of Antibacterial Proteins. Front. Immunol. 2021;12:679767. doi: 10.3389/fimmu.2021.679767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan W.J., Miao L.H., Lin Y., Huang X., Ge X.P., Moosa S.L., Liu B., Ren M.C., Zhou Q.L., Liang H.L., et al. Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala) Fish Shellfish Immunol. 2017;70:66–75. doi: 10.1016/j.fsi.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Fawole F.J., Sahu N.P., Shamna N., Phulia V., Emikpe B.O., Adeoye A.A., Aderolu A.Z., Popoola O.M. Effects of detoxified Jatropha curcas protein isolate on growth performance, nutrient digestibility and physio-metabolic response of Labeo rohita fingerlings. Aquacult. Nutr. 2018;24:1223–1233. doi: 10.1111/anu.12660. [DOI] [Google Scholar]
- 46.Zhou X.X., Tian Z.Q., Wang Y.B., Li W.F. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol. Biochem. 2010;36:501–509. doi: 10.1007/s10695-009-9320-z. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M.Z., Pan L.Q., Fan D.P., He J.J., Su C., Gao S., Zhang M.Y. Study of fermented feed by mixed strains and their effects on the survival, growth, digestive enzyme activity and intestinal flora of Penaeus vannamei. Aquaculture. 2021;530:735703. doi: 10.1016/j.aquaculture.2020.735703. [DOI] [Google Scholar]
- 48.Catalán N., Villasante A., Wacyk J., Ramírez C., Romero J. Fermented Soybean Meal Increases Lactic Acid Bacteria in Gut Microbiota of Atlantic Salmon (Salmo salar) Probiot. Antimicrob. Proteins. 2018;10:566–576. doi: 10.1007/s12602-017-9366-7. [DOI] [PubMed] [Google Scholar]
- 49.Cheng A.C., Yeh S.P., Hu S.Y., Lin H.L., Liu C.H. Intestinal microbiota of white shrimp, Litopenaeus vannamei, fed diets containing Bacillus subtilis E20-fermented soybean meal (FSBM) or an antimicrobial peptide derived from B. subtilis E20-FSBM. Aquacult. Res. 2020;51:41–50. doi: 10.1111/are.14345. [DOI] [Google Scholar]
- 50.Tran N.T., Li Z.Z., Wang S.Q., Zheng H.P., Aweya J.J., Wen X.B., Li S.K. Progress and perspectives of short-chain fatty acids in aquaculture. Rev. Aquacult. 2020;12:283–298. doi: 10.1111/raq.12317. [DOI] [Google Scholar]
- 51.Rasmussen-Ivey C.R., Figueras M.J., McGarey D., Liles M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016;7:1337. doi: 10.3389/fmicb.2016.01337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z.M., He Z.F., Emara A.M., Gan X., Li H.J. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chem. 2019;288:405–412. doi: 10.1016/j.foodchem.2019.02.126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).