Abstract
Inflammatory bowel diseases (IBD) pose a growing public health challenge with unclear etiology and limited efficacy of traditional pharmacological treatments. Alternative therapies, particularly antioxidants, have gained scientific interest. This systematic review analyzed studies from MEDLINE, Cochrane, Web of Science, EMBASE, and Scopus using keywords like “Inflammatory Bowel Diseases” and “Antioxidants.” Initially, 925 publications were identified, and after applying inclusion/exclusion criteria—covering studies from July 2015 to June 2024 using murine models or clinical trials in humans and evaluating natural or synthetic substances affecting oxidative stress markers—368 articles were included. This comprised 344 animal studies and 24 human studies. The most investigated antioxidants were polyphenols and active compounds from medicinal plants (n = 242; 70.3%). The review found a strong link between oxidative stress and inflammation in IBD, especially in studies on nuclear factor kappa B and nuclear factor erythroid 2-related factor 2 pathways. However, it remains unclear whether inflammation or oxidative stress occurs first in IBD. Lipid peroxidation was the most studied oxidative damage, followed by DNA damage. Protein damage was rarely investigated. The relationship between antioxidants and the gut microbiota was examined in 103 animal studies. Human studies evaluating oxidative stress markers were scarce, reflecting a major research gap in IBD treatment. PROSPERO registration: CDR42022335357 and CRD42022304540.
Keywords: oxidative stress, biomarkers, complementary and alternative medicine, ulcerative colitis, Crohn’s disease
1. Introduction
The etiology of inflammatory bowel disease (IBD), which is mostly represented by Crohn’s disease (CD) and ulcerative colitis (UC), is largely unknown. IBD is regarded as a global public health concern. It is believed to be caused by changes in the immune system, gut microbiota, and external environment in addition to an individual’s genetic vulnerability [1]. Its incidence stabilized in the 21st century, although it is still a major epidemiological concern in economically developed nations like those in North America and Europe. Conversely, the incidence and prevalence of UC and CD have been rising gradually in developing countries, approaching Western nation rates with each passing decade [2].
Treatment is directly impacted, as previously said, by the absence of specific pharmacological targets in the etiology of IBD. Among the drugs used today are the following: (i) Aminosalicylates, of which sulfasalazine is the most well-known, which has been used for treating IBD for 80 years. Its mechanisms of action include changes in the metabolism of arachidonic acid, the removal of reactive oxygen species (ROS), effects on white blood cells, and a decrease in the production of cytokines [3]. (ii) Corticosteroids, which are used to induce remission in patients with moderate to severe inflammation or who are not responsive to mesalazine. They work by interacting with pro-inflammatory transcription factors such as activator protein 1 (AP-1) and nuclear factor kappa B (NF-κB), which inhibits the transcription of some inflammatory genes and directly reduces cytokines like interleukin—IL-1α, IL-1ß, and IL-8 [4]; (iii) immunosuppressive therapy, which inhibits antigen-induced lymphokine secretion by binding to Ca2+ calmodulin-dependent protein phosphatase calcineurin [5]; and (iv) biological treatments, such as integrin antagonists and tumor necrosis factor-alpha (TNF-α) inhibitors, which were introduced in the late 1990s and are directly linked to improved quality of life for individuals with inflammatory bowel disease [6].
Many side effects continue despite the different pharmacological alternatives that make up the therapeutic pyramid for individuals with IBD. These include abdominal pain, nausea, vomiting, anorexia, headache, hemolysis, pancreatitis, and worsening diarrhea (aminosalicylates) [7]; hyperglycemia, hyperlipidemia, Cushingoid appearance, and metabolic fatty liver disease (corticosteroids) [8]; myelotoxicity, gastrointestinal intolerance, hepatotoxicity, malignancy, pancreatitis, fever, and skin rash (immunomodulators) [9]; uveitis, vasculitis, psoriasis, sarcoidosis, and even the paradoxical effects of inducing or exacerbating conditions intended to be treated (biological therapy) [10].
Due to the numerous side effects and the fact that many patients do not respond to traditional treatment, often requiring surgical procedures, several alternative therapies are being researched as adjuncts to standard IBD medication. Among these, the use of substances with antioxidant properties stands out. Reactive oxygen and nitrogen species (RONS) and the inflammatory process are directly linked to oxidative imbalance, which is closely linked to the onset and progression of inflammatory bowel disease (IBD). This is because oxidative and nitrosative stress not only affects the inflammatory intestinal mucosa but also penetrates deeper intestinal wall layers and is reflected in the body’s circulation [11].
In 2015, our research group published a systematic review of 134 articles evaluating the action of oral antioxidant therapy on oxidative stress markers in IBD, with 130 studies on murine models and only 4 on humans [12].
Despite these findings, several issues remain unresolved, the most important of which is: Should patients with IBD receive antioxidant therapy? Consequently, this update’s goal is to analyze the antioxidant and anti-inflammatory properties of antioxidant therapies that have been studied for IBD in both human and murine models.
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
The search was conducted from July 2015 to June 2024 across the following databases: MEDLINE (via PubMed), Cochrane Controlled Register of Trials (CENTRAL), Web of Science, EMBASE, and Scopus. The following keywords were used: Inflammatory Bowel Diseases; Antioxidants; Antioxidant Therapy; Crohn’s Disease; Ulcerative Colitis; Biomarkers; Oxidative Stress. Boolean operators “OR” and “AND” were applied, with the complete search strategy, including the following terms: “inflammatory bowel disease” AND “antioxidant” AND (“therapy” OR “treatment”). All retrieved records had their titles and abstracts screened. Subsequently, two independent researchers reviewed the titles to remove duplicate records. Articles cited by the authors of the found studies were also included.
2.2. Eligibility of Clinical Research
Inclusion Criteria:
-
(i)
Studies with rodents (rats or mice, due to biological similarity to humans); experimental IBD induced by drugs or genetic modification (knockout animals); acute or chronic IBD models, published from July 2015, in English, Spanish, or Portuguese.
-
(ii)
Studies with patients diagnosed with CD or UC.
-
(iii)
Use of natural or synthetic substances/compounds, or food/plants with oral/gavage antioxidant action isolated or combined with traditional therapies.
-
(iv)The antioxidant action was determined when the studied compounds exhibited one of the following actions:
- Action on ROS/RNS: such as nitrite, nitric oxide, and others.
- Effect on RONS synthesis: influence on activity, protein expression, or gene expression of inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (NOX), lipoxygenase (LOX), myeloperoxidase (MPO), NF-κB or IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha), and nuclear factor erythroid 2-related factor 2 (Nrf2).
- Effect on oxidative damage: damage to cell membranes (thiobarbituric acid reactive substances—TBARS, malondialdehyde—MDA, 4-hydroxynonenal—HNE, isoprostanes, and others), protein damage (protein carbonylation, advanced glycation end-products—AGE, and others), and DNA damage (8-oxo-2′-deoxyguanosine, 8-oxoguanine, and others).
- Action on antioxidant defense: enzymatic (superoxide dismutase—SOD, catalase—CAT, glutathione peroxidase—GPx, glutathione reductase—GR) and non-enzymatic (reduced glutathione—GSH and GSH/GSSG ratio) endogenous antioxidant defense and total antioxidant status (TAS) or total antioxidant capacity (TAC).
- Effect on intestinal microbiota modulation.
Exclusion Criteria:
-
(i)
Studies utilizing a combination of antioxidant substances, except plants or foods, or medicaments + antioxidant.
Classification of Antioxidant Substances:
The studies were categorized into the following therapeutic topics:
-
(i)
Hormones
-
(ii)
Synthetic or hemisynthetic compounds.
-
(iii)
Chemical products derived from non-plant sources.
-
(iv)
Polyphenols and other active compounds from medicinal plants.
-
(v)
Functional foods and antioxidant nutrients.
-
(vi)
Probiotics.
-
(vii)
Others.
Record:
This systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) nº CDR42022335357 and CRD42022304540.
2.3. Data Extraction
The following data were extracted from the studies: supplement presentation; doses; duration of supplementation; route of administration; treatment groups; effects on oxidative stress biomarkers; and action on the intestinal microbiota. Studies with multiple supplementation doses were allocated according to the highest dose.
2.4. Risk of Bias and Quality of Evidence Assessment
2.4.1. Animal Studies
The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) tool was used to assess the risk of bias. This tool evaluates ten domains: random sequence generation, baseline characteristics, allocation concealment, random housing, blinding of caregivers and researchers, random outcome assessment, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Each category was rated as having low risk, high risk, or unclear risk of bias using the software RevMan 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark).
2.4.2. Randomized Clinical Trials (RCTs)
The risk of bias in randomized clinical trials (RCTs) was evaluated using the Cochrane risk of bias tool, which assesses six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcomes data (intention-to-treat or per-protocol analysis), and selective reporting. Studies that did not present a registered clinical protocol were classified as high-risk in the “selective outcome report” domain.
2.4.3. Non-Randomized Controlled Studies
For non-randomized controlled studies, the ROBINS-I (Reduction of Bias In Non-randomized Studies of Interventions) tool was used (available at https://www.riskofbias.info/, accessed on 20 July 2024), evaluating seven domains: confounding, participant selection, classification of interventions, deviations from intended interventions, missing data, outcome measurement, and selection of the reported result.
3. Results and Discussion
Initially, 925 studies were identified after duplicate removal and exclusion criteria evaluation. Following title and abstract screening and full-text reading, 369 studies were included in this review, comprising 344 (93.4%) animal model studies and 24 (6.5%) clinical studies involving humans (Figure 1). The most investigated category of antioxidants in animals was “polyphenols and other active compounds from medicinal plants” (n = 242; 70.3%), followed by functional foods, antioxidant nutrients, probiotics, among others (n = 67; 19.4%), and hormones, synthetic or hemisynthetic compounds, and chemical products derived from non-plant sources (n = 36; 10.4%).
Figure 1.
Flowchart with the main results of the database search.
In human studies, the most investigated category of antioxidants was “functional foods, antioxidant nutrients” (n = 12; 50%), followed by “polyphenols and other active compounds from medicinal plants” (n = 11; 45.8%), and “others” (n = 1; 4.1%).
3.1. Risk of Bias
The elements of allocation concealment, randomization of treatment allocation, blinding of caregivers and researchers, randomized outcome assessment, and blinding of outcome assessors are fundamental in scientific research. They help ensure the comparability of experimental groups and prevent bias from influencing results. In our systematic review of animal studies, as shown in Supplementary Figure S1A, these elements were identified as having a high risk of bias due to a lack of information on whether and how these procedures were implemented, which compromises the validity and reliability of the study results.
The analysis of bias risk in randomized clinical trials is provided in Supplementary Figure S1B. Most categories exhibit a low risk of bias, with only a small proportion classified as uncertain risk. In most cases, the necessary information to assess risk was available in the analyzed categories. However, studies that lacked a registered clinical protocol scored high in the “selective outcome reporting” category.
For non-randomized clinical trials, while some categories of bias—such as outcome blinding—are better controlled, others, such as random sequence generation and selective reporting, present concerning levels of risk. These issues could undermine the integrity of the trial results (see Supplementary Figure S1B).
3.2. Oxidative Stress and Inflammation in IBD: Which Comes First?
Given that oxidative stress plays a crucial role in the pathogenesis of IBD, various antioxidant therapeutic strategies are being explored, including reactive species removal, increased synthesis of antioxidant enzymes, and inhibition of abnormal redox signaling for reactions. Even though oxidative stress is considered a potential pathogenic and critical factor in the initiation, progression, and severity of IBD by actively participating in chronic mucosal inflammation, the underlying mechanisms remain far from being fully elucidated. Based on laboratory reports and clinical trials, a growing number of antioxidants, such as RONS generation inhibitors, hormones, synthetic substances, polyphenols, plant extracts, functional foods, micronutrients, and probiotics, are being investigated as potential therapeutic strategies aimed at minimizing oxidative stress in IBD (Table 1 and Table S1) [13,14,15].
However, understanding the sequence of events—whether oxidative stress or inflammation occurs first—is crucial for the development of effective treatments. In IBD, as in other diseases, redox imbalance is a mechanism intrinsically related to its etiopathogenesis. At the intestinal level, oxidative stress regulates gene expression directly involved with both innate and adaptive immune responses. One consequence of the interaction between RONS and endobiotics is molecular and tissue damage, impairing molecule and tissue functionality, increasing permeability, and fostering chronic inflammation in the intestinal mucosa [16]. This occurs through the activation of pro-inflammatory pathways such as NF-κB, which acts on pro-inflammatory genes and cytokine and chemokine encoding, and the inactivation of anti-inflammatory mediators like Nrf2, nuclear factor, which induces the biosynthesis of a set of antioxidants and detoxifying enzymes, such as GPx, SOD, and CAT [17].
At the same time, NF-κB and the mitogen-activated protein kinase (MAPK) pathways are activated in response to inflammatory activity, such as the binding of TNF-α to its membrane receptor, TNFR. This activation triggers a cascade of other pro-inflammatory and pro-oxidant enzymes, such as NOX and iNOS, thereby increasing the generation of O2•- and NO• [18].
However, recent discoveries have shown that NF-κB activation in specific microenvironments, such as tumors and colitis, can lead to an antioxidant effect by recruiting M2 macrophages, which have anti-inflammatory properties, unlike M1 phenotype macrophages. Additionally, NF-κB activation mediated by ROS does not trigger the classical IL-6 synthesis pathway, further contributing to its anti-inflammatory role [19].
Therefore, it is evident that NF-κB has diverse involvements depending on the environment in which it is activated. This underscores the necessity of investigating this nuclear factor as a therapeutic target when testing new pharmacological or non-pharmacological interventions for the treatment of IBD.
Another strong connection between oxidative stress and inflammation is observed in the dysregulation of the mitochondrial electron transport chain in patients with IBD, which explains the elevated production of O2•- in these individuals. Interestingly, this mitochondrial regulation is re-established following the use of TNF-α inhibitors. However, the mechanism underlying this interaction remains unclear and warrants further investigation [20].
RONS can disrupt tight junctions (TJ) and adherens junctions (AJ) in the intestinal epithelial intercellular junctions. Chronic inflammatory processes, resulting from epithelial integrity disruption, allow the migration of bacteria from the intestinal lumen to the internal environment, leading to the inflammatory histological changes characteristic of IBD [12,21].
With the infiltration of mucosal tissue by activated phagocytic immune cells generating RONS, a shift towards pro-oxidants occurs, disrupting cellular homeostasis and contributing to cellular injury and increased mucosal barrier permeability. This redox imbalance is closely connected to the activation of phagocytic immune cells during inflammatory processes characteristic of IBD. Lipopolysaccharides (LPSs) present in pathogenic intestinal bacteria, which increase during the dysbiosis occurring in IBD, are pathogen-associated molecular patterns (PAMPs) that activate enzymes generating O2•- (NADPH oxidase) and NO• (iNOS). Subsequently, this O2•- is dismutated into H2O2, which can react with Fe2+ to generate •OH or with Cl- to produce hypochlorous acid (HClO), both of which have high antimicrobial activity [22]. Thus, reactive species are released from phagocytes into the extracellular environment, where they exert their antimicrobial function but can also contribute to tissue damage and increased mucosal barrier permeability [23]. This accelerates and perpetuates ongoing inflammation. Various genetic risk loci relevant to oxidative stress associated with IBD have been identified, further implicating oxidative stress in the pathogenesis of IBD [24].
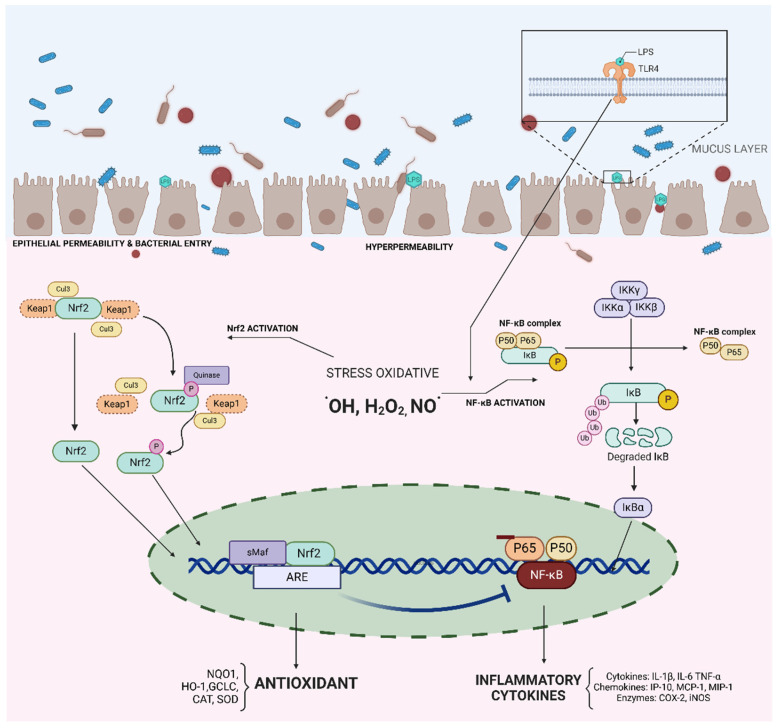
Nitro-oxidative stress (increased especially due to O2•-, H2O2, •OH, HClO, •NO, OONO-, N2O3, N2O4, and others) not only directly damages intestinal epithelial cells but also triggers pro-inflammatory pathways sensitive to reactive species in immune cells. The NF-κB and Nrf2 signaling pathways are two key transcription routes controlling numerous biological functions responding to oxidative stress and inflammation (Figure 2). NF-κB influences pro-inflammatory genes and cytokines and chemokines encoding, while Nrf2 induces antioxidants and detoxifying enzymes [25].
Figure 2.
Interaction between nuclear factor erythroid 2 (Nrf2) and nuclear factor kappa B (NF-κB) in the modulation of the oxidative stress and inflammation Legend: Molecular mechanisms involved in the inflammatory and antioxidant response in intestinal epithelial cells, following recognition of bacterial LPS (lipopolysaccharide) by TLR4 (Toll-like receptor 4). TLR4 activation triggers two main pathways: (1) The Nrf2 pathway (nuclear factor erythroid 2-related factor 2), with the activation of the transcription of antioxidant genes, such as HO-1 (heme oxygenase-1), GCLC (glutamate-cysteine ligase catalase), CAT (catalase), and SOD (superoxide dismutase), which combat oxidative stress induced by reactive oxygen species (•OH, H2O2, and NO•). (2) NF-κB (nuclear factor kappa B) pathway: activation of the transcription of pro-inflammatory genes, including cytokines (IL-1β, IL-6, and TNF-α), chemokines (IP-10, MCP-1, and MIP-1), and enzymes (COX-2 and iNOS), which amplify the inflammatory response. The figure illustrates the complex interaction between the Nrf2 and NF-κB signaling pathways, which modulate the cellular response to oxidative stress and inflammation in the intestinal epithelium. The balance between these pathways is crucial for maintaining intestinal homeostasis and preventing tissue damage.
NF-κB is implicated in the pathogenesis of IBD due to dysregulation in its upstream signaling pathways. Immune receptors stimulating NF-κB, like nucleotide-binding oligomerization domain containing 2 (NOD2), and negative regulatory genes, such as IL-12 and IL-23, are found in the inflamed colonic tissues of IBD patients [15]. Conversely, Nrf2 downregulates pro-inflammatory responses and mucosal compromise through an antioxidant mechanism. Antioxidant-induced transcription can protect against the accumulation of overproduced reactive species. In oxidative stress, Nrf2 has been reported as a suppressor of protein kinase C and a diminisher of oxidase activation, increasing GSH levels [1].
In this study, we observed that some compounds had decreased NF-κB (n = 188) and increased Nrf2 expression (n = 55), as seen in Figure 3. However, in most studies, these markers are evaluated in isolation, making it challenging to interpret the interrelationship and impact of one on the other. This represents a significantly higher number than reported in the 2015 review, where only a few studies assessed these markers.
Table 1.
General characteristics of the studies in animals and results: summary of the main characteristics of the included studies and presentation of the results of the studies, including the effects of the interventions on oxidative stress biomarkers.
| Author | Compound | Antioxidant Action | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓RONS | ↓ RONS Synthesis | ↓ RONS Damage | Improved Antioxidant Defense | |||||||||||||
| NO | RONS | iNOS | COX2 LPO NOX |
MPO | NF-κB/ Iκ-Bα |
MDA LP |
PTN | DNA | SOD | CAT | GPx | GR GSH GST |
Nrf2 | TAC/ TAS |
||
| Hormones | ||||||||||||||||
| [26] | Melatonin | X | ||||||||||||||
| [27] | Dehydroepiandrosterone (DHEA) | X | ||||||||||||||
| [28] | Obestatin | X | X | X | X | X | ||||||||||
| Synthetic compounds | ||||||||||||||||
| [29] | GL-V9 synthetic flavonoid |
X | X | X | X | X | X | |||||||||
| [30] | Glucose-lysine MRPs | X | X | X | X | X | X | X | X | |||||||
| [31] | ZnO nanoparticles (ZnONP) and ZnO microparticles (ZnOMP) | X | X | X | X | |||||||||||
| [32] | Chromium-D-phenylalanine complex (Cr(D-phe) |
X | X | X | ||||||||||||
| [33] | Hydrogen-rich water (HRW) | X | X | X | ||||||||||||
| [34] | P-chloro-phenylselene cholesterol (PCS) | X | X | X | ||||||||||||
| [35] | Selenium nanoparticles (ULP-SeNPs) | X | X | X | X | X | X | |||||||||
| [36] | Hydroxyproline | X | X | X | X | X | X | X | ||||||||
| [37] | LL202 (synthetic flavonoid) | X | X | X | X | |||||||||||
| [38] | RSV imine (IRA), 2-methoxyl-3,6-dihydroxyl-IRA 3,4,5,4-tetramethoxystilbene (C33) | X | ||||||||||||||
| [39] | FA-97 (synthetic phenolic compound) | X | X | X | X | |||||||||||
| [40] | Unconjugated bilirubin (UCB) | X | X | |||||||||||||
| [41] | Taurine-loaded chitosan pectin nanoparticles (Tau-CS-PT-nps) and chitosan pectin nanoparticles (CS-PT-nps) | X | X | |||||||||||||
| [42] | Turmeric-derived nanoparticles | X | ||||||||||||||
| [43] | (R,R)-BD-AcAc2 | X | X | X | X | X | ||||||||||
| [44] | Lawesson’s reagent | X | X | |||||||||||||
| [45] | Nano-selenium modified with Eucommia ulmoides polysaccharide | X | X | X | X | X | X | X | ||||||||
| [46] | Galactosylated polymeric nanocargoes | X | X | X | X | X | X | |||||||||
| [47] | Edible nanoparticles similar to exosomes from Portulaca oleracea L. | X | ||||||||||||||
| [48] | Ferulic acid | X | X | X | X | |||||||||||
| [49] | Ferulic acid | X | X | X | X | X | X | X | ||||||||
| [50] | Res-CDF (cross-linked organic cyclodextrin-metal structure encapsulating resveratrol) |
X | X | X | X | X | X | |||||||||
| [51] | Lipoic acid and/or N-acetylcysteine | X | X | X | X | |||||||||||
| Chemical products derived from sources other than plants | ||||||||||||||||
| [52] | Phycocyanin | X | X | X | X | |||||||||||
| [53] | Lycopene | X | X | X | X | X | X | |||||||||
| [54] | Melitin | X | X | X | X | |||||||||||
| [55] | Shrimp peptide (SP) | X | X | |||||||||||||
| [56] | Polysaccharopeptide from sanghuang mushroom | X | ||||||||||||||
| [57] | Inosine | X | X | X | X | X | X | X | X | |||||||
| [58] | Sodium butyrate | X | X | X | ||||||||||||
| [59] | Sea conch peptides hydrolysate | X | ||||||||||||||
| [60] | Astaxanthin | X | X | X | ||||||||||||
| [61] | Astaxanthin | X | X | |||||||||||||
| Polyphenols and other natural active compounds from medicinal plants | ||||||||||||||||
| [62] | P. argentea methanolic extract (PAME) | X | X | X | ||||||||||||
| [63] | Pfaffia paniculata extract (Brazilian ginseng) | X | ||||||||||||||
| [64] | American ginseng | X | ||||||||||||||
| [65] | Panaxynol (bioactive component of American ginseng) | X | X | X | ||||||||||||
| [66] | Phyllanthus niruri L. spray-dried extract | X | ||||||||||||||
| [67] | Turmeric | X | X | |||||||||||||
| [68] | Grape pomace extract | X | X | X | ||||||||||||
| [69] | Grape seed proanthocyanidin extract | X | ||||||||||||||
| [70] | Grape seed proanthocyanidin extract | X | X | X | ||||||||||||
| [71] | Isoliquiritigenin | X | X | |||||||||||||
| [72] | Isoquercitrin | X | X | |||||||||||||
| [73] | Quercitrin | X | ||||||||||||||
| [74] | Apple peel polyphenols (dried apple peel) | X | X | X | X | X | ||||||||||
| [75] | Concentrated apple extract (CAE) | X | X | X | ||||||||||||
| [76] | Luteolin | X | X | X | X | X | ||||||||||
| [77] | Luteolin | X | X | X | ||||||||||||
| [78] | Fisetin | X | X | X | X | X | ||||||||||
| [79] | Myrtus communis hydroalcoholic extract or essential oil | X | ||||||||||||||
| [80] | Myrtus communis subspecies communis extract | X | X | X | X | |||||||||||
| [81] | Epicatechin | X | X | X | X | X | X | |||||||||
| [82] | Methyl gallate | X | X | |||||||||||||
| [83] | Ethanolic extract (etohe) and hexane phase (hexp) from the leaves of Combretum duarteanum (Cd) | X | X | X | ||||||||||||
| [84] | Carvacrol (5-isopropyl-2-methylphenol) | X | X | X | X | X | ||||||||||
| [85] | Tuber of Amorphophallus paeoniifolius (Dennst.) Nicolson (Araceae) | X | X | X | X | X | ||||||||||
| [86] | Rosmarinic acid | X | X | X | ||||||||||||
| [87] | P-Cymene (p-C) and rosmarinic acid (RA) | X | X | X | X | X | X | X | ||||||||
| [88] | Rosmarinic acid-loaded nanovesicles | X | X | |||||||||||||
| [89] | Moringa seed extract (Moringa oleiferalam) | X | X | X | X | X | ||||||||||
| [90] | Phoenix loureiroi Kunth methanolic extracts | X | X | X | X | |||||||||||
| [91] | Pongamia pinnata (Karanja) | X | X | X | X | X | X | |||||||||
| [92] | Averrhoa bilimbi L. extract | X | X | X | X | X | ||||||||||
| [93] | Olea europaea leaf extract | X | X | |||||||||||||
| [94] | Morusin | X | X | |||||||||||||
| [95] | Soy isoflavones | X | X | X | X | |||||||||||
| [96] | Geniposide | X | X | X | X | |||||||||||
| [97] | Geniposide | X | X | |||||||||||||
| [98] | Geniposide | X | X | X | X | |||||||||||
| [99] | Hyperoside (hyp) | X | X | X | X | |||||||||||
| [100] | Ocimum gratissimum leaves polyphenol-rich extract | X | X | X | X | X | X | |||||||||
| [101] | Ocimum gratissimum Linn. | X | X | |||||||||||||
| [102] | Sesbania grandiflora | X | X | X | X | X | ||||||||||
| [103] | Oligonol | X | X | X | X | |||||||||||
| [104] | Thymol | X | X | |||||||||||||
| [105] | Thymol | X | X | X | X | |||||||||||
| [106] | Brazilian Berry (Myrciaria jaboticaba) peel aqueous extract | X | X | X | X | X | X | |||||||||
| [107] | Epigallocatechin gallate (EGCG) | X | ||||||||||||||
| [108] | (-)-Epigallocatechin-3-gallate | X | ||||||||||||||
| [109] | Epigallocatechin gallate (EGCG) | X | ||||||||||||||
| [110] | Carum copticum L. Extract | X | X | |||||||||||||
| [111] | Hesperidin | X | X | X | ||||||||||||
| [112] | Hesperidin (HMC) | X | X | X | ||||||||||||
| [113] | Hesperidin | X | X | X | ||||||||||||
| [114] | Mother tincture (MT) from fresh, young, nonwoody Thuja occidentalis L. | X | ||||||||||||||
| [115] | Phloretin | X | ||||||||||||||
| [116] | Phloretin | X | X | |||||||||||||
| [117] | Curcumin-galactomannoside | X | X | X | X | X | X | |||||||||
| [118] | Curcumin | X | ||||||||||||||
| [119] | Curcumin in hydroxyethyl starch microspheres | X | ||||||||||||||
| [120] | Curcumin | X | ||||||||||||||
| [121] | Honey polyphenols | X | X | X | X | X | X | |||||||||
| [122] | Glochidion ellipticum Wight extracts | X | X | X | X | X | ||||||||||
| [123] | Galangin | X | X | |||||||||||||
| [124] | Galangin | X | ||||||||||||||
| [125] | Taxifolin | X | X | |||||||||||||
| [126] | Flos lonicerae | X | X | X | X | |||||||||||
| [127] | Oroxindin | X | ||||||||||||||
| [128] | Polyphenolic maqui extract (Aristotelia chilensis) | X | X | X | ||||||||||||
| [129] | Polyphenolic maqui extract (Aristotelia chilensis) | X | ||||||||||||||
| [130] | Acacetin | X | X | |||||||||||||
| [131] | Flavonoid composition rich P. Subpeltata Ortega | X | X | X | X | X | ||||||||||
| [132] | Juglone (JUG) | X | X | X | X | |||||||||||
| [133] | Hydroxytyrosol (HYT) from olive leaves extract (OLE) | X | X | X | X | |||||||||||
| [134] | Lingonberry (LB) | X | X | X | ||||||||||||
| [135] | Caragana sinica extract | X | X | X | X | |||||||||||
| [136] | Quercus brantii (QB) extract | X | X | |||||||||||||
| [137] | T. Occidentalis leaf extract (ato) | X | X | X | X | X | X | |||||||||
| [138] | Dilodendron bipinnatum Radlk. extract | X | X | X | ||||||||||||
| [139] | Copaifera malmei leaf infusion extract (iecm) | X | X | X | ||||||||||||
| [140] | Kaempferol (kae) | X | ||||||||||||||
| [141] | 6-Paradol from seeds of Aframomum melegueta | X | X | X | ||||||||||||
| [142] | Maesa lanceolata hydroethanolic extract | X | X | X | ||||||||||||
| [143] | Garcinia mangostana and α-mangostin extract | X | X | X | X | X | ||||||||||
| [144] | Garcinia pedunculata bark (AEGP) aqueous extract | X | X | X | X | |||||||||||
| [145] | Troxerutin | X | X | X | X | X | X | |||||||||
| [146] | P. Lentiscus leaf aqueous extract | X | X | X | ||||||||||||
| [147] | Wogonin | X | X | X | X | X | ||||||||||
| [148] | Plant polyphenols (gallic acid, proanthocyanidin, ellagic acid, and tannic acid) | X | ||||||||||||||
| [149] | Gallic acid | X | X | X | X | |||||||||||
| [150] | Gallic acid | X | ||||||||||||||
| [151] | Gallic acid | X | ||||||||||||||
| [152] | Arum maculatum | X | ||||||||||||||
| [153] | Coumaric acid and syringic acid | X | ||||||||||||||
| [154] | Syringic acid | X | X | |||||||||||||
| [155] | Syringic acid | X | X | X | ||||||||||||
| [156] | Apigenin | X | X | X | X | |||||||||||
| [157] | Safranal | X | ||||||||||||||
| [158] | Resveratrol | X | ||||||||||||||
| [159] | Resveratrol in polysaccharide-zein nanoparticles from Mesona chinensis | X | ||||||||||||||
| [160] | Oxiresveratrol (OXY) | X | X | X | ||||||||||||
| [161] | Ligustroside | X | ||||||||||||||
| [162] | Isoimperatorin | X | X | |||||||||||||
| [163] | Forsythia suspensa polyphenols | X | ||||||||||||||
| [164] | m Callicarpa nudiflora Hook flavonoids | X | ||||||||||||||
| [165] | Calliandra haematocephala extracts | X | X | X | ||||||||||||
| [166] | Diosmin | X | X | X | X | X | ||||||||||
| [167] | Ziziphus jujuba Mill polyphenol extracts | X | X | X | ||||||||||||
| [168] | Geraniol | X | X | X | X | X | X | |||||||||
| [169] | Terminalia catappa Linn | X | X | X | ||||||||||||
| [170] | L. Dentata or L. Stoechas | X | X | X | X | |||||||||||
| [171] | Demethyleneberberine—DMB (component from Cortex Phellodendri Chinensis) |
X | X | |||||||||||||
| [172] | Raphanus sativus L. seeds aqueous extract | X | X | X | X | |||||||||||
| [173] | Myrrh | X | X | X | ||||||||||||
| [174] | Gegen qinlian | X | ||||||||||||||
| [175] | Ursolic acid | X | X | X | X | |||||||||||
| [176] | Lion’s Mane Medicinal Mushroom and Hericium erinaceus (Agaricomycetes) ethanol extract | X | X | X | ||||||||||||
| [177] | Polysaccharide from cultured mycelium of Hericium erinaceus | X | X | |||||||||||||
| [178] | Polysaccharides from the edible mushroom Hericium erinaceus | X | X | X | ||||||||||||
| [179] | Amphipterygium adstringens extract | X | X | |||||||||||||
| [180] | Mangiferin | X | X | X | X | X | ||||||||||
| [181] | Portulaca oleracea L. | X | X | X | X | |||||||||||
| [182] | Portulaca oleracea L. | X | X | X | X | X | ||||||||||
| [183] | Andrographolide | X | X | X | ||||||||||||
| [184] | Carpolobia lutea G. Don (Polygalaceae) | X | X | X | ||||||||||||
| [185] | Lagerstroemia speciosa | X | X | X | ||||||||||||
| [186] | Atractylodes macrocephala and Taraxacum herba extracts | X | X | X | ||||||||||||
| [187] | Aronia berry | X | X | |||||||||||||
| [188] | Aronia berry extract | X | ||||||||||||||
| [189] | Aronia melanocarpa (Michx.) Elliott. | X | X | X | X | X | ||||||||||
| [190] | Decursin and decursinol | X | ||||||||||||||
| [191] | Perilla frutescens extract (PE) | X | X | |||||||||||||
| [192] | Glyceollins from daidzein in soybean | X | X | X | X | X | ||||||||||
| [193] | Catalpol | X | X | X | ||||||||||||
| [194] | D-limonene | X | X | X | X | X | ||||||||||
| [195] | Liriodendrin | X | X | X | X | X | ||||||||||
| [196] | Yuzu (Citrus junos Tanaka) | X | ||||||||||||||
| [197] | Veronica polita | X | X | X | X | X | ||||||||||
| [198] | Ziziphus spina-christi fruit extract | X | X | X | X | X | X | X | X | X | ||||||
| [199] | Red raspberries | X | X | |||||||||||||
| [200] | Plumieride | X | X | X | X | |||||||||||
| [201] | Ipomoea asarifolia aqueous extract | X | X | X | X | X | ||||||||||
| [202] | Osthole | X | X | X | X | X | X | |||||||||
| [203] | Polygonum cuspidatum Siebold & Zucc root Extract | X | X | X | X | X | X | |||||||||
| [204] | Alpinia officinarum | X | X | X | X | X | X | X | ||||||||
| [205] | Magnolol | X | X | |||||||||||||
| [206] | Magnolol contained in a butyrate-derived polymer nanoplatform | X | ||||||||||||||
| [207] | Trichilia catigua ethyl-acetate fraction | X | ||||||||||||||
| [208] | 4-methylesculetin | X | X | |||||||||||||
| [209] | 4-methylesculetin | X | X | |||||||||||||
| [210] | Fargesin | X | X | X | X | |||||||||||
| [211] | Sinomenine | X | X | X | ||||||||||||
| [212] | Sinomenine | X | X | X | X | X | X | X | X | X | X | |||||
| [213] | Stevioside | X | X | X | X | X | X | X | ||||||||
| [214] | Sesamin | X | X | X | ||||||||||||
| [215] | Terminalia arjuna hydroalcoholic extract | X | X | X | X | X | ||||||||||
| [216] | Persea americana Mill. Avocado extract | X | X | X | X | |||||||||||
| [217] | Quercetin aglycone | X | X | X | X | X | ||||||||||
| [218] | 2-O-β-d-Glucopyranosyl-l-ascorbic acid, an ascorbic acid derivative isolated from the fruits of Lycium Barbarum L. | X | X | |||||||||||||
| [219] | Apocynin | X | X | X | ||||||||||||
| [220] | Apocynin | X | X | X | X | X | ||||||||||
| [221] | Crocin | X | X | X | ||||||||||||
| [222] | Crocin | X | X | |||||||||||||
| [223] | Crocina | X | X | |||||||||||||
| [224] | Salidroside (salt) | X | X | X | ||||||||||||
| [225] | Freeze-dried fruit powder of Actinidia arguta | X | X | X | ||||||||||||
| [226] | Tagetes erecta L. flowers hydroalcoholic extract | X | X | X | X | |||||||||||
| [227] | Daidzein | X | X | |||||||||||||
| [228] | Ajuga chamaepitys (L.) Schreber subsp. Chia (Schreber) | X | ||||||||||||||
| [229] | Sorbus domestica | X | X | X | X | X | X | |||||||||
| [230] | Bryophyllum pinnatum (Lamarck) leaf extract | X | X | X | X | X | ||||||||||
| [231] | Piper umbellatum L. (Piperaceae) | X | X | X | X | X | X | |||||||||
| [232] | Myristicin | X | X | X | X | X | ||||||||||
| [233] | Bruguiera gymnorrhiza leaves | X | X | X | X | |||||||||||
| [234] | Arjunarishta | X | X | X | X | X | ||||||||||
| [235] | Antrocaryon micraster | X | X | X | ||||||||||||
| [236] | Piperine | X | X | X | X | X | ||||||||||
| [237] | Puerarin | X | X | X | X | X | X | X | X | X | ||||||
| [238] | Rumex japonicus Houtt. | X | ||||||||||||||
| [239] | Gloeostereum incarnatum | X | X | X | X | X | ||||||||||
| [240] | Sinapic acid | X | X | X | X | X | ||||||||||
| [241] | Nerolidol | X | X | X | X | X | X | |||||||||
| [242] | Nerolidol (NRD) | X | X | X | X | X | ||||||||||
| [243] | Jasonia glutinosa (L.) DC. extract | X | X | X | X | |||||||||||
| [244] | Sanhuang shu’ai | X | X | X | ||||||||||||
| [245] | Flammuliana velutipes polysaccharide | X | X | X | X | |||||||||||
| [246] | of the Syringa oblata Lindl Iridoid glycosides | X | X | |||||||||||||
| [247] | Mucilage Garden cress | X | ||||||||||||||
| [248] | Otostegia fruticosa leaves crude extract | X | X | |||||||||||||
| [249] | Dracocephalum kotschyi methanol extract | X | X | X | ||||||||||||
| [250] | Fruit of Rosa odorata doce var.gigantea | X | X | X | X | X | ||||||||||
| [251] | Artemisia argyi extract | X | X | X | X | |||||||||||
| [252] | Inula viscosa ethanolic extract | X | X | X | X | X | ||||||||||
| [253] | Cepharanthine | X | X | X | X | |||||||||||
| [254] | Saposhnikovia divaricata | X | X | |||||||||||||
| [255] | Trigonellafoenum-graecum L. seeds aqueous extract | X | X | X | X | |||||||||||
| [256] | Quinic acid | X | X | X | X | X | X | X | ||||||||
| [257] | Echinacea purpurea extract | X | ||||||||||||||
| [258] | Echinacea purpurea polysaccharide | X | X | X | X | |||||||||||
| [259] | Daphnetin | X | X | |||||||||||||
| [260] | Oxyberberin | X | X | X | X | X | ||||||||||
| [261] | Curculigoside (CUR), from Curculigo orchioides Gaertn | X | X | X | X | X | X | |||||||||
| [262] | Scrophularia striata Boiss aqueous and hydroalcoholic Extracts | X | X | |||||||||||||
| [263] | Picralima nitida seeds crude alkaloidal extract | X | X | X | X | |||||||||||
| [264] | Mesua assamica (King&Prain) kosterm. Bark ethanolic extract | X | X | X | X | X | X | X | ||||||||
| [265] | Higenamine | X | X | |||||||||||||
| [266] | Carboxymethyl Poria Polysaccharides | X | X | |||||||||||||
| [267] | 1,8-cineol (eucaliptol) | X | X | X | X | X | X | X | ||||||||
| [268] | Polygonatum Cyrtonema Hua Oligosaccharides | X | X | X | X | |||||||||||
| [269] | Polysaccharide from the fermented mycelium of Inonotus obliquus | X | X | X | X | |||||||||||
| [270] | Pinus eldarica aqueous and hydroalcoholic extracts | X | X | |||||||||||||
| [271] | Terminalia chebula ethyl acetate extract | X | X | X | X | X | ||||||||||
| [272] | Ecklonia cava extract | X | X | |||||||||||||
| [273] | Salvia verbenaca extract | X | X | X | ||||||||||||
| [274] | Commiphora leptophloeos extract | X | X | X | X | |||||||||||
| [275] | Sagittaria sagittifolia L. polysaccharides | X | X | X | X | |||||||||||
| [276] | Tetrastigma hemsleyanum root extract | X | X | |||||||||||||
| [277] | Gilaburu (Viburnum opulus L.) fruit extract | X | X | X | X | |||||||||||
| [278] | Acidic polysaccharide from Selaginella uncinata (Desv.) Spring | X | X | X | X | |||||||||||
| [279] | Lizhong decoction | X | X | X | X | X | ||||||||||
| [280] | (-)-Syringaresinol | X | ||||||||||||||
| [281] | Aegeline | X | X | X | X | |||||||||||
| [282] | Fraxetin | X | X | X | X | |||||||||||
| [283] | Honeysuckle | X | X | |||||||||||||
| [284] | Passiflora edulis | X | ||||||||||||||
| [285] | Jinxiang garlic (Allium sativum L.) | X | ||||||||||||||
| [286] | Cinnamaldehyde and hesperetin | X | X | X | X | X | ||||||||||
| [287] | Anacardium occidentale L. | X | X | X | X | |||||||||||
| [288] | Loganic acid | X | X | X | X | X | X | |||||||||
| [289] | Four sanshools of Zanthoxylum fruit | X | X | X | X | X | ||||||||||
| [290] | Sea buckthorn | X | X | X | X | X | X | |||||||||
| Functional foods and nutrients | ||||||||||||||||
| [291] | Oat β-glucan | X | X | |||||||||||||
| [292] | Blueberry | X | X | X | X | X | X | |||||||||
| [293] | Riboflavin | X | X | X | ||||||||||||
| [294] | Goat whey | X | X | |||||||||||||
| [295] | Garlic oil | X | X | X | X | |||||||||||
| [296] | Red bean | X | X | X | ||||||||||||
| [297] | Lecithin | X | X | X | ||||||||||||
| [298] | Coenzyme Q10 | X | X | X | X | |||||||||||
| [299] | Coenzyme Q10 | X | X | X | X | X | ||||||||||
| [300] | Honey | X | X | X | ||||||||||||
| [301] | Flaxseed extract | X | X | X | X | X | X | X | X | |||||||
| [302] | Β-glucans from Lentinus edodes | X | X | X | X | |||||||||||
| [303] | Selenium | X | X | |||||||||||||
| [304] | Selenocysteine and selenocystine | X | X | X | ||||||||||||
| [305] | Selenium in biogenic nanoparticles | X | X | X | X | |||||||||||
| [306] | Camellia oil | X | X | X | ||||||||||||
| [307] | Walnut extract | X | X | |||||||||||||
| [308] | Aqueous cinnamon extract | X | X | X | X | |||||||||||
| [309] | Cinnamon (Cinnamomum japonicum) subcritical water extract | X | ||||||||||||||
| [310] | Tocotrienol (alpha-tocopherol) | X | X | X | X | X | ||||||||||
| [311] | Omega 3 | X | X | X | X | |||||||||||
| [312] | Eicosapentaenoic acid (EPA) | X | X | X | X | X | ||||||||||
| [313] | Noni juice-fortified yogurt | X | X | |||||||||||||
| [314] | Isolated from fish skin gelatin hydrolysate (fsghf3) |
X | X | X | X | X | X | |||||||||
| [315] | Mannoglucan (Chinese yam.) |
X | ||||||||||||||
| [316] | Alpha-tocopherylquinone | X | ||||||||||||||
| [317] | Β-carotene | X | ||||||||||||||
| [318] | Momordica charantia | X | X | X | X | |||||||||||
| [319] | Virgin coconut oil | X | X | |||||||||||||
| [320] | Pumpkin polysaccharides | X | ||||||||||||||
| [321] | Fermented yogurt | X | ||||||||||||||
| Probiotics | ||||||||||||||||
| [322] | NTU 101; L. rhamnosus BCRC 16000; L. paracasei subsp. paracasei BCRC 14023 | X | X | X | X | X | ||||||||||
| [323] | Lactobacillus plantarum (CAU1054 OR CAU1055, OR CAU1064) OR Lactobacillus salivarius CAU1301 | X | X | |||||||||||||
| [324] | MegaSporeBiotic TM (MSB) probiotic capsules and MegaMucosa TM (MM) powder | X | X | |||||||||||||
| [325] | Bifidobacterium bifidum ATCC 29521 | X | X | X | X | |||||||||||
| [326] | Lactobacillus acidophilus XY27 | X | X | X | X | X | X | |||||||||
| [327] | Minas Frescal probiotic cheese containing L. lactis | X | X | |||||||||||||
| [328] | Probiotic yeast Saccharomyces boulardii (s. Boulardii) |
X | X | X | X | X | ||||||||||
| [329] |
Lactobacillus acidophilus KDSL 1.0901, Lactobacillus helveticus KDSL 1.8701, Lactobacillus plantarum KDSL 1.0318, and mixed lactobacilli |
X | ||||||||||||||
| [330] | Lactobacillus gasseri 4M13 | X | X | X | X | |||||||||||
| [331] | L. pentosus A14-6 and L. pentosus CMY46 | X | ||||||||||||||
| [332] | Lactobacillus acidophilus C4 | X | X | X | ||||||||||||
| [333] | Exopolysaccharide Ropy Bifidobacterium pseudocatenulatum Bi-OTA128 | X | X | X | X | |||||||||||
| Others | ||||||||||||||||
| [334] | Insect (cockroach) Periplaneta americana | X | X | |||||||||||||
| [335] | MicroRNAs | X | X | X | X | X | X | |||||||||
| [336] | Insect (cockroach) Periplaneta americana | X | X | X | X | X | X | |||||||||
| [337] | Aspergillus awamori | X | X | X | X | X | ||||||||||
| [338] | Maggot extracts | X | ||||||||||||||
| [339] | Meroterpene algae 11-hydroxy-1′-O—methylamadione | X | X | X | ||||||||||||
| [340] | Arthrospira (Spirulina) platensis | X | X | |||||||||||||
| [341] | hydroalcoholic extracts (HA) of cyanobacterium Spirulina platensis | X | ||||||||||||||
| [342] | Chinese propolis | X | X | X | X | |||||||||||
| [343] | Saccharina japonica | X | X | |||||||||||||
| [344] | Aphanizomenon flos-aquae | X | X | X | X | X | ||||||||||
| [345] | Melanin from Sepia pharaonis ink | X | X | X | X | |||||||||||
| [346] | Tuna bioactive peptides (TBP) | X | X | |||||||||||||
| [347] | Turtle peptide | X | X | X | ||||||||||||
| [348] | Oxylipin-containing lyophilized biomass from a microalga | X | X | X | X | |||||||||||
| [349] | Fermented Mekabu aqueous solution by Lactobacillus plantarum Sanriku-SU7 |
X | ||||||||||||||
Legend: CAT = catalase; COX2 = cyclooxigenase type 2; DNA = deoxyribonucleic acid; ERON = reactive oxygen and nitrogen species; GPx = glutathione peroxidase; GR = glutathione reductase; GSH = glutathione; GST = glutathione S-transferase; iNOS = inducible nitric oxide synthase; Iκ-Bα = nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; LP = lipid peroxidation; LPO = lipoxygenase; MDA = malondialdehyde; MPO = myeloperoxidase; NF-Κb = nuclear factor kappa-light-chain-enhancer of activated B cells; NO = nitric oxide; NOX = nicotinamide adenine dinucleotide phosphate-oxidase; Nrf2 = nuclear factor erythroid 2; PTN = protein; RONS = reactive oxygen and nitrogen species; SOD = superoxide dismutase; TAC = total antioxidant capacity.
However, as reported in the previous review [12], all studies found were in animal models primarily focusing on tissue expression, indicating a lack of human studies to corroborate findings from murine models.
Nrf2 is considered a critical factor in maintaining redox equilibrium, essential for expressing various antioxidant enzymes, providing direct protective action, and inducing cellular damage repair and tissue regeneration [17]. Thus, interest in modulating Nrf2 has been growing in recent years to maintain and restore human health [350].
Nevertheless, comprehending oxidative stress is not a straightforward task. The involvement of various molecules and cellular pathways, each responding to different stimuli depending on the tissue or compartment analyzed, complicates the evaluation of the antioxidant therapy. Therefore, it is essential to assess the specific targets of each tested compound. Only by understanding these targets can we safely prescribe combinations of different substances that act on distinct targets, thereby potentially enhancing therapeutic efficacy, whether used alone or in conjunction with combined therapy.
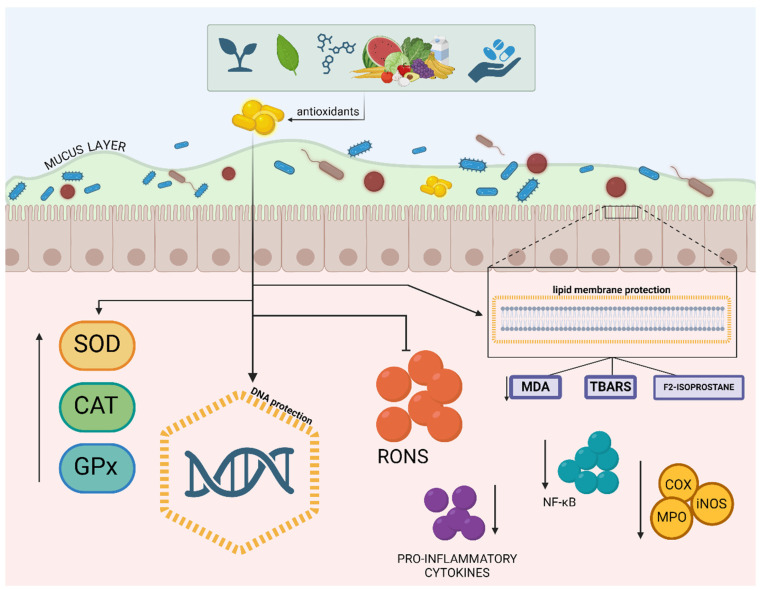
Figure 3.
Mechanisms of action of antioxidants in the modulation of the intestinal inflammatory response. Legend: Mechanisms of antioxidant protection in the intestinal epithelium. Dietary and endogenously produced antioxidants neutralize reactive oxygen and nitrogen species (RONS), protecting deoxyribonucleic acid (DNA), membrane lipids (reducing malondialdehyde [351], thiobarbituric acid reactive substances [TBARS], and F-2 isoprostane), and mucus, increasing the production of the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which also combat oxidative stress. Antioxidants attenuate effects of nuclear factor kappa B (NF-κB), reducing the production of pro-inflammatory cytokines and the expression of enzymes such as cyclooxygenase (COX), inducible nitric oxide synthase (iNOS), and myeloperoxidase (MPO), which can lead to tissue damage if left unchecked; ↑ (increased); ↓ (decreased).
3.3. Action on Reactive Oxygen and Nitrogen Species and Their Generation
It is well known that RONS are produced both endogenously (mitochondria, peroxisomes, NOX, lipoxygenases, cytochrome P450, XO, COX, NOS, MPO, inflammatory cytokines, among others) and exogenously (ultraviolet light, radiation, chemotherapy, toxins, microorganisms, etc.). Low levels of these species impair various physiological functions, leading to issues in proliferative responses, immune system dysfunction, and defects in vasodilation. These alterations can result in conditions such as chronic granulomatous disease and certain autoimmune diseases [352].
In homeostasis, RONS are controlled by the enzymatic and non-enzymatic antioxidant defense system (eustress), which includes defenses naturally produced by the body, such as SOD, CAT, GPx, GR, thioredoxin (Trx), and peroxiredoxins. Additionally, certain metals, such as zinc and selenium, play crucial roles in antioxidant enzyme function, and natural chelators, including thiols, help neutralize metal ions that catalyze oxidative reactions. Dietary antioxidants like polyphenols, carotenoids, flavonoids, coenzyme Q10, vitamins C (ascorbic acid), and E (α-tocopherol) also contribute to this system [353]. Micromolecular antioxidants such as amino acids (e.g., cysteine, methionine, and glutamine) and other small molecules (e.g., uric acid and bilirubin) also play crucial roles in mitigating oxidative stress [354,355]. In a balanced state, redox signaling is essential. Under physiologic range (eustress or “good stress”), the reactive species generated play a critical role in microbial control by inhibiting the overgrowth of microbial populations, thus reducing microbial attacks. These reactive species aid in the programmed destruction of altered cells, contribute to embryogenesis, facilitate muscle exercise, and are crucial for the nervous system by enabling nerve impulse transmission between neurons [356].
However, in harmful situations (distress or “bad stress”), excessive release of RONS that overwhelms these defense mechanisms plays a crucial role in the development of diseases, aging, and carcinogenesis. This occurs when these antioxidant defenses fail [357]. Despite the ongoing discussion by Bienertova-Vasku and Scheringer (2020) [358] about the effectiveness of these concepts (eustress and distress) and suggesting that both are forms of oxidative stress to varying degrees, the concepts introduced by Selye in 1975 remain widely accepted and used [356].
Among the various animal models included in this review, oxidative stress/redox imbalance was present in both acute [30,35,36,48,78,89,91,92,96,215,221,311,359], and chronic situations [52,97,106,128,139], regardless of the colitis-inducing agent, whether acetic acid [117,202,249,301,303,306], dextran sodium sulfate [73,201,240,314,360], 2,4,6-trinitrobenzene sulfonic acid (TNBS) [136,183,207,236,361], among others. This indicates that oxidative stress needs to be controlled to minimize the effects of the disease, regardless of the phase, type, and severity of IBD.
The groups of “polyphenols and other active natural compounds derived from plants” (n = 242; 70.3%) were the most investigated classifications of antioxidants in animal models in this review. It was observed that the antioxidant effects of polyphenols involved the reduction of RONS concentration and synthesis, control of oxidative damage, improvement of antioxidant defenses, and effects on the intestinal microbiota, suggesting their broad action in controlling redox imbalance [67,117,118,362].
Among the compounds tested in animal models that demonstrated scavenging radical activity, particular attention should be given to those that act on reactive nitrogen species (RNS), reducing NO• levels (n = 46; 13.3%). Noteworthy compounds include zinc oxide nanoparticles (ZnONPs) and zinc oxide microparticles (ZnOMPs) [31], galactosylated polymeric nanocarriers [46], phycocyanin [52], aphanizomenon flos-aquae algae [344], ferulic acid [48], Myrtus communis subspecies extract [80], epicatechin [81], and Moringa oleifera Lam seed extract [89], among others.
Antioxidant vegetal natural compounds, isolated or in mixtures, such as geraniol [168], Vaccinium corymbosum [292], Ziziphus spina-christi fruit extract [198], Polygonum cuspidatum root extract [203], and Alpinia officinarum [204], demonstrated a wide range of actions, including reduction of RONS, decreased synthesis of reactive species (inhibiting NOX and iNOS), and improved antioxidant defense by increase in levels of SOD, CAT, GPX, and GSH.
Some antioxidants’ mechanisms of action, which are based on this review, can be seen in Figure 2. It is important to note that reducing oxidative stress is crucial in situations where this stress is harmful or in cases of distress, such as in cardiovascular and inflammatory diseases. However, careful attention is needed when the treatment goal is to enhance oxidative stress, as is the case with antimicrobial actions or cancer treatment. Thus, it is essential to accurately assess when reducing RONS generation is a therapeutic target in a given clinical scenario.
3.4. Action on Antioxidant Defenses
Several studies confirmed reduced antioxidant defenses in both drug-induced colitis animal models [28,45,87,138,175,200,212,234,300,363] and in humans [363,364,365,366,367,368,369,370,371,372,373]. Among the 218 animal studies evaluating the action of compounds on antioxidant defenses, once again, the group of “polyphenols and other natural active compounds derived from plants” was the group studied that proved to be the most efficient in improving antioxidant defense (n = 151; 43.8%), with notable compounds, including gallic acid [149], Ziziphus na-christi fruit extract [198], plumieride [200], Alpinia officinarum [204], stevioside [213], sesamin [214], myristicin [232], sinapic acid [240], Lizhong decoction (a classical Chinese herbal) [279], and sinomenine [212], which showed beneficial effects on three or more biomarkers of antioxidant defenses. According to these findings, the markers SOD, CAT, and GSH were the most impacted by the antioxidants tested in the murine models of induced colitis.
In humans, as shown in Table 2 and Table 3, compounds such as omega-3 [363,364], pycnogenol [373], curcumin and piperine [366], spiruline [367], zinc aspartate [372], Urtica dioica leaf extract [368], resveratrol [369], and saffron [371] had effects on various antioxidant defenses, such as improvement in the antioxidant activity of CAT [363], a significant increase in the activity of SOD in whole blood, and an improvement in the antioxidant status of individuals [365]. TAS or TAC was also analyzed and increased with the use of vitamin D [374], spiruline [367], Nigella sativa [375], zingiber [376], Pistacia lentisco [377], and resveratrol [369]. However, it is essential to emphasize that these findings are challenging to compare due to the significant diversity in the methods used to evaluate them, which limits the ability to interpret the results collectively [378].
Table 2.
General characteristics of the studies: summary of the main characteristics of the included studies.
| Author | Ibd | Study | Intervention | Dose and Time of Intervention | Group Subjects, n and Age |
|---|---|---|---|---|---|
| Functional foods and nutrients | |||||
| [364] | UC mild or moderate phase | Blind randomized clinical trial | Omega-3 | 4.3 g (4800 mg—4 capsules of 1200 mg per day) 8 weeks |
70 elderly Placebo: n = 35; age = 69.7 Intervention: n = 35; age = 69.7 |
| [374] | UC mild or moderate phase | Randomized crossover clinical trial, placebo control | Omega-3 | 4.5 g/d (90 mg EPA + 60 mg DHA) 8 weeks |
18 adults Placebo: n = 9; age = not informed intervention: n = 9; age = 40 |
| [365] | CD in remission | Double-blind randomized controlled clinical trial | Antioxidant complex (AO) AO complex + omega 3 (n-3) | 12 weeks | 17 adults Placebo: n = 8; age = 38 AO: n = 8; age = 43 AO + omega 3: n = 9; age = 41 |
| [379] | CD mild phase or remission | Double-blind randomized controlled clinical trial | Vitamin E + vitamin C supplement | Vitamin E (800 IU) Vitamin C (1000 mg) 4 weeks |
57 adults Placebo: n = 29; age = 36.5 Intervention: n = 28; age = 38.3 |
| [380] | Active CD | Double-blind randomized controlled clinical trial | Glutamine-enriched diet | Polymeric diet enriched with glutamine (42% of the amino acid composition) 4 weeks |
15 children Placebo: n = 8; age = 10.5 Intervention: n = 7; age = 12.2 |
| [381] | UC | Double-blind randomized controlled clinical trial | Intervention 1: ground flaxseed (GF) Intervention 2: Linseed oil (FO) |
GF: 30,000 mg/d FO: 10,000 g/d 2 weeks |
75 adults Placebo: n = 25; age = 35.2 GF: n = 25; age = 29.9 FO: n = 25; age = 32.2 |
| [382] | UC mild or moderate phase | Randomized, multicenter, double-blind clinical study | Selenium | 200 mcg/d 10 weeks |
100 adults Placebo: n = 50; age = 37.9 Intervention: n = 50; age = 34.5 |
| [374] | UC mild or moderate phase | Double-blind randomized controlled clinical trial | Vitamin D | Intervention 1: 1000 IU/d (I1) Intervention 2: 2000 IU (I2) 12 weeks |
46 adults I1: n = 22; age = 39.7 I2: n = 24; age = 34 |
| [383] | CD | Randomized controlled trial | Fish oil (EPA and DHA) | 2.7 g/d 24 weeks |
61 adults Placebo: n = 31; age = 40.5 Intervention: n = 31; age = 45.4 |
| [384] | CD or UC | Prospective study | Riboflavin | 100 mg/d 3 weeks |
70 adults Group 1 (fecal Calprotectin < 200 µg/g): n = 40; age = 44.2 Group 2 (fecal calprotectin > 200 µg/g): n = 30; age = 38.8 |
| [376] | UC mild or moderate phase | Double-blind randomized controlled clinical trial | Zingiber | 2000 mg/d 12 weeks |
46 adults Placebo: n = 24; age = 39.2 Intervention: n = 22; age = 41.4 |
| [372] | Inactive to moderate CD or UC | Double-blind randomized controlled clinical trial | Zinc aspartate | 300 mg 4 weeks |
36 adults Placebo: n = 22; age = 38 Intervention: n = 14; age = 42 |
| Polyphenols and other natural active compounds from medicinal plants | |||||
| [370] | UC mild or moderate phase | Randomized, double-blind, controlled pilot study | Resveratrol | 500 mg/d 6 weeks |
49 adults Placebo: n = 24; age = 39 Intervention: n = 25; age = 37.7 |
| [369] | UC mild or moderate phase | Randomized, double-blind, controlled pilot study | Resveratrol | 500 mg/d 6 weeks |
56 adults Placebo: n = 28; age = 38.8 Intervention: n = 28; age = 37.4 |
| [366] | Mild or moderate CD or UC | Double-blind randomized controlled clinical trial | Curcumin (doses of 1000 mg/day) or Curcumin + piperine |
Curcumin: 1000 mg/day Piperine: 10 mg/day 12 weeks |
Placebo: n = 19; 50.9 Curcumin: n = 20; 46.9 Curcumin + piperine: n = 19; 44.7 years |
| [385] | UC mild or moderate phase |
Double-blind randomized controlled clinical trial | Saffron | 100 mg 8 weeks |
75 adults Placebo: n = 35; age = 40.97 Intervention: n = 40; age = 40.55 |
| [371] | UC mild or moderate phase | Randomized controlled trial | Saffron | 100 mg/d 8 weeks |
75 adults Placebo: n = 35; age = 41.0 Intervention: n = 40; age = 40.5 |
| [386] | CD mild or moderate stage | Pilot study | Mastic gum (Pistacia lentiscus) | 0.37 g 4 weeks |
18 adults Placebo: n = 8; age = 31.5 Intervention: n = 10; age = 36.9 |
| [377] | CD or UC in remission | Double-blind randomized controlled clinical trial | Pistacia lentiscus | 2800 mg/d 12 weeks |
60 adults Placebo: n = 27; age = 45 Intervention: n = 33; age = 38.2 |
| [373] | CD in remission | Pilot study | Pycnogenol | 2 mg/d 12 weeks |
29 teenagers Control: n = 15; age = 13.9 Intervention: n = 14; age = 16.3 |
| [368] | Mild or moderate CD or UC | Double-blind randomized controlled clinical trial | Urtica dioica leaf extract | 400 mg 12 weeks |
59 adults Placebo: n = 29; age = 38.3 Intervention: n = 30; age = 36.6 |
| [375] | UC mild or moderate phase | Double-blind randomized controlled clinical trial | Nigella sativa | 2000 mg/d 6 weeks |
48 adults Placebo: n = 24; age = 39.2 Intervention: n = 24; age = 34.8 |
| [387] | CD or UC in remission | Randomized, single-blind, controlled study | Medicinal mushroom extract based on Agaricus blazei Murill | 30 mL (twice a day) 3 weeks |
50 adults Placebo: n = 25; age = not informed Intervention: n = 25: age = not informed |
| Others | |||||
| [367] | UC mild or moderate phase | Double-blind randomized controlled clinical trial | Spirulina | 1 g/day (two 500 mg capsules/day) 8 weeks. |
73 adults Placebo: n = 37; age = 39.5 Intervention: n = 36; age = 37.8 |
Legend: CD = Crohn’s disease; UC = ulcerative disease.
Table 3.
Study results: presentation of the results of the studies, including the effects of the interventions on oxidative stress biomarkers and levels of pro- and anti-inflammatory cytokines.
| Author | Intervention | Oxidative Stress Markers | Cytokines | General Effects | ||||
|---|---|---|---|---|---|---|---|---|
| SOD | GSH Complex | TAC | LP | OTHER | ||||
| Functional Foods and Nutrients | ||||||||
| [364] | Omega-3 | ↑ | ↓ | ↓ TNF-α, IL-2, IL-1α, and IL-1β | Improved serum levels of oxidative, antioxidant, and inflammatory markers, diastolic and systolic pressure, while AGE, MDA, Ox-LDL, catalase, superoxide dismutase, and TNF-α were altered in the control group | |||
| [363] | Omega-3 | ↑ | ↓ | Cat: NS | No significant difference in laboratory indicators, sigmoidoscopy, or histology scores, and no significant difference in plasma MDA, erythrocyte LP, and CAT levels | |||
| [365] | Antioxidant complex (AO) AO complex + omega 3 (n-3) | ↑ | ↑ total oxidant status | Increased serum concentrations of vitamin E, vitamin C, and SOD activity. TAS increased significantly after supplementation with AO | ||||
| [379] | Vitamin E + vitamin C supplement | ↓ | Significant increase in their plasma levels compared to the placebo group. | |||||
| [380] | Glutamine-enriched diet | ↓ | Did not change plasma concentrations of antioxidants | |||||
| [381] | Intervention 1: ground flaxseed (GF) Intervention 2: linseed oil (FO) |
↓ IL-6 and IFN-γ | Significant reduction in fecal calprotectin, Mayo score, ESR, INF-γ, IL-6, waist circumference, DBP, and systolic blood pressure, and a significant increase in TGF-β and IBDQ-9 score. | |||||
| [382] | Selenium | ↓ IL-17 IL-10: NS |
Led to remission and improved quality of life. The concentration of IL-17 decreased. IL-10 levels did not show any considerable change between the two groups | |||||
| [374] | Vitamin D | ↑ | ↓ total oxidant status | Decreased TOS concentration in high doses, and increased IBDQ-9 scores compared to low-dose participants. However, no significant changes were observed in serum TAC or SCCAI scores. | ||||
| [383] | Fish oil (EPA and DHA) | ↓ TNF-α | Increased EPA and DHA incorporation into PBMCs, while reducing arachidonic acid incorporation and IFN production by mitogen-stimulated and lipopolysaccharide-stimulated PBMCs. | |||||
| [384] | Riboflavin | ↑ Free thiols | ↓ IL-6, IL-10, IL-2, and TNF-α IL-1β: NS |
Reduced oxidative stress and clinical symptoms. Decreased Enterobacteriaceae abundance, despite no evident changes in the fecal microbiome. | ||||
| [376] | Zingiber | ↑ | ↓ | Improved disease severity index and oxidative stress | ||||
| [372] | Zinc aspartate | NS | No changes were found in the plasma and erythrocyte metallothionein | |||||
| Polyphenols and other natural active compounds from medicinal plants | ||||||||
| [370] | Resveratrol | ↓ NF-κB | ↓ TNF-α | Inflammatory markers can be reduced through attenuation of NF-kB activity, potentially improving quality of life. | ||||
| [369] | Resveratrol | ↑ | ↑ | ↓ | Improve quality of life by reducing oxidative stress. | |||
| [366] | Curcumin (doses of 1000 mg/day) or curcumin + piperine |
↑ | ↓ | MPO, CAT and H2O2: NS | TNF-α, IL-17A, IL-22, and IL-10 | Significantly increased SOD levels compared to a placebo group. However, the three-month treatment time did not significantly alter other biomarkers. | ||
| [385] | Saffron | ↓ TNFα and IL17 ↑ IL10: |
Significant decreases in serum TNF, hs-CRP, and IL-10 levels compared to the placebo group, while no significant difference was found in ESR, IL-17, and IBDQ-9 scores. | |||||
| [371] | Saffron | ↑ | ↑ | ↓ | Reduced disease activity and increased serum levels of TAC, SOD, and GPX. | |||
| [386] | Mastic gum (Pistacia lentiscus) | ↓ TNF-α | ↓ IL-6 | Significant decreases in CDAI, plasma IL-6, and CRP, and increase in TAP, with no side effects observed. | ||||
| [377] | Pistacia lentiscus | ↓ | ↓ Ox-LDL | Decreased oxLDL/HDL, oxLDL/LDL, and oxLDL/LDL | ||||
| [373] | Pycnogenol | ↑ | ↓ | Serum AOC negatively correlated with disease activity and with CRP and fecal calprotectin | ||||
| [368] | Urtica dioica leaf extract | ↑ | Significantly reduced hs-CRP levels, increased SOD levels, and increased scores on the IBDQ-9 questionnaire | |||||
| [375] | Nigella sativa | ↑ | ↓ | NFκB: NS | TNF-α: NS | No significant differences in serum antioxidant capacity, NF-kB levels, or scores of IBDQ-9. | ||
| [387] | Medicinal mushroom extract based on Agaricus blazei Murill | ↑ IL-1ß, IL-2, IL-4, IL-5, IL-7, IL-10, IL-13, IL-17, and TNF-α ↓ IL-6, IL-8: ↓ IL-12, and IFN-γ |
Influenced cytokine levels and weak systemic anti-inflammatory effect | |||||
| Others | ||||||||
| [367] | Spirulina | NS | ↑ | ↓ | Serum TAC levels increased, leading to a higher health-related quality of life score, but no significant changes in disease activity score. | |||
Legend: CAT = catalase; IFN-γ = interferon-gamma; IL-1β = interleukin-1 beta; IL-2 = interleukin-2; IL-4 = interleukin-4; IL-6 = interleukin-6; IL-7 = interleukin-7; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-12 = interleukin-12; IL-13 = interleukin-13; IL-17 = interleukin-17; IL-17 = interleukin-17A; MPO = myeloperoxidase; NFκB = nuclear factor kappa-light-chain-enhancer of activated B cells; NS = not significant; Ox-LDL = oxidized LDL; TNF-α = tumor necrosis factor alpha.
Several studies with induced colitis included in the systematic review (n = 55; 15.9%) observed a positive effect of the analyzed compounds, increasing Nrf2 activity, suggesting that the tested compounds could protect against the accumulation of overproduced reactive species. Notable compounds include hesperidin [111], troxerutin [145], luteolin [76], crocin [221], and American ginseng [64], among others.
3.5. Effect on Oxidative Damage
3.5.1. Lipid Peroxidation (LP)
Inflammation, a hallmark of IBD, is closely linked to the generation of metabolites such as RONS, which contribute to nitro-oxidative damage present in the peripheral blood of IBD patients. These biomarker alterations occur as early as the onset of the disease, even in mild cases, and intensify as the disease progresses [388], particularly with the onset of severe complications, such as extraintestinal [389,390] manifestations (irreversible oxidative and inflammatory damage in the colon, liver, and kidney) and colorectal cancer (CRC) [17].
LP has emerged as a critical biological process resulting from nitro-oxidative stress as well as intestinal inflammation. Malondialdehyde (MDA) is one of the final products of lipid peroxidation and is the most measured metabolite in experimental and clinical research, especially in the form of thiobarbituric acid reactive substances (TBARS), evaluated in intestinal tissue.
High levels of MDA have been detected in the intestinal tissues of IBD patients, indicating increased LP and, consequently, oxidative/inflammatory damage. In this context, MDA quantification can serve as an indicator of the degree of stress and damage associated with IBD and provide useful information for assessing the severity of the disease and the efficacy of antioxidant therapeutic interventions [391,392]. However, TBARS, a traditional method for evaluating LP, has some limitations, such as a lack of specificity, as they can react with other compounds, leading to a superestimation of oxidative damage. TBARS are highly unstable and easily degradable, making comparison between different studies difficult. Another limitation is the intake of foods rich in polyunsaturated fatty acids (PUFAs), which can influence TBARS levels regardless of inflammatory activity.
Another promising biomarker of lipid membrane damage is F2-isoprostane, a metabolite of prostaglandin F2α (PGF2α), generated non-enzymatically by the peroxidation of PUFAs. Its urinary and plasma concentrations are increased in IBD patients and directly correlate with disease severity and inflammatory activity. Studies point to the superiority of F2-isoprostane over TBARS as a biomarker of oxidative damage due to its greater specificity and stability [393,394,395].
In this review, we found 69 studies (20%) reporting a decrease in damage caused by RONS. All these articles measured MDA or TBARS and observed positive effects of various compounds in minimizing lipid peroxidation. Substances such as carvacrol (5-isopropyl-2-methylphenol) [84] and omega 3 [311], among others like garlic oil [295], oat β-glucan [291], mangiferin [180], Veronica polita [197], red raspberries [199], and osthole [202], were particularly effective in reducing lipid damage.
In human studies, 11 studies (45.8%) reported natural products present in so-called medicinal plants and their chemical compounds that reduced LP as indicated by decreased levels of MDA levels: zingiber [376], resveratrol [369], saffron [371], and oxidized LDL, using Pistacia lentiscus [377].
Isoprostane F2α type III (iPF2α-III or 15-F2t IsoP) can be measured in biological fluids and tissues. iPF2α-III is one of several urinary markers studied as potential predictors of IBD activity. Urinary excretion of iPF2α-III has been correlated with clinical relapse and inflammation in CD patients [396]. A study investigating urinary iPF2α-III concentrations as an index of LP in CD patients compared to healthy controls and tested whether LP correlates with clinical relapse and inflammation in patients showed elevated urinary iPF2α-III concentrations in CD patients. Those with clinical relapse had the highest levels, while patients in clinical remission had levels similar to controls, suggesting that increased LP is associated with clinical relapse [397].
The combination of vitamin E (800 IU) and vitamin C (1000 mg) daily demonstrated a similar baseline effect and significantly reduced plasma F2-isoprostane levels after 4 weeks of vitamin supplementation compared to the placebo group. The plasma LP product was significantly higher in the vitamin group at baseline. However, the change after 4 weeks was significantly more favorable in the vitamin group compared to the placebo group [379].
3.5.2. Protein
As explained previously, the presence of RONS disrupts various proteins that constitute the mucosal epithelial barrier. These species can compromise TJ and AJ, which form the intercellular junctions of the intestinal epithelium, along with desmosomes. Epithelial TJs are known to form a barrier against allergens, toxins, and pathogens. The dephosphorylation of adherent proteins results in the breakdown of intercellular junctions, leading to barrier dysfunction and consequently increased intestinal permeability [12,21].
Carbonylated proteins, formed on various amino acid residues, such as arginine, histidine, lysine, proline, threonine, and cysteine, are the most widely used biomarkers for measuring protein oxidation. Due to their formation on multiple amino acid residues, carbonylation is more extensive and detectable compared to alterations on single amino acids, making it a critical marker for assessing oxidative damage in proteins [398]. Despite its importance, in this review, carbonylated proteins were not analyzed by the included studies, highlighting an unexplored gap in the research.
3.5.3. DNA
RONS, such as H2O2, O2-•, NO•, and free oxygen radicals, can cause DNA damage in IBD through various mechanisms, contributing to inflammation progression, tissue injury, and complications associated with these conditions. RONS can induce structural alterations and conformational changes in DNA, which impair the integrity of the genetic material [399]. These species can cause lipid peroxidation, leading to disruptions in the cell membrane, which further exacerbate oxidative stress. Additionally, RONS decrease the efficiency of DNA polymerase and DNA repair enzymes by causing oxidative damage to proteins and genes involved in these processes. This can impede DNA replication and repair mechanisms (apoptosis and necrosis), leading to accumulated mutations. RONS also activate cytoplasmic and nuclear signal transduction pathways, stimulating cell proliferation [400]. Additionally, RONS can oxidize nitrogenous bases, producing metabolites like 8-hydroxy-2-deoxyguanosine (8-OHdG), a widely evaluated biomarker for detecting DNA strand breaks [401,402,403].
Compounds such as melanin from Sepia pharaonis ink [345], riboflavin [293], glyceollin [192], aqueous extract of Raphanus sativus L. seeds [172], and Sorbus domestica [229] were identified in this review as antioxidants with protective effects on DNA. However, all these compounds were tested exclusively in animal models, with no human studies available, highlighting a significant gap in the research on antioxidants in human IBD.
3.6. Effect on Gut Microbiota
The role of the gut microbiota, comprising bacteria, viruses, fungi, and protozoa, in IBD has been the focus of extensive research over the years. However, defining and diagnosing dysbiosis remains a challenge, making the evaluation of the therapeutic properties of various substances an unresolved area of investigation [404].
The relationship between gut microbiota and oxidative stress is bidirectional. As discussed, the generation of reactive species without adequate neutralization, particularly •OH, NO•, and ONOO-, causes significant damage to macromolecules, especially lipid membranes. This leads to the destruction of epithelial tissue and increased intestinal permeability, allowing bacteria and bacterial products, such as lipopolysaccharides (LPS) produced by Gram-negative bacteria, to penetrate the previously sterile layer of the intestine (lamina propria), further exacerbating inflammation and oxidative stress [405].
The entry of bacteria activates immune cells such as neutrophils and natural killer lymphocytes and increases the generation of pro-inflammatory cytokines like TNF-α, IL-22, IL-6, and IL-17, which perpetuate the oxidative cascade through NF-κB activation. Additionally, LPS from intestinal bacteria is recognized by toll-like receptor type 4 (TLR4), which, through myeloid differentiation factor 88 (MyD88), activates NF-κB and stimulates the mitochondrial electron transport chain. As a consequence, there is increased generation of O2-•, reduced antioxidant defense, and thus, an enhanced redox imbalance [406].
In this review, 29.9% (n = 103) of the studies (Table 4) evaluated the action of antioxidants on the intestinal microbiota in experimental models. One study evaluated the use of the hormone melatonin [26] in animals with DSS-induced colitis. The authors identified through intestinal microbiota taxonomy that the feces of these animals contained seven phyla, with Bacteroidetes (58.9%) being the most abundant in the diseased group, followed by Firmicutes (31.5%) and Proteobacteria (8.0%). In contrast, Firmicutes were the most abundant phyla in the melatonin-treated group (49.5%), followed by Bacteroidetes (41.6%) and Proteobacteria (7.5%).
Similarly, Zhang and collaborators (2020) tested Flammulina velutipes polysaccharide (FVP) in preventing DSS-induced colitis and observed higher concentrations of Firmicutes, Bacteroidetes, and Proteobacteria in the diseased animals, which were restored to levels like healthy animals after FVP treatment.
Several studies included in this review demonstrated the effect of other active natural compounds from medicinal plants on the intestinal microbiota [70,116,148,258,269,306,329,407,408,409,410]. According to Xuan et al. (2020), it was observed that short-chain fatty acids (SCFAs) decreased after DSS-induced colitis, and treatment with galangin reversed these changes, increasing acetate and butyrate levels, which serve as essential fuel for colonocytes, thereby improving intestinal health. Additionally, galangin enriched specific bacterial populations that promoted SCFA production, such as Butyricimonas spp., mediated by its effects on intestinal microbiota remodeling.
Table 4.
General characteristics of animal studies that investigated action on the intestinal microbiota.
| Action on the Intestinal Microbiota | ||
|---|---|---|
| Author | Components | Dose/Time |
| Hormones | ||
| [26] | Melatonin | 0.2 mg/L melatonin in water 7 days |
| Synthetic compounds | ||
| [31] | ZnO nanoparticles (ZnONP) and ZnO microparticles (ZnOMP) | 0.5, 5, and 50 mg/kg ZnONPs; 50 mg/kg of ZnOMPs 7 days |
| [43] | (R,R)-BD-AcAc2 | Standard rodent food mixed with 4% (R,R)-BD-AcAc2 24 days |
| [45] | Nano-selenium modified with Eucommia ulmoides polysaccharide | 200 µL 5 days |
| [47] | Edible nanoparticles similar to exosomes from Portulaca oleracea L. | 20 mg/µL 5 days |
| Chemical products derived from sources other than plants | ||
| [57] | Inosine | 100 and 800 mg/kg 7 days |
| Polyphenols and other natural active compounds from medicinal plants | ||
| [70] | Grape seed proanthocyanidin extract | 50 mg/kg 21 days |
| [74] | Apple peel polyphenols (dried apple peel) | 200 and 400 mg/kg 10 days |
| [87] | p-Cymene (p-C) and rosmarinic acid (RA) | 25, 50, 100, and 200 mg/kg 48, 24, and 1 h before TNBS administration and 24 h after induction of inflammation. |
| [94] | Morusin | 12, 5, 25, or 50 mg/kg 5 days |
| [106] | Brazilian Berry (Myrciaria jaboticaba) Peel (EJP) aqueous extract | Short-term EJP (weeks 6 and 7) OR Long-term EJP (weeks 2 to 7) |
| [115] | Phloretin | 25, 50, and 100 mg/kg 7 days |
| [116] | Phloretin | 60 mg/kg 10 days for colitis 17 days for microbiota |
| [118] | Curcumin | 50 or 150 mg kg 7 days |
| [119] | Curcumin in hydroxyethyl starch microspheres | 6.8 mg/kg 7 days |
| [121] | Honey polyphenols | 10.5 mg/kg 7 days |
| [124] | Galangin | 15 mg/kg 7 days |
| [125] | Taxifolin | 100 mg/kg 14 days |
| [130] | Acacetin | 50 and 150 mg/kg 9 days |
| [132] | Juglone (JUG) | 0.04 mg/mL juglone 17 days |
| [140] | Kaempferol (Kae) | 50 mg/kg/day 14 days |
| [148] | Plant polyphenols (gallic acid, proanthocyanidin, ellagic acid, and tannic acid) | 100 mg/kg of each polyphenol 6 days |
| [158] | Resveratrol | 100 mg/kg 9 days |
| [159] | Resveratrol in polysaccharide-zein nanoparticles from Mesona chinensis | 10 mg/kg 14 days |
| [161] | Ligustroside | 1, 2, and 4 mg/kg 7 days |
| [163] | Forsythia suspensa polyphenols | 200, 400, and 600 mg/kg 7 days |
| [164] | Flavonoids from Callicarpa nudiflora Hook | 400 mg/kg 17 days |
| [166] | Diosmin | 100 and 200 mg/kg 7 days |
| [167] | Polyphenol extracts from Ziziphus jujuba Mill | 200 mg/mL 7 days |
| [178] | Polysaccharides from the edible mushroom Hericium erinaceus | 200, 300, and 400 mg/kg 7 days |
| [182] | Portulaca oleracea L. | 400 and 800 mg/kg 8 days |
| [189] | Aronia melanocarpa (Michx.) Elliott. | 100, 300, and 600 mg/kg 21 days |
| [199] | Red raspberries | 6 g/kg 6 weeks |
| [201] | Ipomoea asarifolia aqueous extract | 25, 50, and 100 mg/kg 3 days |
| [206] | Magnolol contained in a butyrate-derived polymer nanoplatform | 5 mg/kg 2, 4, 12, and 24 h |
| [215] | Terminalia arjuna hydroalcoholic extract | 125, 250, and 500 mg/kg 28 days |
| [217] | Quercetin aglycone (QUE) | QUE: 0.21%, QMQ: 0.36% 14 days |
| [218] | 2-O-β-d-Glucopyranosyl-l-ascorbic acid, an ascorbic acid derivative isolated from the fruits of Lycium Barbarum L. | 300 mg/kg 8 days |
| [233] | Bruguiera gymnorrhiza leaves | 25, 50, and 100 mg/kg 7 days |
| [234] | Arjunarishta | 1.8, 0.9, and 0.45 mL/kg 28 days |
| [235] | Antrocaryon micraster | 30, 100, and 300 mg/kg 3 days |
| [237] | Puerarin | 10 or 50 mg/kg 7 days |
| [238] | Rumex japonicus Houtt. | 100 mg/kg 14 days |
| [240] | Sinapic acid | 10 or 50 mg/kg 7 days |
| [244] | Sanhuang shu’ai | 0.8 or 1.6 g/kg 7 days |
| [245] | Flammuliana velutipes Polysaccharide | 50, 100, and 200 mg/kg 14 days |
| [253] | Cepharanthine | 10 mg/kg 7 days |
| [254] | Saposhnikovia divaricata | 50, 100, and 200 mg/kg 9 days |
| [258] | Echinacea purpurea polysaccharide | 200 mg/kg 21 days |
| [266] | Carboxymethyl Poria polysaccharides | 300 mg/kg/day |
| [268] | Polygonatum Cyrtonema Hua oligosaccharides | 0.5, 2, and 5 mg/kg 5 days |
| [269] | Polysaccharide from the fermented mycelium of Inonotus obliquus | 100, 200, and 400 mg/kg 7 days |
| [271] | Terminalia chebula ethyl acetate extract | 100 and 200 mg/kg 7 days |
| [272] | Ecklonia cava extract | 50, 100, 200, mg/kg 21 days |
| [275] | Sagittaria sagittifolia L. polysaccharides | 100, 200, and 400 mg/kg 14 days |
| [276] | Tetrastigma hemsleyanum root extract | 100 and 500 mg/kg 7 days |
| [278] | Selaginella uncinata (Desv.) Spring acidic polysaccharide | 50 and 100 mg/kg 14 days |
| [282] | Fraxetin | 10, 30, and 60 mg/kg 25 days |
| [283] | Honeysuckle | Extract added to the feed at 0.15, 0.75, and 1.5 g/kg 15 days |
| [284] | Passiflora edulis | 8 mg/mL in drinking water 5 days |
| [289] | Four sanshools of Zanthoxylum fruit | 2.5 mg/kg 7 days |
| [290] | Sea buckthorn | 200 mg/kg 21 days |
| [341] | Cyanobacterium Spirulina platensis Hydroalcoholic extracts | 100 or 200 mg/kg 15 days |
| [362] | Curcumin or resveratrol | Curcumin: 50 mg/kg or resveratrol: 80 mg/kg 7 days |
| [411] | Casein-quaternary chitosan complexes induced the soft assembly of egg white peptide and curcumin | 15 mg/kg 7 days |
| [412] | Pc-Fe nanozyme (procyanidin and free iron) |
100 mg/kg 7 days |
| [413] | Saffron | 20 mg/kg 10 days |
| [360] | Crocetin | 10 ou 40 mg/kg/dia 21 days |
| [414] | Hydroxysaffor yellow A | 3 ou 6 mg/mL 7 days |
| [408] | Phlorizin | 20, 40, and 80 mg/kg 7 days |
| [415] | Bay Laurel (Laurus nobilis L.) | Basal diet supplemented with 1 to 3% laurel 7 days |
| [416] | Okanin (Coreopsis tinctoria Nutt) | 10 mg/kg 7 days |
| [417] | Alhagi honey Polysaccharide | 200 and 400 mg/kg 7 days |
| [418] | Rhein (Rheum rhabarbarum) | 50 mg/kg and 100 mg/kg 15 days |
| [419] | Schisandrin | 20, 40, and 80 mg/kg 3 days before and 7 days after |
| [420] | Hypericum sampsonii Hance | 3, 6, and 12 mg/kg 8 days |
| Functional foods and nutrients | ||
| [285] | Jinxiang garlic (Allium sativum L.) | 200 or 400 mg/kg/day 14 days |
| [305] | Selenium in biogenic nanoparticles | 800 ng/kg 5 days |
| [306] | Camellia oil | 2 mL/kg 20 days |
| [309] | Cinnamon (Cinnamomum japonicum) subcritical water extract | 100, 300, or 500 mg/kg 21 days |
| [315] | Mannoglucan (Chinese yam) | 300 mg/kg per day 7 days |
| [317] | β-carotene | 50 mg/kg 7 days |
| [69] | Pumpkin polysaccharides | 50 and 100 mg/kg 7 days |
| [421] | Quinoa | 907 g/kg 7 days |
| [422] | α-tocopherol (αT) and tocopherol-rich γ-tocopheres (γTmT) |
0.05% in the diet 21 days |
| [423] | Ornithine α-ketoglutarate | 0.5%, 1.0%, and 1.5% 21 days |
| [424] | Sichuan pepper powder | Diet supplemented with 5% HJ powder 7 days |
| [425] | Ficus carica | 150 and 300 mg/kg 35 days |
| Probiotics | ||
| [325] | Bifidobacterium bifidum ATCC 29521 | 2 × 108 CFU/day 27 days |
| [329] |
Lactobacillus acidophilus KDSL 1.0901, Lactobacillus helveticus KDSL 1.8701, Lactobacillus plantarum KDSL 1.0318, and mixed lactobacilli |
1 × 109 CFU mL−1
21 days |
| [330] | Lactobacillus gasseri 4M13 | 750 mg kg 14 days |
| [331] | L. pentosus A14-6 and L. pentosus CMY46 |
L. pentosus A14-6, L. pentosus CMY46, L. pentosus A14-6 plus XOS, and L. pentosus CMY46 plus GOS. 1 × 109 CFU/200 μL/day. 7 days |
| [332] | Lactobacillus acidophilus C4 | 1 × 109 CFU/mL 7 days |
| [333] | Exopolysaccharide Ropy Bifidobacterium pseudocatenulatum Bi-OTA128 | 2 × 109 CFU in 0.2 mL of saline 21 days |
| [426] | Lactobacillus brevis Bmb6 | 109 UFC in 100 µL of PBS 14 days |
| Others | ||
| [334] | Insect (cockroach) Periplaneta americana | 200 mg/kg and 100 mg/kg 7 days |
| [343] | Saccharina japonica | 1, 2, and 4 g/kg 14 days |
| [345] | Melanin from Sepia pharaonis ink | 75, 150, and 300 mg/kg 9 days |
| [346] | Tuna bioactive peptides | 200 and 500 mg/kg 7 days |
| [347] | Turtle peptide | 500 mg/kg 7 days |
| [427] | Sargassum horneri | 100 mg/kg 4 semanas |
Legend: CFU = colony-forming unit; GOS = galactooligosaccharides; TNBS = 2,4,6-trinitrobenzenesulfonic acid; XOS = xylooligosaccharides.
4. Conclusions and Perspectives
Compared to our group’s last review in 2015, there has been a notable increase in clinical trials assessing oxidative stress markers in human subjects. Despite remaining questions—such as the appropriateness of antioxidant therapy during the active phase, the molecular action of antioxidants in humans versus animal models, the optimal duration for supplementation, and the most suitable antioxidants for CD or UC—important advances have been made in understanding antioxidant therapy. Key findings include: (1) antioxidant action primarily enhances defenses such as SOD and TAS/TAC, protecting against lipid membrane oxidative damage; and (2) antioxidants appear to be safe and effective for nonhospitalized IBD patients, improving both oxidative stress and inflammation markers.
A critical challenge that remains is the absence of population reference values for oxidative stress markers, which hampers the interpretation of clinical results in human studies. Without established reference points for normality, it is difficult to determine whether antioxidant therapy successfully normalizes or brings these markers closer to normal levels following treatment.
Future research must prioritize testing antioxidant substances isolated or combined with pharmacological treatment in human subjects. While discovering new therapeutic substances is vital, the significant discrepancy between the number of substances tested in animal models and those tested in humans must be addressed. Promoting clinical research with rigorous methodological designs is crucial. This includes detailed sample preparation protocols, ensuring adequate sample sizes, and repeating experiments to verify reproducibility. In fact, it is essential to thoroughly evaluate the advantages and disadvantages of different analytical methods used for assessing nitro-oxidative stress and antioxidant efficacy and mainly consider chemical properties, sensitivity, specificity, and the context in which each method performs best. Furthermore, incorporating supplemental materials such as detailed protocols, technical videos, and instructional content in publications or laboratory reports would significantly contribute to advancing the quality and transparency of antioxidant research.
This responsibility lies with public and private research centers and scientific journals. Only through comprehensive, high-quality data can we conduct meta-analyses that will lead to recommendations by international societies, ensuring the prescription of these substances, alone or along with conventional therapies. Thus, despite the significant progress made in the past 9 years, we are still far from reaching a definitive answer regarding the prescription of antioxidants for individuals with IBD. More rigorous and well-structured clinical trials are necessary to establish clear guidelines and determine the real impact of antioxidant therapy in these patients.
Acknowledgments
Scientific Initiation Program/Professor Alberto Antunes University Hospital (PIC/HUPAA), Scientific Initiation Program/UFAL (PBIC/UFAL), Natural Resources Chemistry Research Laboratory (LPqRN).
List of Abbreviations
5-ASA—5-aminosalicylic acid; ACG—American College of Gastroenterology; AJ—Adherens Junctions; CAM—Complementary and Alternative Medicine; CAT—catalase; CD—Crohn’s disease; CFU—colony-forming unit; COX—cyclooxygenase; CU—ulcerative colitis; DHEA—dehydroepiandrosterone; DNA—deoxyribonucleic acid; GIT—gastrointestinal tract; GOS—galactooligosaccharides; GPx—glutathione peroxidase; GR—glutathione reduced; GSH—reduced glutathione; GSSG—oxidized glutathione; IBD—inflammatory bowel diseases; IL—interleukin; INF-γ—interferon gamma; iNOS—inducible nitric oxide synthase; IκBα—nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; JAK—Janus kinase inhibitors; LOX—lipoxygenases; MAPK—mitogen-activated protein kinase; MDA—malondialdehyde; MPO—myeloperoxidase; MTX—methotrexate; MZ—mesalazine; NAC—N-acetylcysteine; NF-κB—nuclear factor kappa B; NOD2—nucleotide-binding oligomerization domain containing 2; NOS—inducible nitric oxide synthase isoforms; NOX—nicotinamide adenine dinucleotide phosphate oxidase; Nrf2—nuclear factor erythroid 2-related factor; PUFA—polyunsaturated fatty acids; RNS—reactive nitrogen species; RONS—reactive oxygen and nitrogen species; ROS—reactive oxygen species; SCFA—short-chain fatty acid(s); SNPs—single nucleotide polymorphisms; SOD—superoxide dismutase; SSZ—sulfasalazine; TAC—total antioxidant capacity; TAS—total antioxidant status; TBARS—thiobarbituric acid reactive substances; TJ—tight junctions; Trx—thioredoxin; TNBS—2,4,6-trinitrobenzenesulfonic acid; TNF-α—tumor necrosis factor alpha; TNFR—TNF-α receptor; TPs—thiopurines; XO—xanthine oxidase; XOS—xylooligosaccharides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13111369/s1, Figure S1A: Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included animal studies; Figure S1B: Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included randomized clinical trials studies; Table S1. Dose and time of oral supplementation of the studies in animals.
Author Contributions
L.E.M.d.S.X., formal analysis, investigation, writing—original draft and writing—review and editing; T.C.G.R., investigation and writing—original draft; A.S.d.P.M., conceptualization, formal analysis and writing—review and editing; J.C.d.F.S., conceptualization, investigation and writing—review and editing; N.B.B., data curation, formal analysis, methodology, and writing—review and editing; M.O.F.G., conceptualization, funding acquisition, supervision and writing—review and editing; and F.A.M., conceptualization, data curation, investigation, methodology, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was registered at Register of Systematic Reviews (PROSPERO) nº CDR42022335357 and CRD42022304540.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is an unpublished study that is not undergoing the submission process in any other scientific journal. All data are privately accessible.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The financial support was provided by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) [435704/2018-4], INCT-Bioanalítica (Instituto Nacional de Ciências e Tecnologia em Bioanalítica) [465389/2014-7], CAPES/PROEX (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Programa de excelência Acadêmica) [88881.894490/2023-01], and FAPEAL/PPSUS (Fundação de Amparo à Pesquisa do Estado de Alagoas/Programa Pesquisa para o SUS, Ministério da Saúde) [60030-00879].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tian T., Wang Z., Zhang J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang R., Li Z., Liu S., Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13:e065186. doi: 10.1136/bmjopen-2022-065186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Z., Wang S., Li J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021;8:765474. doi: 10.3389/fmed.2021.765474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quera R., Nunez P., Sicilia B., Flores L., Gomollon F. Corticosteroids in inflammatory bowel disease: Are they still a therapeutic option? Gastroenterol. Hepatol. 2023;46:716–726. doi: 10.1016/j.gastrohep.2022.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Bentin J. Mechanism of action of cyclosporin in rheumatoid arthritis. Clin. Rheumatol. 1995;14:22–25. doi: 10.1007/BF02215854. [DOI] [PubMed] [Google Scholar]
- 6.Na S.-Y., Moon W. Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease. Gut Liver. 2019;13:604–616. doi: 10.5009/gnl19019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima H., Munakata A., Yoshida Y. Adverse effects of sulfasalazine and treatment of ulcerative colitis with mesalazine. J. Gastroenterol. 1995;30((Suppl. S8)):115–117. [PubMed] [Google Scholar]
- 8.Katz J.A. Treatment of inflammatory bowel disease with corticosteroids. Gastroenterol. Clin. N. Am. 2004;33:171–189. doi: 10.1016/j.gtc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Yewale R.V., Ramakrishna B.S., Doraisamy B.V., Basumani P., Venkataraman J., Jayaraman K., Murali A., Premkumar K., Kumar A.S. Long-term safety and effectiveness of azathioprine in the management of inflammatory bowel disease: A real-world experience. JGH Open. 2023;7:599–609. doi: 10.1002/jgh3.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihai I.R., Burlui A.M., Rezus I.I., Mihai C., Macovei L.A., Cardoneanu A., Gavrilescu O., Dranga M., Rezus E. Inflammatory Bowel Disease as a Paradoxical Reaction to Anti-TNF-α Treatment—A Review. Life. 2023;13:1779. doi: 10.3390/life13081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourgonje A.R., Feelisch M., Faber K.N., Pasch A., Dijkstra G., van Goor H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020;26:1034–1046. doi: 10.1016/j.molmed.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Moura F.A., de Andrade K.Q., dos Santos J.C., Araujo O.R., Goulart M.O. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015;6:617–639. doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Biasi F., Astegiano M., Maina M., Leonarduzzi G., Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011;18:4851–4865. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 15.Liu P., Li Y., Wang R., Ren F., Wang X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines. 2021;10:85. doi: 10.3390/biomedicines10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmus I.M., Ciobica A., Trifan A., Stanciu C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi, J. Gastroenterol. 2016;22:3–17. doi: 10.4103/1319-3767.173753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moura F.A., Goulart M.O.F., Campos S.B.G., da Paz Martins A.S. The Close Interplay of Nitro-Oxidative Stress, Advanced Glycation end Products and Inflammation in Inflammatory Bowel Diseases. Curr. Med. Chem. 2020;27:2059–2076. doi: 10.2174/0929867325666180904115633. [DOI] [PubMed] [Google Scholar]
- 18.Tobon-Velasco J.C., Cuevas E., Torres-Ramos M.A. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets. 2014;13:1615–1626. doi: 10.2174/1871527313666140806144831. [DOI] [PubMed] [Google Scholar]
- 19.Canton M., Sánchez-Rodríguez R., Spera I., Venegas F.C., Favia M., Viola A., Castegna A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021;12:734229. doi: 10.3389/fimmu.2021.734229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranjan K. Intestinal Immune Homeostasis and Inflammatory Bowel Disease: A Perspective on Intracellular Response Mechanisms. Gastrointest. Disord. 2020;2:246–266. doi: 10.3390/gidisord2030024. [DOI] [Google Scholar]
- 21.Alves A.J.T., Jr., Goto E.F.K., Pereira J.A., Domingues F.A., Ávila M.G.d., Coy C.S.R., Martinez C.A.R. Expressão de e-caderina e claudina-3 no epitélio cólico após terapia com infliximabe: Modelo experimental de colite de exclusão. ABCD Arq. Bras. Cir. Dig. 2021;34:e1639. doi: 10.1590/0102-672020210002e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterbourn C.C., Kettle A.J., Hampton M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 23.Mourenza Á., Gil J.A., Mateos L.M., Letek M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants. 2020;9:361. doi: 10.3390/antiox9050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krzystek-Korpacka M., Kempiński R., Bromke M.A., Neubauer K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics. 2020;10:601. doi: 10.3390/diagnostics10080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu D., Ma Y., Ding S., Jiang H., Fang J. Effects of Melatonin on Intestinal Microbiota and Oxidative Stress in Colitis Mice. BioMed Res. Int. 2018;2018:2607679. doi: 10.1155/2018/2607679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J., Lu M., Yan W., Li L., Ma H. Dehydroepiandrosterone alleviates intestinal inflammatory damage via GPR30-mediated Nrf2 activation and NLRP3 inflammasome inhibition in colitis mice. Free Radic. Biol. Med. 2021;172:386–402. doi: 10.1016/j.freeradbiomed.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Elhassan Y.H. Anti-inflammatory, anti-apoptotic, and antioxidant effects of obestatin on the colonic mucosa following acetic acid-induced colitis. Folia Morphol. 2023;82:641–655. doi: 10.5603/FM.a2022.0071. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y., Sun Y., Ding Y., Wang X., Zhou Y., Li W., Huang S., Li Z., Kong L., Guo Q., et al. GL-V9, a new synthetic flavonoid derivative, ameliorates DSS-induced colitis against oxidative stress by up-regulating Trx-1 expression via activation of AMPK/FOXO3a pathway. Oncotarget. 2015;6:26291–26307. doi: 10.18632/oncotarget.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong C.-O., Rhee C.H., Pyo M.C., Lee K.-W. Anti-inflammatory effect of glucose-lysine Maillard reaction products on intestinal inflammation model in vivo. Int. Immunopharmacol. 2017;52:324–332. doi: 10.1016/j.intimp.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Chen H., Wang B., Cai C., Yang X., Chai Z., Feng W. ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci. Rep. 2017;7:43126. doi: 10.1038/srep43126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagarjun S., Dhadde S.B., Veerapur V.P., Thippeswamy B., Chandakavathe B.N. Ameliorative effect of chromium-d-phenylalanine complex on indomethacin-induced inflammatory bowel disease in rats. Biomed. Pharmacother. 2017;89:1061–1066. doi: 10.1016/j.biopha.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 33.Shen N.-Y., Bi J.-B., Zhang J.-Y., Zhang S.-M., Gu J.-X., Qu K., Liu C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J. Gastroenterol. 2017;23:1375–1386. doi: 10.3748/wjg.v23.i8.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarzecki M.S., Bortolotto V.C., Poetini M.R., Araujo S.M., de Paula M.T., Roman S.S., Spiazzi C., Cibin F.W., Rodrigues O.E., Jesse C.R., et al. Anti-Inflammatory and Anti-Oxidant Effects of p-Chloro-phenyl-selenoesterol on TNBS-Induced Inflammatory Bowel Disease in Mice. J. Cell. Biochem. 2017;118:709–717. doi: 10.1002/jcb.25670. [DOI] [PubMed] [Google Scholar]
- 35.Zhu C., Zhang S., Song C., Zhang Y., Ling Q., Hoffmann P.R., Li J., Chen T., Zheng W., Huang Z. Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-κB mediated hyper inflammation. J. Nanobiotechnol. 2017;15:20. doi: 10.1186/s12951-017-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji Y., Dai Z., Sun S., Ma X., Yang Y., Tso P., Wu G., Wu Z. Hydroxyproline Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice: Involvment of the NF-κB Signaling and Oxidative Stress. Mol. Nutr. Food Res. 2018;62:e1800494. doi: 10.1002/mnfr.201800494. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y., Bai D., Zhao Y., Zhu Q., Zhou Y., Li Z., Lu N. LL202 ameliorates colitis against oxidative stress of macrophage by activation of the Nrf2/HO-1 pathway. J. Cell. Physiol. 2019;234:10625–10639. doi: 10.1002/jcp.27739. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y., Zheng Z., Li C., Pan Y., Tang X., Wang X.J. Synthetic Imine Resveratrol Analog 2-Methoxyl-3,6-Dihydroxyl-IRA Ameliorates Colitis by Activating Protective Nrf2 Pathway and Inhibiting NLRP3 Expression. Oxidative Med. Cell. Longev. 2019;2019:7180284. doi: 10.1155/2019/7180284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei Y., Wang Z., Zhang Y., Wan T., Xue J., He W., Luo Y., Xu Y., Bai X., Wang Q. FA-97, a New Synthetic Caffeic Acid Phenethyl Ester Derivative, Ameliorates DSS-Induced Colitis Against Oxidative Stress by Activating Nrf2/HO-1 Pathway. Front. Immunol. 2019;10:2969. doi: 10.3389/fimmu.2019.02969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng J.D., He Y., Yu H.Y., Liu Y.L., Ge Y.X., Li X.T., Li X., Wang Y., Guo M.-R., Qu Y.-L., et al. Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation. World J. Gastroenterol. 2019;25:1865–1878. doi: 10.3748/wjg.v25.i15.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed O., Abdel-Halim M., Farid A., Elamir A. Taurine loaded chitosan-pectin nanoparticle shows curative effect against acetic acid-induced colitis in rats. Chem. -Biol. Interact. 2022;351:109715. doi: 10.1016/j.cbi.2021.109715. [DOI] [PubMed] [Google Scholar]
- 42.Liu C., Yan X., Zhang Y., Yang M., Ma Y., Zhang Y., Xu Q., Tu K., Zhang M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022;20:206. doi: 10.1186/s12951-022-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saber S., Alamri M.M.S., Alfaifi J., Saleh L.A., Abdel-Ghany S., Aboregela A.M., Farrag A.A., Almaeen A.H., Adam M.I.E., AlQahtani A.A.J., et al. (R,R)-BD-AcAc2 Mitigates Chronic Colitis in Rats: A Promising Multi-Pronged Approach Modulating Inflammasome Activity, Autophagy, and Pyroptosis. Pharmaceuticals. 2023;16:28. doi: 10.3390/ph16070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Török S., Almási N., Veszelka M., Börzsei D., Szabó R., Varga C. Protective Effects of H2S Donor Treatment in Experimental Colitis: A Focus on Antioxidants. Antioxidants. 2023;12:1025. doi: 10.3390/antiox12051025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye R., Guo Q., Huang J., Wang Z., Chen Y., Dong Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnol. 2023;21:222. doi: 10.1186/s12951-023-01965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeeshan M., Ain Q.U., Weigmann B., Story D., Smith B.R., Ali H. Dual pH and microbial-sensitive galactosylated polymeric nanocargoes for multi-level targeting to combat ulcerative colitis. Asian J. Pharm. Sci. 2023;18:100831. doi: 10.1016/j.ajps.2023.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu M.-Z., Xu H.-M., Liang Y.-J., Xu J., Yue N.-N., Zhang Y., Tian C.M., Yao J., Wang L.S., Nie Y.Q., et al. Edible exosome-like nanoparticles from Portulaca oleracea L. mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J. Nanobiotechnol. 2023;21:19. doi: 10.1186/s12951-023-02065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadar S.S., Vyawahare N.S., Bodhankar S.L. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, iNOs, COX-2, and apoptosis in laboratory rats. EXCLI J. 2016;15:482–499. doi: 10.17179/excli2016-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghasemi-Dehnoo M., Amini-Khoei H., Lorigooini Z., AnjomShoa M., Rafieian-Kopaei M. Ferulic acid ameliorates ulcerative colitis in a rat model via the inhibition of two LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways and thus alleviating the inflammatory, oxidative and apoptotic conditions in the colon tissue. Inflammopharmacology. 2023;31:2587–2597. doi: 10.1007/s10787-023-01277-y. [DOI] [PubMed] [Google Scholar]
- 50.Chen T., Chen L., Luo F., Xu Y., Wu D., Li Y., Zhao R., Hua Z., Hu J. Efficient oral delivery of resveratrol-loaded cyclodextrin-metal organic framework for alleviation of ulcerative colitis. Int. J. Pharm. 2023;646:123496. doi: 10.1016/j.ijpharm.2023.123496. [DOI] [PubMed] [Google Scholar]
- 51.Moura F.A., de Andrade K.Q., de Araújo O.R.P., Nunes-Souza V., Santos J.C.d.F., Rabelo L.A., Goulart M.O. Colonic and Hepatic Modulation by Lipoic Acid and/or N-Acetylcysteine Supplementation in Mild Ulcerative Colitis Induced by Dextran Sodium Sulfate in Rats. Oxidative Med. Cell. Longev. 2016;2016:4047362. doi: 10.1155/2016/4047362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu C., Ling Q., Cai Z., Wang Y., Zhang Y., Hoffmann P.R., Zheng W., Zhou T., Huang Z. Selenium-Containing Phycocyanin from Se-Enriched Spirulina platensis Reduces Inflammation in Dextran Sulfate Sodium-Induced Colitis by Inhibiting NF-κB Activation. J. Agric. Food Chem. 2016;64:5060–5070. doi: 10.1021/acs.jafc.6b01308. [DOI] [PubMed] [Google Scholar]
- 53.Li Y., Pan X., Yin M., Li C., Han L. Preventive Effect of Lycopene in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice through the Regulation of TLR4/TRIF/NF-κB Signaling Pathway and Tight Junctions. J. Agric. Food Chem. 2021;69:13500–13509. doi: 10.1021/acs.jafc.1c05128. [DOI] [PubMed] [Google Scholar]
- 54.Ahmedy O.A., Ibrahim S.M., Salem H.H., Kandil E.A. Antiulcerogenic effect of melittin via mitigating TLR4/TRAF6 mediated NF-κB and p38MAPK pathways in acetic acid-induced ulcerative colitis in mice. Chem. -Biol. Interact. 2020;331:109276. doi: 10.1016/j.cbi.2020.109276. [DOI] [PubMed] [Google Scholar]
- 55.Xiang X., Jiang Q., Shao W., Li J., Zhou Y., Chen L., Deng S., Zheng B., Chen Y. Protective Effects of Shrimp Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Nutr. 2021;8:773064. doi: 10.3389/fnut.2021.773064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo K., Tang K., Liang Y., Xu Y., Sheng K., Kong X., Wang J., Zhu F., Zha X., Wang Y. Purification and antioxidant and anti-Inflammatory activity of extracellular polysaccharopeptide from sanghuang mushroom, Sanghuangporus lonicericola. J. Sci. Food Agric. 2021;101:1009–1020. doi: 10.1002/jsfa.10709. [DOI] [PubMed] [Google Scholar]
- 57.Guo W., Tang X., Zhang Q., Zhao J., Mao B., Zhang H., Cui S. Mitigation of Dextran-Sodium-Sulfate-Induced Colitis in Mice through Oral Administration of Microbiome-Derived Inosine and Its Underlying Mechanisms. Int. J. Mol. Sci. 2023;24:13852. doi: 10.3390/ijms241813852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silveira A.K., Gomes H.M., Fröhlich N.T., Possa L., Santos L., Kessler F., Martins A., Rodrigues M.S., De Oliveira J., do Nascimento N.D., et al. Sodium Butyrate Protects Against Intestinal Oxidative Damage and Neuroinflammation in the Prefrontal Cortex of Ulcerative Colitis Mice Model. Immunol. Investig. 2023;52:796–814. doi: 10.1080/08820139.2023.2244967. [DOI] [PubMed] [Google Scholar]
- 59.Ullah H., Deng T., Ali M., Farooqui N.A., Alsholi D.M., Siddiqui N.Z., Rehman A.U., Ali S., Ilyas M., Wang L., et al. Sea Conch Peptides Hydrolysate Alleviates DSS-Induced Colitis in Mice through Immune Modulation and Gut Microbiota Restoration. Molecules. 2023;28:21. doi: 10.3390/molecules28196849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W., Zhang X., Lv X., Qu A., Liang W., Wang L., Zhao P., Wu Z. Oral Delivery of Astaxanthin via Carboxymethyl Chitosan-Modified Nanoparticles for Ulcerative Colitis Treatment. Molecules. 2024;29:1291. doi: 10.3390/molecules29061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakai S., Nishida A., Ohno M., Inatomi O., Bamba S., Sugimoto M., Kawahara M., Andoh A. Astaxanthin, a xanthophyll carotenoid, prevents development of dextran sulphate sodium-induced murine colitis. J. Clin. Biochem. Nutr. 2019;64:66–72. doi: 10.3164/jcbn.18-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adjadj M., Boumerfeg S., Noureddine C., Baghiani A., Khennouf S., Arrar L., Mubarak M.S. Protective Effect of Paronychia argentea L. on Acetic Acid Induced Ulcerative Colitis in Mice by Regulating Antioxidant Parameters and inflammatory Markers. Wulfenia J. 2015;22:148–172. [Google Scholar]
- 63.Costa C., Tanimoto A., Quaglio A., Almeida L., Severi J., Di Stasi L.C. Anti-inflammatory effects of Brazilian ginseng (Pfaffia paniculata) on TNBS-induced intestinal inflammation: Experimental evidence. Int. Immunopharmacol. 2015;28:459–469. doi: 10.1016/j.intimp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Chaparala A., Tashkandi H., Chumanevich A.A., Witalison E.E., Windust A., Cui T., Nagarkatti M., Nagarkatti P., Hofseth L.J. Molecules from American Ginseng Suppress Colitis through Nuclear Factor Erythroid-2-Related Factor 2. Nutrients. 2020;12:1850. doi: 10.3390/nu12061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaparala A., Poudyal D., Tashkandi H., Witalison E.E., Chumanevich A.A., Hofseth J.L., Nguyen I., Hardy O., Pittman D.L., Wyatt M.D., et al. Panaxynol, a bioactive component of American ginseng, targets macrophages and suppresses colitis in mice. Oncotarget. 2020;11:2026–2036. doi: 10.18632/oncotarget.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Melo M.N., Soares L.A.L., Porto C.R.d.C., de Araújo A.A., Almeida M.d.G., de Souza T.P., Petrovick P.R., de Araújo R.F., Jr., Guerra G.C. Spray-dried extract of Phyllanthus niruri L. reduces mucosal damage in rats with intestinal inflammation. J. Pharm. Pharmacol. 2015;67:1107–1118. doi: 10.1111/jphp.12408. [DOI] [PubMed] [Google Scholar]
- 67.Bastaki S.M.A., Al Ahmed M.M., Al Zaabi A., Amir N., Adeghate E. Effect of turmeric on colon histology, body weight, ulcer, IL-23, MPO and glutathione in acetic-acid-induced inflammatory bowel disease in rats. BMC Complement. Altern. Med. 2016;16:72. doi: 10.1186/s12906-016-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boussenna A., Joubert-Zakeyh J., Fraisse D., Pereira B., Vasson M.-P., Texier O., Felgines C. Dietary Supplementation with a Low Dose of Polyphenol-Rich Grape Pomace Extract Prevents Dextran Sulfate Sodium-Induced Colitis in Rats. J. Med. Food. 2016;19:755–758. doi: 10.1089/jmf.2015.0124. [DOI] [PubMed] [Google Scholar]
- 69.Chu L., Zhang S., Wu W., Gong Y., Chen Z., Wen Y., Wang Y., Wang L. Grape seed proanthocyanidin extract alleviates inflammation in experimental colitis mice by inhibiting NF-κB signaling pathway. Environ. Toxicol. 2024;39:2572–2582. doi: 10.1002/tox.24129. [DOI] [PubMed] [Google Scholar]
- 70.Sheng K., Zhang G., Sun M., He S., Kong X., Wang J., Zhu F., Zha X., Wang Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020;11:7817–7829. doi: 10.1039/D0FO01418D. [DOI] [PubMed] [Google Scholar]
- 71.Choi Y.H., Bae J.-K., Chae H.-S., Nhoek P., Choi J.-S., Nhoek P., Choi J.S., Chin Y.W. Isoliquiritigenin ameliorates dextran sulfate sodium-induced colitis through the inhibition of MAPK pathway. Int. Immunopharmacol. 2016;31:223–232. doi: 10.1016/j.intimp.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 72.Cibiček N., Roubalová L., Vrba J., Zatloukalová M., Ehrmann J., Zapletalová J., Večeřa R., Křen V., Ulrichová J. Protective effect of isoquercitrin against acute dextran sulfate sodium-induced rat colitis depends on the severity of tissue damage. Pharmacol. Rep. 2016;68:1197–1204. doi: 10.1016/j.pharep.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Dönder Y., Arikan T.B., Baykan M., Akyüz M., Öz A.B. Effects of quercitrin on bacterial translocation in a rat model of experimental colitis. Asian, J. Surg. 2018;41:543–550. doi: 10.1016/j.asjsur.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Denis M.-C., Roy D., Yeganeh P.R., Desjardins Y., Varin T., Haddad N., Amre D., Sané A.T., Garofalo C., Furtos A., et al. Apple peel polyphenols: A key player in the prevention and treatment of experimental inflammatory bowel disease. Clin. Sci. 2016;130:2217–2237. doi: 10.1042/CS20160524. [DOI] [PubMed] [Google Scholar]
- 75.Pastrelo M.M., Ribeiro C.C.D., Duarte J.W., Gollucke A.P.B., Artigiani-Neto R., Ribeiro D.A., Miszputen S.J., Fujiyama Oshima C.T., Ribeiro Paiotti A.P. Effect of concentrated apple extract on experimental colitis induced by acetic acid. Int. J. Mol. Cell. Med. 2017;6:38–49. [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Shen L., Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016;40:24–31. doi: 10.1016/j.intimp.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 77.Li B., Guo Y., Jia X., Cai Y., Zhang Y., Yang Q. Luteolin alleviates ulcerative colitis in rats via regulating immune response, oxidative stress, and metabolic profiling. Open Med. 2023;18:20230785. doi: 10.1515/med-2023-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sahu B.D., Kumar J.M., Sistla R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-kappa B signaling. J. Nutr. Biochem. 2016;28:171–182. doi: 10.1016/j.jnutbio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 79.Khosropour P., Sajjadi S.E., Talebi A., Minaiyan M. Anti-inflammatory effect of Myrtus communis hydroalcoholic extract and essential oil on acetic acid-induced colitis in rats. J. Rep. Pharm. Sci. 2019;8:204–210. [Google Scholar]
- 80.Sen A., Yuksel M., Bulut G., Bitis L., Ercan F., Ozyilmaz-Yay N., Ozben A., Hamit C., Sevil O., Goksel S. Therapeutic Potential of Myrtus communis Subsp.communis Extract Against Acetic ACID-Induced Colonic Inflammation in Rats. J. Food Biochem. 2017;41:e12297. doi: 10.1111/jfbc.12297. [DOI] [Google Scholar]
- 81.Zhang H., Deng A., Zhang Z., Yu Z., Liu Y., Peng S., Wu L., Qin H., Wang W. The protective effect of epicatechin on experimental ulcerative colitis in mice is mediated by increasing antioxidation and by the inhibition of NF-κB pathway. Pharmacol. Rep. 2016;68:514–520. doi: 10.1016/j.pharep.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Anzoise M.L., Basso A.R., Del Mauro J.S., Carranza A., Ordieres G.L., Gorzalczany S. Potential usefulness of methyl gallate in the treatment of experimental colitis. Inflammopharmacology. 2018;26:839–849. doi: 10.1007/s10787-017-0412-6. [DOI] [PubMed] [Google Scholar]
- 83.de Morais Lima G.R., Machado F.D., Périco L.L., de Faria F.M., Luiz-Ferreira A., Souza Brito A.R., Pellizzon C.H., Hiruma-Lima C.A., Tavares J.F., Barbosa Filho J.M., et al. Anti-inflammatory intestinal activity of Combretum duarteanum Cambess. in trinitrobenzene sulfonic acid colitis model. World J. Gastroenterol. 2017;23:1353–1366. doi: 10.3748/wjg.v23.i8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Santana Souza M.T., Teixeira D.F., de Oliveira J.P., Oliveira A.S., Quintans-Júnior L.J., Correa C.B., Camargo E.A. Protective effect of carvacrol on acetic acid-induced colitis. Biomed. Pharmacother. 2017;96:313–319. doi: 10.1016/j.biopha.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 85.Dey Y.N., Sharma G., Wanjari M.M., Kumar D., Lomash V., Jadhav A.D. Beneficial effect of Amorphophallus paeoniifolius tuber on experimental ulcerative colitis in rats. Pharm. Biol. 2017;55:53–62. doi: 10.1080/13880209.2016.1226904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin B.R., Chung K.S., Cheon S.Y., Lee M., Hwang S., Noh Hwang S., Rhee K.J., An H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017;7:46252. doi: 10.1038/srep46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Formiga R.d.O., Júnior E.B.A., Vasconcelos R.C., Guerra G.C.B., de Araújo A.A., de Carvalho T.G., Garcia V.B., de Araújo Junior R.F., Gadelha F.A.A.F., Vieira G.C., et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020;21:5870. doi: 10.3390/ijms21165870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marinho S., Illanes M., Ávila-Román J., Motilva V., Talero E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules. 2021;11:162. doi: 10.3390/biom11020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim Y., Wu A.G., Jaja-Chimedza A., Graf B.L., Waterman C., Verzi M.P., Raskin I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE. 2017;12:e0184709. doi: 10.1371/journal.pone.0184709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murugan R., Saravanan S., Parimelazhagan T. Study of intestinal anti-inflammatory activity of Phoenix loureiroi Kunth (Arecaceae) fruit. Biomed. Pharmacother. 2017;93:156–164. doi: 10.1016/j.biopha.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 91.Patel P.P., Trivedi N.D. Effect of karanjin on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in Balb/c mice. Indian, J. Pharmacol. 2017;49:161–167. doi: 10.4103/ijp.IJP_234_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suluvoy J.K., Sakthivel K.M., Guruvayoorappan C., Grace V.B. Protective effect of Averrhoa bilimbi L. fruit extract on ulcerative colitis in wistar rats via regulation of inflammatory mediators and cytokines. Biomed. Pharmacother. 2017;91:1113–1121. doi: 10.1016/j.biopha.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 93.Vezza T., Algieri F., Rodríguez-Nogales A., Garrido-Mesa J., Utrilla M.P., Talhaoui N., Gómez-Caravaca A.M., Segura-Carretero A., Rodríguez-Cabezas M.E., Monteleone G., et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017;61:1601066. doi: 10.1002/mnfr.201601066. [DOI] [PubMed] [Google Scholar]
- 94.Vochyánová Z., Pokorná M., Rotrekl D., Smékal V., Fictum P., Suchý P., Gajdziok J., Šmejkal K., Hošek J. Prenylated flavonoid morusin protects against TNBS-induced colitis in rats. PLoS ONE. 2017;12:e0182464. doi: 10.1371/journal.pone.0182464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang B., Wu C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp. Ther. Med. 2017;14:276–282. doi: 10.3892/etm.2017.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu B., Li Y.L., Xu M., Yu C.C., Lian M.Q., Tang Z.Y., Li C.X., Lin Y. Geniposide ameliorates TNBS-induced experimental colitis in rats via reducing inflammatory cytokine release and restoring impaired intestinal barrier function. Acta Pharmacol. Sinica. 2017;38:688–698. doi: 10.1038/aps.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Z., Li Y., Shen P., Li S., Lu X., Liu J., Cao Y., Liu B., Fu Y., Zhang N. Administration of geniposide ameliorates dextran sulfate sodium-induced colitis in mice via inhibition of inflammation and mucosal damage. Int. Immunopharmacol. 2017;49:168–177. doi: 10.1016/j.intimp.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 98.Zhuge X., Jin X., Ji T., Li R., Xue L., Yu W., Quan Z., Tong H., Xu F. Geniposide ameliorates dextran sulfate sodium-induced ulcerative colitis via KEAP1-Nrf2 signaling pathway. J. Ethnopharmacol. 2023;314:116626. doi: 10.1016/j.jep.2023.116626. [DOI] [PubMed] [Google Scholar]
- 99.Yang L., Shen L., Li Y., Li Y., Yu S., Wang S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J. Inflamm. 2017;14:25. doi: 10.1186/s12950-017-0172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alabi Q.K., Akomolafe R.O., Omole J.G., Adefisayo M.A., Ogundipe O.L., Aturamu A., Sanya J.O. Polyphenol-rich extract of Ocimum gratissimum leaves ameliorates colitis via attenuating colonic mucosa injury and regulating pro-inflammatory cytokines production and oxidative stress. Biomed. Pharmacother. 2018;103:812–822. doi: 10.1016/j.biopha.2018.04.071. [DOI] [PubMed] [Google Scholar]
- 101.Abiodun O.O., Nwadike N., Ogunleye F.N., Sosanya A.S. Ocimum gratissimum Linn. (Lamiaceae) protects wistar rats against inflammation and oxidative stress in trinitrobenzene sulfonic acid-induced colitis. Thai J. Pharm. Sci. 2020;44:136–144. doi: 10.56808/3027-7922.2445. [DOI] [Google Scholar]
- 102.Gupta R.A., Motiwala M.N., Mahajan U.N., Sabre S.G. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. J. Ethnopharmacol. 2018;219:222–232. doi: 10.1016/j.jep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 103.Kim Kim K.J., Park J.M., Lee J.S., Kim Y.S., Kangwan N., Han Y.M., Kang E.A., An J.M., Park Y.K., Hahm K.B. Oligonol prevented the relapse of dextran sulfate sodium-ulcerative colitis through enhancing NRF2-mediated antioxidative defense mechanism. J. Physiol. Pharmacol. 2018;69:359–371. doi: 10.26402/jpp.2018.3.03. [DOI] [PubMed] [Google Scholar]
- 104.Chamanara M., Abdollahi A., Rezayat S.M., Ghazi-Khansari M., Dehpour A., Nassireslami E., Rashidian A. Thymol reduces acetic acid-induced inflammatory response through inhibition of NF-kB signaling pathway in rat colon tissue. Inflammopharmacology. 2019;27:1275–1283. doi: 10.1007/s10787-019-00583-8. [DOI] [PubMed] [Google Scholar]
- 105.Tahmasebi P., Froushani S.M.A., Ahangaran N.A. Thymol has beneficial effects on the experimental model of ulcerative colitis. Avicenna J. Phytomed. 2019;9:538–550. doi: 10.22038/AJP.2019.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.da Silva-Maia J.K., Batista G., Cazarin C.B.B., Soares E.S., Junior S.B., Leal R.F., da Cruz-Höfling M.A., Maróstica Junior M.R. Aqueous Extract of Brazilian Berry (Myrciaria jaboticaba) Peel Improves Inflammatory Parameters and Modulates Lactobacillus and Bifidobacterium in Rats with Induced-Colitis. Nutrients. 2019;11:2776. doi: 10.3390/nu11112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du Y., Ding H., Vanarsa K., Soomro S., Baig S., Hicks J., Mohan C. Low dose Epigallocatechin Gallate Alleviates Experimental Colitis by Subduing Inflammatory Cells and Cytokines, and Improving Intestinal Permeability. Nutrients. 2019;11:1743. doi: 10.3390/nu11081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bitzer Z.T., Elias R.J., Vijay-Kumar M., Lambert J.D. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016;60:2267–2274. doi: 10.1002/mnfr.201501042. [DOI] [PubMed] [Google Scholar]
- 109.Bing X., Xuelei L., Wanwei D., Linlang L., Keyan C. EGCG Maintains Th1/Th2 Balance and Mitigates Ulcerative Colitis Induced by Dextran Sulfate Sodium through TLR4/MyD88/NF-κB Signaling Pathway in Rats. Can. J. Gastroenterol. Hepatol. 2017;2017:3057268. doi: 10.1155/2017/3057268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Firoozi D., Nekooeian A.A., Tanideh N., Mazloom Z., Mokhtari M., Sartang M.M. The Healing Effects of Hydroalcoholic Extract of Carum Copticum L. on Experimental Colitis in Rats. Iran. J. Med. Sci. 2019;44:501–510. doi: 10.30476/ijms.2019.44961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guo K., Ren J., Gu G., Wang G., Gong W., Wu X.W., Ren H., Hong Z., Li J. Hesperidin Protects Against Intestinal Inflammation by Restoring Intestinal Barrier Function and Up-Regulating Treg Cells. Mol. Nutr. Food Res. 2019;63:e1800975. doi: 10.1002/mnfr.201800975. [DOI] [PubMed] [Google Scholar]
- 112.Guazelli C.F., Fattori V., Ferraz C.R., Borghi S.M., Casagrande R., Baracat M.M., Verri W.A.J. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Interact. 2021;333:109315. doi: 10.1016/j.cbi.2020.109315. [DOI] [PubMed] [Google Scholar]
- 113.Shafik N.M., Gaber R.A., Mohamed D.A., Ebeid A.M. Hesperidin modulates dextran sulfate sodium-induced ulcerative colitis in rats: Targeting sphingosine kinase-1-sphingosine 1 phosphate signaling pathway, mitochondrial biogenesis, inflammation, and apoptosis. J. Biochem. Mol. Toxicol. 2019;33:e22312. doi: 10.1002/jbt.22312. [DOI] [PubMed] [Google Scholar]
- 114.Stan M.S., Voicu S.N., Caruntu S., Nica I.C., Olah N.-K., Burtescu R., Balta C., Rosu M., Herman H., Hermenean A., et al. Antioxidant and Anti-Inflammatory Properties of a Thuja occidentalis Mother Tincture for the Treatment of Ulcerative Colitis. Antioxidants. 2019;8:416. doi: 10.3390/antiox8090416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Li S., Cao H., Shen P., Liu J., Fu Y., Cao Y., Zhang N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2018;10:422–431. doi: 10.1039/C8FO01699B. [DOI] [PubMed] [Google Scholar]
- 116.Wu M., Li P., An Y., Ren J., Yan D., Cui J., Li D., Li M., Wang M., Zhang G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019;150:104489. doi: 10.1016/j.phrs.2019.104489. [DOI] [PubMed] [Google Scholar]
- 117.Sheethal S., Ratheesh M., Jose S.P., Asha S., Krishnakumar I.M., Sandya S., Girishkumar B., Grace J. Anti-Ulcerative Effect of Curcumin-Galactomannoside Complex on Acetic Acid-Induced Experimental Model by Inhibiting Inflammation and Oxidative Stress. Inflammation. 2020;43:1411–1422. doi: 10.1007/s10753-020-01218-9. [DOI] [PubMed] [Google Scholar]
- 118.Guo X., Xu Y., Geng R., Qiu J., He X. Curcumin Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice Through Regulating Gut Microbiota. Mol. Nutr. Food Res. 2022;66:2100943. doi: 10.1002/mnfr.202100943. [DOI] [PubMed] [Google Scholar]
- 119.Huang D., Wang Y., Xu C., Zou M., Ming Y., Luo F., Xu Z., Miao Y., Wang N., Lin Z., et al. Colon-targeted hydroxyethyl starch-curcumin microspheres with high loading capacity ameliorate ulcerative colitis via alleviating oxidative stress, regulating inflammation, and modulating gut microbiota. Int. J. Biol. Macromol. 2024;266:131107. doi: 10.1016/j.ijbiomac.2024.131107. [DOI] [PubMed] [Google Scholar]
- 120.Erarslan A.S., Ozmerdivenli R., Sirinyıldız F., Cevik O., Gumus E., Cesur G. Therapeutic and prophylactic role of vitamin D and curcumin in acetic acid-induced acute ulcerative colitis model. Toxicol. Mech. Methods. 2023;33:480–489. doi: 10.1080/15376516.2023.2187729. [DOI] [PubMed] [Google Scholar]
- 121.Zhao H.A., Cheng N., Zhou W.Q., Chen S.N., Wang Q., Gao H., Xue X., Wu L., Cao W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol. Nutr. Food Res. 2019;63:1900638. doi: 10.1002/mnfr.201900638. [DOI] [PubMed] [Google Scholar]
- 122.Hossen I., Hua W., Mehmood A., Raka R.N., Jingyi S., Jian-Ming J., Min X., Shakoor A., Yanping C., Wang C., et al. Glochidion ellipticum Wight extracts ameliorate dextran sulfate sodium-induced colitis in mice by modulating nuclear factor kappa-light-chain-enhancer of activated B cells signalling pathway. J. Pharm. Pharmacol. 2021;73:410–423. doi: 10.1093/jpp/rgaa044. [DOI] [PubMed] [Google Scholar]
- 123.Gerges S.H., Tolba M.F., Elsherbiny D.A., El-Demerdash E. The natural flavonoid galangin ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Effect on Toll-like receptor 4, inflammation and oxidative stress. Basic Clin. Pharmacol. 2020;127:10–20. doi: 10.1111/bcpt.13388. [DOI] [PubMed] [Google Scholar]
- 124.Xuan H., Ou A., Hao S., Shi J., Jin X.L. Galangin Protects against Symptoms of Dextran Sodium Sulfate-Induced Acute Colitis by Activating Autophagy and Modulating the Gut Microbiota. Nutrients. 2020;12:347. doi: 10.3390/nu12020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hou J., Hu M., Zhang L., Gao Y., Ma L., Xu Q. Dietary Taxifolin Protects Against Dextran Sulfate Sodium-Induced Colitis via NF-κB Signaling, Enhancing Intestinal Barrier and Modulating Gut Microbiota. Front. Immunol. 2020;11:631809. doi: 10.3389/fimmu.2020.631809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu D., Yu X., Sun H., Zhang W., Liu G., Zhu L. Flos lonicerae flavonoids attenuate experimental ulcerative colitis in rats via suppression of NF-κB signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020;393:2481–2494. doi: 10.1007/s00210-020-01814-4. [DOI] [PubMed] [Google Scholar]
- 127.Liu Q., Zuo R., Wang K., Nong F.F., Fu Y.J., Huang S.W., Pan Z.F., Zhang X.L., Deng X.L., Zhang X.X., et al. Oroxindin inhibits macrophage NLRP3 inflammasome activation in DSS-induced ulcerative colitis in mice via suppressing TXNIP-dependent NF-κB pathway. Acta Pharmacol. Sinica. 2020;41:771–781. doi: 10.1038/s41401-019-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ortiz T., Argüelles-Arias F., Illanes M., García-Montes J.-M., Talero E., Macías-García L., Alcudia A., Vázquez-Román V., Motilva V., De-Miguel M. Polyphenolic Maqui Extract as a Potential Nutraceutical to Treat TNBS-Induced Crohn’s Disease by the Regulation of Antioxidant and Anti-Inflammatory Pathways. Nutrients. 2020;12:1752. doi: 10.3390/nu12061752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ortiz-Cerda T., Argüelles-Arias F., Macías-García L., Vázquez-Román V., Tapia G., Xie K., García-García M.D., Merinero M., García-Montes J.M., Alcudia A., et al. Effects of polyphenolic maqui (Aristotelia chilensis) extract on the inhibition of NLRP3 inflammasome and activation of mast cells in a mouse model of Crohn’s disease-like colitis. Front. Immunol. 2024;14:1229767. doi: 10.3389/fimmu.2023.1229767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren J., Yue B., Wang H., Zhang B., Luo X., Yu Z., Zhang J., Ren Y., Mani S., Wang Z., et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2021;11:577237. doi: 10.3389/fphys.2020.577237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shanmugam S., Thangaraj P., Lima B.d.S., Trindade G.G., Narain N., Mara de Oliveira E.S.A., Santin J.R., Broering M.F., Serafini M.R., Quintans-Júnior L.J., et al. Protective effects of flavonoid composition rich P. subpeltata Ortega. on indomethacin induced experimental ulcerative colitis in rat models of inflammatory bowel diseases. J. Ethnopharmacol. 2020;248:112350. doi: 10.1016/j.jep.2019.112350. [DOI] [PubMed] [Google Scholar]
- 132.Chen S., Wu X., Yu Z. Juglone Suppresses Inflammation and Oxidative Stress in Colitis Mice. Front. Immunol. 2021;12:674341. doi: 10.3389/fimmu.2021.674341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elmaksoud H.A.A., Motawea M.H., Desoky A.A., Elharrif M.G., Ibrahimi A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021;142:112073. doi: 10.1016/j.biopha.2021.112073. [DOI] [PubMed] [Google Scholar]
- 134.Jeon Y.-D., Lee J.-H., Kang S.-H., Myung H., Jin J.-S. Lingonberry Fruit Ethanol Extract Ameliorates DSS-Induced Ulcerative Colitis In Vivo and In Vitro. Appl. Sci. 2021;11:7955. doi: 10.3390/app11177955. [DOI] [Google Scholar]
- 135.Li T., Zou Q.P., Huang F., Cheng G.G., Mao Z.W., Wang T., Dong F.W., Li B.J., He H.P., Li Y.P. Flower extract of Caragana sinica. ameliorates DSS-induced ulcerative colitis by affecting TLR4/NF-KB and TLR4/MAPK signaling pathway in a mouse model. Iran. J. Basic Med. Sci. 2021;24:595–603. doi: 10.22038/IJBMS.2021.53847.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Naini M.A., Mehrvarzi S., Zargari-Samadnejadi A., Tanideh N., Ghorbani M., Dehghanian A., Hasanzarrini M., Banaee F., Koohi-Hosseinabadi O., Irajie C., et al. The Antioxidant and Anti-Inflammatory Effects of Quercus brantii Extract on TNBS-Induced Ulcerative Colitis in Rats. Evid. -Based Complement. Altern. Med. 2021;2021:3075973. doi: 10.1155/2021/9945244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oladele J.O., Anyim J.C., Oyeleke O.M., Olowookere B.D., Bamigboye M.O., Oladele O.T., Oladiji A.T. Telfairia occidentalis mitigates dextran sodium sulfate-induced ulcerative colitis in rats via suppression of oxidative stress, lipid peroxidation, and inflammation. J. Food Biochem. 2021;45:e13873. doi: 10.1111/jfbc.13873. [DOI] [PubMed] [Google Scholar]
- 138.de Oliveira R.G., Damazo A.S., Antonielli L.F., Miyajima F., Pavan E., Duckworth C.A., Lima J.C.D.S., Arunachalam K., Martins D.T.O. Dilodendron bipinnatum Radlk. extract alleviates ulcerative colitis induced by TNBS in rats by reducing inflammatory cell infiltration, TNF-α and IL-1β concentrations, IL-17 and COX-2 expressions, supporting mucus production and promotes an antioxidant effect. J. Ethnopharmacol. 2021;269:113735. doi: 10.1016/j.jep.2020.113735. [DOI] [PubMed] [Google Scholar]
- 139.Pavan E., Damazo A.S., Arunachalam K., Almeida P.O.d.A., Oliveira D.M., Venturini C.L., Figueiredo F.F., Cruz T.C.D.D., Silva J.V.D., Martins D.T.O. Copaifera malmei Harms leaves infusion attenuates TNBS-ulcerative colitis through modulation of cytokines, oxidative stress and mucus in experimental rats. J. Ethnopharmacol. 2021;267:113499. doi: 10.1016/j.jep.2020.113499. [DOI] [PubMed] [Google Scholar]
- 140.Qu Y.F., Li X.Y., Xu F.Y., Zhao S.M., Wu X.M., Wang Y.Z., Xie J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-kappa B Axis. Front. Immunol. 2021;12:679897. doi: 10.3389/fimmu.2021.679897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rafeeq M., Murad H.A.S., Abdallah H.M., El-Halawany A.M. Protective effect of 6-paradol in acetic acid-induced ulcerative colitis in rats. BMC Complement. Med. Ther. 2021;21:28. doi: 10.1186/s12906-021-03203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tagne M.A.F., Tchoffo A., Noubissi P.A., Mazo A.G., Kom B., Mukam J.N., Sokeng Dongmo S., Kamgang R. Effects of hydro-ethanolic extract of leaves of Maesa lanceolata (Mursinaceae) on acetic acid-induced ulcerative colitis in rats. Inflammopharmacology. 2021;29:1211–1223. doi: 10.1007/s10787-021-00825-8. [DOI] [PubMed] [Google Scholar]
- 143.Tatiya-Aphiradee N., Chatuphonprasert W., Jarukamjorn K. Ethanolic Garcinia mangostana extract and α-mangostin improve dextran sulfate sodium-induced ulcerative colitis via the suppression of inflammatory and oxidative responses in ICR mice. J. Ethnopharmacol. 2021;265:113384. doi: 10.1016/j.jep.2020.113384. [DOI] [PubMed] [Google Scholar]
- 144.Mundugaru R., Udaykumar P., Kumar K.N.S., Nayak S., Jacob T., Alfarhan A.H., Rajakrishnan R. Protective effect of garcinia pedunculata fruit rind in acetic acid induced ulcerative colitis. Farmacia. 2019;67:160–166. doi: 10.31925/farmacia.2019.1.22. [DOI] [Google Scholar]
- 145.Wang X., Gao Y., Wang L., Yang D., Bu W., Gou L., Huang J., Duan X., Pan Y., Cao S., et al. Troxerutin Improves Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2021;69:2729–2744. doi: 10.1021/acs.jafc.0c06755. [DOI] [PubMed] [Google Scholar]
- 146.Zahouani Y., Ben Rhouma K., Kacem K., Sebai H., Sakly M. Aqueous Leaf Extract of Pistacia lentiscus Improves Acute Acetic Acid-Induced Colitis in Rats by Reducing Inflammation and Oxidative Stress. J. Med. Food. 2021;24:697–708. doi: 10.1089/jmf.2020.0020. [DOI] [PubMed] [Google Scholar]
- 147.Zhou Y.A.-O., Dou F., Song H., Liu T. Anti-ulcerative effects of wogonin on ulcerative colitis induced by dextran sulfate sodium via Nrf2/TLR4/NF-κB signaling pathway in BALB/c mice. Environ. Toxicol. 2022;37:954–963. doi: 10.1002/tox.23457. [DOI] [PubMed] [Google Scholar]
- 148.Chen H., Li Y., Wang J., Zheng T., Wu C., Cui M., Feng Y., Ye H., Dong Z., Dang Y. Plant Polyphenols Attenuate DSS-induced Ulcerative Colitis in Mice via Antioxidation, Anti-inflammation and Microbiota Regulation. Int. J. Mol. Sci. 2023;24:10828. doi: 10.3390/ijms241310828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pandurangan A.K., Mohebali N., Norhaizan M.E., Looi C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015;9:3923–3934. doi: 10.2147/DDDT.S86345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu L., Gu P., Shen H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019;67:129–137. doi: 10.1016/j.intimp.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 151.Bayramoglu G., Senturk H., Kanbak G., Canbek M., Bayramoglu A., Dokumacioglu E., Engür S. Gallic Acid Reduces Experimental Colitis in Rats by Downregulation of Cathepsin and Oxidative Stress. Erciyes Med. J. 2020;42:213–217. [Google Scholar]
- 152.Doğan G.T., Kepekçi R.A., Bostancıeri N., Tarakçıoğlu M. Protective effect of Arum maculatum against dextran sulfate sodium induced colitis in rats. Biotech. Histochem. 2023;98:456–465. doi: 10.1080/10520295.2023.2225226. [DOI] [PubMed] [Google Scholar]
- 153.Ekhtiar M., Ghasemi-Dehnoo M., Mirzaei Y., Azadegan-Dehkordi F., Amini-Khoei H., Lorigooini Z., Samiei-Sefat A., Bagheri N. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int. Immunopharmacol. 2023;120:110309. doi: 10.1016/j.intimp.2023.110309. [DOI] [PubMed] [Google Scholar]
- 154.Ghasemi-Dehnoo M., Amini-Khoei H., Lorigooini Z., AnjomShoa M., Bijad E., Rafieian-Kopaei M. Inhibition of TLR4, NF-κB, and INOS pathways mediates ameliorative effect of syringic acid in experimental ulcerative colitis in rats. Inflammopharmacology. 2023;32:795–808. doi: 10.1007/s10787-023-01387-7. [DOI] [PubMed] [Google Scholar]
- 155.Fang W., Zhu S., Niu Z., Yin Y. The protective effect of syringic acid on dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Dev. Res. 2019;80:731–740. doi: 10.1002/ddr.21524. [DOI] [PubMed] [Google Scholar]
- 156.Shibrya E.E., Rashed R.R., El Fattah M.A.A., El-Ghazaly M.A., Kenawy S.A. Apigenin and Exposure to Low Dose Gamma Radiation Ameliorate Acetic Acid-Induced Ulcerative Colitis in Rats. Dose-Response. 2023;21:15593258231155787. doi: 10.1177/15593258231155787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lertnimitphun P., Jiang Y., Kim N., Fu W., Zheng C., Tan H., Zhou H., Zhang X., Pei W., Lu Y., et al. Safranal Alleviates Dextran Sulfate Sodium-Induced Colitis and Suppresses Macrophage-Mediated Inflammation. Front. Pharmacol. 2019;10:1281. doi: 10.3389/fphar.2019.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu X., Ocansey D.K.W., Pei B., Zhang Y., Wang N., Wang Z., Mao F. Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur. J. Med. Res. 2023;28:319. doi: 10.1186/s40001-023-01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang J., Lin J., Zhang W., Shen M., Wang Y., Xie J. Resveratrol-loaded pH-responsive Mesona chinensis polysaccharides-zein nanoparticles for effective treatment of ulcerative colitis. J. Sci. Food Agric. 2024;104:3992–4003. doi: 10.1002/jsfa.13282. [DOI] [PubMed] [Google Scholar]
- 160.Yeom J., Ma S., Kim J.-K., Lim Y.-H. Oxyresveratrol Ameliorates Dextran Sulfate Sodium-Induced Colitis in Rats by Suppressing Inflammation. Molecules. 2021;26:2630. doi: 10.3390/molecules26092630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao R., Ren Y., Xue P., Sheng Y., Yang Q., Dai Y., Zhang X., Lin Z., Liu T., Geng Y., et al. Protective Effect of the Polyphenol Ligustroside on Colitis Induced with Dextran Sulfate Sodium in Mice. Nutrients. 2024;16:522. doi: 10.3390/nu16040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goodarzi S., Abdolghaffari A.H., Najafi B., Hamedani M.P., Tavakoli S., Marvi M., Baeeri M., Yassa N., Hadjiakhoondi A., Hadjiakhoondi M., et al. Isoimperatorin alleviates acetic acid-induced colitis in rats. Asian Pac. J. Trop. Biomed. 2024;14:147–153. doi: 10.4103/apjtb.apjtb_851_23. [DOI] [Google Scholar]
- 163.Lv W., Jin W., Lin J., Wang Z., Ma Y., Zhang W., Zhu Y., Hu Y., Qu Q., Guo S. Forsythia suspensa polyphenols regulate macrophage M1 polarization to alleviate intestinal inflammation in mice. Phytomedicine. 2024;125:155336. doi: 10.1016/j.phymed.2024.155336. [DOI] [PubMed] [Google Scholar]
- 164.Nong K., Qin X., Liu Z., Wang Z., Wu Y., Zhang B., Chen W., Fang X., Liu Y., Wang X., et al. Potential effects and mechanism of flavonoids extract of Callicarpa nudiflora Hook on DSS-induced colitis in mice. Phytomedicine. 2024;128:155523. doi: 10.1016/j.phymed.2024.155523. [DOI] [PubMed] [Google Scholar]
- 165.Rehman I.U., Saleem M., Raza S.A., Bashir S., Muhammad T., Asghar S., Qamar M.U., Shah T.A., Bin Jardan Y.A., Mekonnen A.B., et al. Anti-ulcerative colitis effects of chemically characterized extracts from Calliandra haematocephala in acetic acid-induced ulcerative colitis. Front. Chem. 2024;12:1291230. doi: 10.3389/fchem.2024.1291230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Salem M.B., El-Lakkany N.M., El-Din S.H.S., Hammam O.A., Samir S. Diosmin alleviates ulcerative colitis in mice by increasing Akkermansia muciniphila abundance, improving intestinal barrier function, and modulating the NF-κB and Nrf2 pathways. Heliyon. 2024;10:e27527. doi: 10.1016/j.heliyon.2024.e27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wei X., Ma N., Yang W., Tian J., Liu H., Fang H. Polyphenol Extracts from Ziziphus jujuba Mill. "Junzao" Attenuates Ulcerative Colitis by Inhibiting the NLRP3 and MAPKs Signaling Pathways and Regulating Gut Microbiota Homeostasis in Mice. Mol. Nutr. Food Res. 2024;68:e2300643. doi: 10.1002/mnfr.202300643. [DOI] [PubMed] [Google Scholar]
- 168.Medicherla K., Sahu B.D., Kuncha M., Kumar J.M., Sudhakar G., Sistla R. Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-κB signaling. Food Funct. 2015;6:2984–2995. doi: 10.1039/C5FO00405E. [DOI] [PubMed] [Google Scholar]
- 169.Abiodun O.O., Rodríguez-Nogales A., Algieri F., Gomez-Caravaca A.M., Segura-Carretero A., Utrilla M.P., Rodriguez-Cabezas M.E., Galvez J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016;192:309–319. doi: 10.1016/j.jep.2016.07.056. [DOI] [PubMed] [Google Scholar]
- 170.Algieri F., Rodriguez-Nogales A., Vezza T., Garrido-Mesa J., Garrido-Mesa N., Utrilla M.P., González-Tejero M.R., Casares-Porcel M., Molero-Mesa J., Del Mar Contreras M., et al. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J. Ethnopharmacol. 2016;190:142–158. doi: 10.1016/j.jep.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 171.Chen Y.Y., Li R.Y., Shi M.J., Zhao Y.X., Yan Y., Xu X.X., Zhang M., Zhao X.T., Zhang Y.B. Demethyleneberberine alleviates inflammatory bowel disease in mice through regulating NF-κB signaling and T-helper cell homeostasis. Inflamm. Res. 2017;66:187–196. doi: 10.1007/s00011-016-1005-3. [DOI] [PubMed] [Google Scholar]
- 172.Choi K.-C., Cho S.-W., Kook S.-H., Chun S.-R., Bhattarai G., Poudel S.B., Kim M.K., Lee K.Y., Lee J.C. Intestinal anti-inflammatory activity of the seeds of Raphanus sativus L. in experimental ulcerative colitis models. J. Ethnopharmacol. 2016;179:55–65. doi: 10.1016/j.jep.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 173.Fatani A.J., Alrojayee F.S., Parmar M.Y., Abuohashish H.M., Ahmed M.M., Al-Rejaie S.S. Myrrh attenuates oxidative and inflammatory processes in acetic acid-induced ulcerative colitis. Exp. Ther. Med. 2016;12:730–738. doi: 10.3892/etm.2016.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li R., Chen Y., Shi M., Xu X., Zhao Y., Wu X., Zhang Y. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine. 2016;23:1012–1020. doi: 10.1016/j.phymed.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 175.Liu B., Piao X., Guo L., Liu S., Chai F., Gao L. Ursolic acid protects against ulcerative colitis via anti-inflammatory and antioxidant effects in mice. Mol. Med. Rep. 2016;13:4779–4785. doi: 10.3892/mmr.2016.5094. [DOI] [PubMed] [Google Scholar]
- 176.Qin M., Geng Y., Lu Z., Xu H., Shi J.S., Xu X., Xu Z.H. Anti-Inflammatory Effects of Ethanol Extract of Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), in Mice with Ulcerative Colitis. Int. J. Med. Mushrooms. 2016;18:227–234. doi: 10.1615/IntJMedMushrooms.v18.i3.50. [DOI] [PubMed] [Google Scholar]
- 177.Wang D., Zhang Y., Yang S., Zhao D., Wang M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int. J. Biol. Macromol. 2019;125:572–579. doi: 10.1016/j.ijbiomac.2018.12.092. [DOI] [PubMed] [Google Scholar]
- 178.Li H., Feng J., Liu C., Hou S., Meng J., Liu J.-Y., Zilong S., Chang M.C. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int. J. Biol. Macromol. 2024;267:131251. doi: 10.1016/j.ijbiomac.2024.131251. [DOI] [PubMed] [Google Scholar]
- 179.Rodriguez-Canales M., Jimenez-Rivas R., Canales-Martinez M.M., Garcia-Lopez A.J., Rivera-Yañez N., Nieto-Yañez O., Ledesma-Soto Y., Sanchez-Torres L.E., Rodriguez-Sosa M., Terrazas L.I., et al. Protective Effect of Amphipterygium adstringens Extract on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Mediat. Inflamm. 2016;2016:8543561. doi: 10.1155/2016/8543561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Somani S., Zambad S., Modi K. Mangiferin attenuates DSS colitis in mice: Molecular docking and in┬ávivo approach. Chemico-Biol. Interact. 2016;253:18–26. doi: 10.1016/j.cbi.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 181.Yang X., Yan Y., Li J., Tang Z., Sun J., Zhang H., Hao S., Wen A., Liu L. Protective effects of ethanol extract from Portulaca oleracea L. on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am. J. Transl. Res. 2016;8:2138–2148. [PMC free article] [PubMed] [Google Scholar]
- 182.Ning K., Shi C., Chi Y.-Y., Zhou Y.-F., Zheng W., Duan Y., Tong W., Xie Q., Xiang H. Portulaca oleracea L. polysaccharide alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal homeostasis. Int. J. Biol. Macromol. 2024;256:128375. doi: 10.1016/j.ijbiomac.2023.128375. [DOI] [PubMed] [Google Scholar]
- 183.Yang Y., Yan H., Jing M., Zhang Z., Zhang G., Sun Y., Shan L., Yu P., Wang Y., Xu L. Andrographolide derivative AL-1 ameliorates TNBS-induced colitis in mice: Involvement of NF-κB and PPAR-γsignaling pathways. Sci. Rep. 2016;6:29716. doi: 10.1038/srep29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abiodun O.O., Oshinloye A.O. Carpolobia lutea G. Don (Polygalaceae) Inhibits Inflammation and Oxidative Stress in an Acetic Acid Induced Model of Rat Colitis. Drug Res. 2017;67:20–24. doi: 10.1055/s-0042-114572. [DOI] [PubMed] [Google Scholar]
- 185.Chaudhary G., Mahajan U.B., Goyal S.N., Ojha S., Patil C.R., Subramanya S.B. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am. J. Transl. Res. 2017;9:1792–1800. [PMC free article] [PubMed] [Google Scholar]
- 186.Han K.H., Park J.M., Jeong M., Han Y.M., Go E.J., Park J., Kim H., Han J.G., Kwon O., Hahm K.B. Heme Oxygenase-1 Induction and Anti-inflammatory Actions of Atractylodes macrocephala and Taraxacum herba Extracts Prevented Colitis and Was More Effective than Sulfasalazine in Preventing Relapse. Gut Liver. 2017;11:655–666. doi: 10.5009/gnl16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pei R., Liu J., Martin D.A., Valdez J.C., Jeffety J., Barrett-Wilt G.A., Liu Z., Bolling B.W. Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress. Nutrients. 2019;11:1316. doi: 10.3390/nu11061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kang S.-H., Jeon Y.-D., Moon K.-H., Lee J.-H., Kim D.-G., Kim W., Myung H., Kim J.S., Kim H.J., Bang K.S., et al. Aronia Berry Extract Ameliorates the Severity of Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. J. Med. Food. 2017;20:667–675. doi: 10.1089/jmf.2016.3822. [DOI] [PubMed] [Google Scholar]
- 189.Li Y., Tsopmejio I.S.N., Diao Z., Xiao H., Wang X., Jin Z., Song H. Aronia melanocarpa (Michx.) Elliott. attenuates dextran sulfate sodium-induced Inflammatory Bowel Disease via regulation of inflammation-related signaling pathways and modulation of the gut microbiota. J. Ethnopharmacol. 2022;292:115190. doi: 10.1016/j.jep.2022.115190. [DOI] [PubMed] [Google Scholar]
- 190.Oh S.R., Ok S., Jung T.S., Jeon S.O., Park J.M., Jung J.W., Ryu D.S. Protective effect of decursin and decursinol angelate-rich Angelica gigas Nakai extract on dextran sulfate sodium-induced murine ulcerative colitis. Asian Pac. J. Trop. Med. 2017;10:864–870. doi: 10.1016/j.apjtm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 191.Park D.D., Yum H.W., Zhong X., Kim S.H., Kim S.H., Kim D.H., Kim D.H., Kim S.J., Na H.K., Sato A., et al. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets. Front. Pharmacol. 2017;8:482. doi: 10.3389/fphar.2017.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seo H., Oh J., Hahn D., Kwon C.-S., Lee J.S., Kim J.-S. Protective Effect of Glyceollins in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Med. Food. 2017;20:1055–1062. doi: 10.1089/jmf.2017.3960. [DOI] [PubMed] [Google Scholar]
- 193.Xiong Y., Shi L., Wang L., Zhou Z., Wang C., Lin Y., Luo D., Qiu J., Chen D. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharmacol. Res. 2017;123:73–82. doi: 10.1016/j.phrs.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 194.Yu L., Yan J., Sun Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017;15:2339–2346. doi: 10.3892/mmr.2017.6241. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Z., Yang L., Wang B., Zhang L., Zhang Q., Li D., Zhang S., Gao H., Wang X. Protective role of liriodendrin in mice with dextran sulphate sodium-induced ulcerative colitis. Int. Immunopharmacol. 2017;52:203–210. doi: 10.1016/j.intimp.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 196.Abe H., Ishioka M., Fujita Y., Umeno A., Yasunaga M., Sato A., Ohnishi S., Suzuki S., Ishida N., Shichiri M., et al. Yuzu (Citrus junos Tanaka) Peel Attenuates Dextran Sulfate Sodium-induced Murine Experimental Colitis. J. Oleo Sci. 2018;67:335–344. doi: 10.5650/jos.ess17184. [DOI] [PubMed] [Google Scholar]
- 197.Akanda M.R., Nam H.H., Tian W., Islam A., Choo B.K., Park B.Y. Regulation of JAK2/STAT3 and NF-κB signal transduction pathways; Veronica polita alleviates dextran sulfate sodium-induced murine colitis. Biomed. Pharmacother. 2018;100:296–303. doi: 10.1016/j.biopha.2018.01.168. [DOI] [PubMed] [Google Scholar]
- 198.Almeer R.S., Mahmoud S.M., Amin H.K., Moneim A.E.A. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. 2018;115:49–62. doi: 10.1016/j.fct.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 199.Bibi S., Kang Y., Du M., Zhu M.-J. Dietary red raspberries attenuate dextran sulfate sodium-induced acute colitis. J. Nutr. Biochem. 2017;51:40–46. doi: 10.1016/j.jnutbio.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 200.Boeing T., de Souza P., Bonomini T.J., Mariano L.N.B., Somensi L.B., Lucinda R.M., Malheiros A., da Silva L.M., Andrade S.F. Antioxidant and anti-inflammatory effect of plumieride in dextran sulfate sodium-induced colitis in mice. Biomed. Pharmacother. 2018;99:697–703. doi: 10.1016/j.biopha.2018.01.142. [DOI] [PubMed] [Google Scholar]
- 201.da Silva V.C., de Araújo A.A., de Souza Araújo D.F., Souza Lima M.C.J., Vasconcelos R.C., de Araújo Júnior R.F., Langasnner S.M.Z., de Freitas Fernandes Pedrosa M., de Medeiros C.A.C.X., Guerra G.C.B. Intestinal Anti-Inflammatory Activity of the Aqueous Extract from Ipomoea asarifolia in DNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2018;19:4016. doi: 10.3390/ijms19124016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khairy H., Saleh H., Badr A.M., Marie M.-A.S. Therapeutic efficacy of osthole against dinitrobenzene sulphonic acid induced-colitis in rats. Biomed. Pharmacother. 2018;100:42–51. doi: 10.1016/j.biopha.2018.01.104. [DOI] [PubMed] [Google Scholar]
- 203.Liu B., Li S., Sui X., Guo L., Liu X., Li H., Gao L., Cai S., Li Y., Wang T., et al. Root Extract of Polygonum cuspidatum Siebold & Zucc. Ameliorates DSS-Induced Ulcerative Colitis by Affecting NF-kappaB Signaling Pathway in a Mouse Model via Synergistic Effects of Polydatin, Resveratrol, and Emodin. Front. Pharmacol. 2018;9:347. doi: 10.3389/fphar.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajendiran V., Natarajan V., Devaraj S.N. Anti-inflammatory activity of Alpinia officinarum hance on rat colon inflammation and tissue damage in DSS induced acute and chronic colitis models. Food Sci. Hum. Wellness. 2018;7:273–281. doi: 10.1016/j.fshw.2018.10.004. [DOI] [Google Scholar]
- 205.Shen P., Zhang Z., He Y., Gu C., Zhu K., Li S., Li Y., Lu X., Liu J., Zhang N., et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018;196:69–76. doi: 10.1016/j.lfs.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 206.Fan X., Zhang Z., Gao W., Pan Q., Luo K., He B., Pu Y. An Engineered Butyrate-Derived Polymer Nanoplatform as a Mucosa-Healing Enhancer Potentiates the Therapeutic Effect of Magnolol in Inflammatory Bowel Disease. ACS Nano. 2024;18:229–244. doi: 10.1021/acsnano.3c05732. [DOI] [PubMed] [Google Scholar]
- 207.Vicentini F.A., Barbosa M.M.C., Fortunato M.C., Amado C.A.B., Comar J.F., Longhini R., de Mello J.C.P., Natali M.R.M. Treatment with Trichilia catigua ethyl-acetate fraction improves healing and reduces oxidative stress in TNBS-induced colitis in rats. Biomed. Pharmacother. 2018;107:194–202. doi: 10.1016/j.biopha.2018.07.160. [DOI] [PubMed] [Google Scholar]
- 208.Witaicenis A., de Oliveira E.C.S., Tanimoto A., Zorzella-Pezavento S.F.G., de Oliveira S.L., Sartori A., Di Stasi L.C. 4-methylesculetin, a coumarin derivative, ameliorates dextran sulfate sodium-induced intestinal inflammation. Chem.-Biol. Interact. 2018;280:59–63. doi: 10.1016/j.cbi.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 209.Tanimoto A., Witaicenis A., Caruso P., Piva H.M., Araujo G.C., Moraes F.R., Fossey M.C., Cornélio M.L., Souza F.P., Di Stasi L.C. 4-Methylesculetin, a natural coumarin with intestinal anti-inflammatory activity, elicits a glutathione antioxidant response by different mechanisms. Chem.-Biol. Interact. 2020;315:108876. doi: 10.1016/j.cbi.2019.108876. [DOI] [PubMed] [Google Scholar]
- 210.Yue B., Ren Y.-J., Zhang J.-J., Luo X.-P., Yu Z.-L., Ren G.-Y., Sun A.N., Deng C., Wang Z.T., Dou W. Anti-Inflammatory Effects of Fargesin on Chemically Induced Inflammatory Bowel Disease in Mice. Molecules. 2018;23:1380. doi: 10.3390/molecules23061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou Y., Liu H., Song J., Cao L., Tang L., Qi C. Sinomenine alleviates dextran sulfate sodium-induced colitis via the Nrf2/NQO-1 signaling pathway. Mol. Med. Rep. 2018;18:3691–3698. doi: 10.3892/mmr.2018.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Niu Z., Li X., Yang X., Sun Z. Protective effects of sinomenine against dextran sulfate sodium-induced ulcerative colitis in rats via alteration of HO-1/Nrf2 and inflammatory pathway. Inflammopharmacology. 2024;32:2007–2022. doi: 10.1007/s10787-024-01455-6. [DOI] [PubMed] [Google Scholar]
- 213.Alavala S., Sangaraju R., Nalban N., Sahu B.D., Jerald M.K., Kilari E.K., Sistla R. Stevioside, a diterpenoid glycoside, shows anti-inflammatory property against Dextran Sulphate Sodium-induced ulcerative colitis in mice. Eur. J. Pharmacol. 2019;855:192–201. doi: 10.1016/j.ejphar.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 214.Bai X., Gou X., Cai P., Xu C., Cao L., Zhao Z., Huang M., Jin J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxidative Med. Cell. Longev. 2019;2019:2432416. doi: 10.1155/2019/2432416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cota D., Mishra S., Shengule S. Beneficial role of Terminalia arjuna hydro-alcoholic extract in colitis and its possible mechanism. J. Ethnopharmacol. 2019;230:117–125. doi: 10.1016/j.jep.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 216.Hong J.Y., Chung K.S., Shin J.S., Park G., Jang Y.P., Lee K.T. Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. Int. J. Mol. Sci. 2019;20:177. doi: 10.3390/ijms20010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hong Z., Piao M. Effect of Quercetin Monoglycosides on Oxidative Stress and Gut Microbiota Diversity in Mice with Dextran Sodium Sulphate-Induced Colitis. BioMed Res. Int. 2018;2018:8343052. doi: 10.1155/2018/8343052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Huang K., Dong W., Liu W., Yan Y., Wan P., Peng Y., Xu Y., Zeng X., Cao Y. 2-O-β-d-Glucopyranosyl-l-ascorbic Acid, an Ascorbic Acid Derivative Isolated from the Fruits of Lycium Barbarum L., Modulates Gut Microbiota and Palliates Colitis in Dextran Sodium Sulfate-Induced Colitis in Mice. J. Agric. Food Chem. 2019;67:11408–11419. doi: 10.1021/acs.jafc.9b04411. [DOI] [PubMed] [Google Scholar]
- 219.Hwang Y.-J., Nam S.-J., Chun W., Kim S.I., Park S.C., Kang C.D., Lee S.J. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS ONE. 2019;14:e0217642. doi: 10.1371/journal.pone.0217642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kouki A., Ferjani W., Dang P.M.-C., Ghanem-Boughanmi N., Souli A., Ben-Attia M., El-Benna J. Preventive Anti-inflammatory Effects of Apocynin on Acetic Acid-Induced Colitis in Rats. Inflammation. 2024;47:438–453. doi: 10.1007/s10753-023-01920-4. [DOI] [PubMed] [Google Scholar]
- 221.Khodir A.E., Said E., Atif H., ElKashef H.A., Islam A., Choo B.K., Park B.Y. Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats. Biomed. Pharmacother. 2019;110:389–399. doi: 10.1016/j.biopha.2018.11.133. [DOI] [PubMed] [Google Scholar]
- 222.Rezaei N., Avan A., Pashirzad M., Rahmani F., Moradi Marjaneh R., Behnam-Rassouli R., Shafiee M., Ryzhikov M., Hashemzehi M., Ariakia F., et al. Crocin as a novel therapeutic agent against colitis. Drug Chem. Toxicol. 2020;43:514–521. doi: 10.1080/01480545.2018.1527850. [DOI] [PubMed] [Google Scholar]
- 223.Albalawi G.A., Albalawi M.Z., Alsubaie K.T., Albalawi A.Z., Elewa M.A., Hashem K.S., Al-Gayyar M.M.H. Curative effects of crocin in ulcerative colitis via modulating apoptosis and inflammation. Int. Immunopharmacol. 2023;118:110138. doi: 10.1016/j.intimp.2023.110138. [DOI] [PubMed] [Google Scholar]
- 224.Li H., Shen L., Lv T., Wang R., Zhang N., Peng H., Diao W. Salidroside attenuates dextran sulfate sodium-induced colitis in mice via SIRT1/FoxOs signaling pathway. Eur. J. Pharmacol. 2019;861:172591. doi: 10.1016/j.ejphar.2019.172591. [DOI] [PubMed] [Google Scholar]
- 225.Lian L., Zhang S., Yu Z., Ge H., Qi S., Zhang X., Long L., Xiong X., Chu D., Ma X., et al. The dietary freeze-dried fruit powder of Actinidia arguta ameliorates dextran sulphate sodium-induced ulcerative colitis in mice by inhibiting the activation of MAPKs. Food Funct. 2019;10:5768–5778. doi: 10.1039/C9FO00664H. [DOI] [PubMed] [Google Scholar]
- 226.Meurer M.C., Mees M., Mariano L.N.B., Boeing T., Somensi L.B., Mariott M., da Silva R.C.M.V.A.F., Dos Santos A.C., Longo B., Santos França T.C., et al. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr. Res. 2019;66:95–106. doi: 10.1016/j.nutres.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 227.Shen J., Li N., Zhang X. Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-κB Signaling. J. Environ. Pathol. Toxicol. Oncol. 2019;38:29–39. doi: 10.1615/JEnvironPatholToxicolOncol.2018027531. [DOI] [PubMed] [Google Scholar]
- 228.Akkol E.K., Ilhan M., Karpuz B., Taştan H., Sobarzo-Sánchez E., Khan H. Beneficial effects of Ajuga chamaepitys (L.) Schreber subsp. chia (Schreber) and its iridoids on the colitis model: Histopathological and biochemical evidence. Food Chem. Toxicol. 2020;144:111589. doi: 10.1016/j.fct.2020.111589. [DOI] [PubMed] [Google Scholar]
- 229.Akkol E.K., Dereli F.T.G., Taştan H., Sobarzo-Sánchez E., Khan H. Effect of Sorbus domestica and its active constituents in an experimental model of colitis rats induced by acetic acid. J. Ethnopharmacol. 2019;251:112521. doi: 10.1016/j.jep.2019.112521. [DOI] [PubMed] [Google Scholar]
- 230.Andrade A.W.L., Guerra G.C.B., Araújo D.F.d.S., Júnior R.F.d.A., de Araújo A.A., de Carvalho T.G., Fernandes J.M., Diez-Echave P., Hidalgo-García L., Rodriguez-Cabezas M.E., et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front. Pharmacol. 2020;11:998. doi: 10.3389/fphar.2020.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Arunachalam K., Damazo A.S., Macho A., Lima J.C.d.S., Pavan E., Figueiredo F.d.F., Oliveira D.M., Cechinel-Filho V., Wagner T.M., Martins D.T.O. Piper umbellatum L. (Piperaceae): Phytochemical profiles of the hydroethanolic leaf extract and intestinal anti-inflammatory mechanisms on 2,4,6 trinitrobenzene sulfonic acid induced ulcerative colitis in rats. J. Ethnopharmacol. 2020;254:112707. doi: 10.1016/j.jep.2020.112707. [DOI] [PubMed] [Google Scholar]
- 232.Badr G., Elsawy H., Amalki M.A., Alfwuaires M., El-Gerbed M.S.A., Abdel-Moneim A.M. Protective effects of myristicin against ulcerative colitis induced by acetic acid in male mice. Food Agric. Immunol. 2020;31:435–446. doi: 10.1080/09540105.2020.1739626. [DOI] [Google Scholar]
- 233.Chen J.F., Luo D.D., Lin Y.S., Liu Y.H., Wu J.Z., Yi X.Q., Wu Y., Zhang Q., Gao C.J., Cai J., et al. Aqueous extract of Bruguiera gymnorrhiza leaves protects against dextran sulfate sodium induced ulcerative colitis in mice via suppressing NF-κB activation and modulating intestinal microbiota. J. Ethnopharmacol. 2020;251:112554. doi: 10.1016/j.jep.2020.112554. [DOI] [PubMed] [Google Scholar]
- 234.Cota D., Mishra S., Shengule S. Arjunarishta alleviates experimental colitis via suppressing proinflammatory cytokine expression, modulating gut microbiota and enhancing antioxidant effect. Mol. Biol. Rep. 2020;47:7049–7059. doi: 10.1007/s11033-020-05766-z. [DOI] [PubMed] [Google Scholar]
- 235.Osafo N., Essel L., Obiri D., Antwi A., Duduyemi M.B. Ulcerative colitis induced with acetic acid is ameliorated by Antrocaryon micraster through reduced serum levels of tumor necrosis factor alpha and interleukin-6 in sprague dawley rats. Pharmacogn. Res. 2020;12:85–91. doi: 10.4103/pr.pr_75_19. [DOI] [Google Scholar]
- 236.Guo G., Shi F., Zhu J., Shao Y., Gong W., Zhou G., Wu H., She J., Shi W. Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-κB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum. Exp. Toxicol. 2020;39:477–491. doi: 10.1177/0960327119892042. [DOI] [PubMed] [Google Scholar]
- 237.Jeon Y.-D., Lee J.-H., Lee Y.-M., Kim D.-K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020;124:109847. doi: 10.1016/j.biopha.2020.109847. [DOI] [PubMed] [Google Scholar]
- 238.Kim H.-Y., Jeon H., Bae C.H., Lee Y., Kim H., Kim S. Rumex japonicus Houtt. alleviates dextran sulfate sodium-induced colitis by protecting tight junctions in mice. Integr. Med. Res. 2020;9:100398. doi: 10.1016/j.imr.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li X., Liu X., Zhang Y., Zhang Y., Liu S., Zhang N., Wang D. Protective effect of Gloeostereum incarnatum on ulcerative colitis via modulation of Nrf2/NF-κB signaling in C57BL/6 mice. Mol. Med. Rep. 2020;22:3418–3428. doi: 10.3892/mmr.2020.11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Qian B., Wang C., Zeng Z., Ren Y., Li D., Song J.-L. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxidative Med. Cell. Longev. 2020;2020:8393504. doi: 10.1155/2020/8393504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Raj V., Venkataraman B., Almarzooqi S., Chandran S., Ojha S.K., Attoub S., Adrian T.E., Subramanya S.B. Nerolidol Mitigates Colonic Inflammation: An Experimental Study Using both In Vivo and In Vitro Models. Nutrients. 2020;12:2032. doi: 10.3390/nu12072032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bastaki S.M.A., Amir N., Adeghate E., Ojha S. Nerolidol, a sesquiterpene, attenuates oxidative stress and inflammation in acetic acid-induced colitis in rats. Mol. Cell. Biochem. 2021;476:3497–3512. doi: 10.1007/s11010-021-04094-5. [DOI] [PubMed] [Google Scholar]
- 243.Valero M.S., González M., Ramón-Gimenez M., Andrade P.B., Moreo E., Les F., Fernandes F., Gómez-Rincón C., Berzosa C., García de Jalón J.A., et al. Jasonia glutinosa (L.) DC.; a traditional herbal medicine, reduces inflammation, oxidative stress and protects the intestinal barrier in a murine model of colitis. Inflammopharmacology. 2020;28:1717–1734. doi: 10.1007/s10787-019-00626-0. [DOI] [PubMed] [Google Scholar]
- 244.Wu Z.-C., Zhao Z.-L., Deng J.-P., Huang J.-T., Wang Y.-F., Wang Z.-P. Sanhuang Shu’ai decoction alleviates DSS-induced ulcerative colitis via regulation of gut microbiota, inflammatory mediators and cytokines. Biomed. Pharmacother. 2020;125:109934. doi: 10.1016/j.biopha.2020.109934. [DOI] [PubMed] [Google Scholar]
- 245.Zhang R., Yuan S., Ye J., Wang X., Zhang X., Shen J., Yuan M., Liao W. Polysaccharide from flammuliana velutipes improves colitis via regulation of colonic microbial dysbiosis and inflammatory responses. Int. J. Biol. Macromol. 2020;149:1252–1261. doi: 10.1016/j.ijbiomac.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 246.Zhang Y., Han D., Yu S., An C., Liu X., Zhong H., Xu Y., Jiang L., Wang Z. Protective Effect of Iridoid Glycosides of the Leaves of Syringa oblata Lindl. on Dextran Sulfate Sodium-Induced Ulcerative Colitis by Inhibition of the TLR2/4/MyD88/NF-κB Signaling Pathway. BioMed Res. Int. 2020;2020:7650123. doi: 10.1155/2020/7650123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Akl E.M., Taha F.S., Mohamed S.S., Mohamed R.S. Characterization of Garden Cress Mucilage and its Prophylactic Effect Against Indomethacin-Induced Enter-Colitis in Rats. Biointerface Res. Appl. Chem. 2021;11:13911–13923. [Google Scholar]
- 248.Ansari M.N., Rehman N.U., Karim A., Soliman G.A., Ganaie M.A., Raish M., Hamad A.M. Role of Oxidative Stress and Inflammatory Cytokines (TNF-alpha and IL-6) in Acetic Acid-Induced Ulcerative Colitis in Rats: Ameliorated by Otostegia fruticosa. Life. 2021;11:195. doi: 10.3390/life11030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Keshavarzi Z., Safari F., Alipour B., Khoshniat A., Azizi R., Vatanchian M., Maghool F. Antioxidant Effects of Methanol Extract of Dracocephalum kotschyi in Acetic Acid Induced Rat Colitis Model. J. Adv. Med. Biomed. Res. 2022;30:39–46. [Google Scholar]
- 250.Liu X., Quan S., Han Q., Li J., Gao X., Zhang J., Liu D. Effectiveness of the fruit of Rosa odorata sweet var. gigantea (Coll. et Hemsl.) Rehd. et Wils in the protection and the healing of ethanol-induced rat gastric mucosa ulcer based on Nrf2/NF-κB pathway regulation. J. Ethnopharmacol. 2022;282:114626. doi: 10.1016/j.jep.2021.114626. [DOI] [PubMed] [Google Scholar]
- 251.Shin J.M., Son Y.-J., Ha I.J., Erdenebileg S., Jung D.S., Song D.-G., Kim Y.S., Kim S.M., Nho C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022;22:64. doi: 10.1186/s12906-022-03536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cellat M., Tekeli O., Türk E., Aydin T., Uyar A., İşler C.T., Gökçek İ., Etyemez M., Güvenç M. Inula viscosa ameliorates acetic acid induced ulcerative colitis in rats. Biotech. Histochem. 2023;98:255–266. doi: 10.1080/10520295.2023.2176923. [DOI] [PubMed] [Google Scholar]
- 253.Chen G., Wen D., Shen L., Feng Y., Xiong Q., Li P., Zhao Z. Cepharanthine Exerts Antioxidant and Anti-Inflammatory Effects in Lipopolysaccharide (LPS)-Induced Macrophages and DSS-Induced Colitis Mice. Molecules. 2023;28:6070. doi: 10.3390/molecules28166070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Erdenebileg S., Son Y.J., Kim M., Oidovsambuu S., Cha K.H., Kwon J., Jung D.S., Nho C.W. Saposhnikovia divaricata root and its major components ameliorate inflammation and altered gut microbial diversity and compositions in DSS-induced colitis. Integr. Med. Res. 2023;12:100998. doi: 10.1016/j.imr.2023.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fathima A., Gangachannaiah S., Bose U., Kabekkodu S.P., Chakraborty R., Praveen Kumar S.E., Padmanabha U., Rachagolla S., Prathap Y., Vidya M. Effect of aqueous extract of Trigonellafoenum-graecum L. seeds on Acetic acid- induced Ulcerative colitis in rats. Res. J. Pharm. Technol. 2023;16:2161–2168. doi: 10.52711/0974-360X.2023.00355. [DOI] [Google Scholar]
- 256.Ghasemi-Dehnoo M., Lorigooini Z., Amini-Khoei H., Sabzevary-Ghahfarokhi M., Rafieian-Kopaei M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun. Inflamm. Dis. 2023;11:e926. doi: 10.1002/iid3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gu D., Wang H., Yan M., Li Y., Yang S., Shi D., Wu L., Liu C. Echinacea purpurea (L.) Moench extract suppresses inflammation by inhibition of C3a/C3aR signaling pathway in TNBS-induced ulcerative colitis rats. J. Ethnopharmacol. 2023;307:116221. doi: 10.1016/j.jep.2023.116221. [DOI] [PubMed] [Google Scholar]
- 258.Wei F.H., Xie W.Y., Zhao P.S., Gao W., Gao F. Echinacea purpurea Polysaccharide Ameliorates Dextran Sulfate Sodium-Induced Colitis by Restoring the Intestinal Microbiota and Inhibiting the TLR4-NF-κB Axis. Nutrients. 2024;16:1305. doi: 10.3390/nu16091305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.He Z., Liu J., Liu Y. Daphnetin attenuates intestinal inflammation, oxidative stress, and apoptosis in ulcerative colitis via inhibiting REG3A-dependent JAK2/STAT3 signaling pathway. Environ. Toxicol. 2023;38:2132–2142. doi: 10.1002/tox.23837. [DOI] [PubMed] [Google Scholar]
- 260.Li C., Liu M., Deng L., Luo D., Ma R., Lu Q. Oxyberberine ameliorates TNBS-induced colitis in rats through suppressing inflammation and oxidative stress via Keap1/Nrf2/NF-κB signaling pathways. Phytomedicine. 2023;116:154899. doi: 10.1016/j.phymed.2023.154899. [DOI] [PubMed] [Google Scholar]
- 261.Li F., Huang H., Zhao P., Jiang J., Ding X., Lu D., Ji L. Curculigoside mitigates dextran sulfate sodium-induced colitis by activation of KEAP1-NRF2 interaction to inhibit oxidative damage and autophagy of intestinal epithelium barrier. Int. J. Mol. Med. 2023;52:107. doi: 10.3892/ijmm.2023.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Minaiyan M., Pasandideh-Fetrat P., Sadeghi-Dinani M., Talebi A. Ameliorative Effect of Aqueous and Hydroalcoholic Extracts of Scrophularia striata Boiss. on Murine Model of Experimental Colitis. Adv. Biomed. Res. 2023;12:105. doi: 10.4103/abr.abr_151_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Otu-Boakye S.A., Yeboah K.O., Boakye-Gyasi E., Oppong-Kyekyeku J., Okyere P.D., Osafo N. Acetic acid-induced colitis modulating potential of total crude alkaloidal extract of Picralima nitida seeds in rats. Immun. Inflamm. Dis. 2023;11:e855. doi: 10.1002/iid3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Puppala E.R., Yalamarthi S.S., Aochenlar S.L., Prasad N., Syamprasad N.P., Singh M., Nanjappan S.K., Ravichandiran V., Tripathi D.M., Gangasani J.K., et al. Mesua assamica (King&Prain) kosterm. Bark ethanolic extract attenuates chronic restraint stress aggravated DSS-induced ulcerative colitis in mice via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1 signaling pathways. J. Ethnopharmacol. 2023;301:115765. doi: 10.1016/j.jep.2022.115765. [DOI] [PubMed] [Google Scholar]
- 265.Shao X.X., Xu Y., Xiao H.Y., Hu Y., Jiang Y. Higenamine improves DSS-induced ulcerative colitis in mice through the Galectin-3/TLR4/NF-κB pathway. Tissue Cell. 2023;82:102111. doi: 10.1016/j.tice.2023.102111. [DOI] [PubMed] [Google Scholar]
- 266.Tan Z., Zhang Q., Zhao R., Huang T., Tian Y., Lin Y. A Comparative Study on the Effects of Different Sources of Carboxymethyl Poria Polysaccharides on the Repair of DSS-Induced Colitis in Mice. Int. J. Mol. Sci. 2023;24:9034. doi: 10.3390/ijms24109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Venkataraman B., Almarzooqi S., Raj V., Bhongade B.A., Patil R.B., Subramanian V.S., Attoub S., Rizvi T.A., Adrian T.E., Subramanya S.B. Molecular Docking Identifies 1,8-Cineole (Eucalyptol) as A Novel PPARγ Agonist That Alleviates Colon Inflammation. Int. J. Mol. Sci. 2023;24:6160. doi: 10.3390/ijms24076160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xu J., Tang C., Din A.U., Lu Y., Ma X., Zhang T., Wu J., Zuoqin D., Luo P., Wu J. Oligosaccharides of Polygonatum Cyrtonema Hua ameliorates dextran sulfate sodium-induced colitis and regulates the gut microbiota. Biomed. Pharmacother. 2023;161:114562. doi: 10.1016/j.biopha.2023.114562. [DOI] [PubMed] [Google Scholar]
- 269.Zhang J., Cheng S., Liang J., Qu J. Polysaccharide from fermented mycelium of Inonotus obliquus attenuates the ulcerative colitis and adjusts the gut microbiota in mice. Microb. Pathog. 2023;177:105990. doi: 10.1016/j.micpath.2023.105990. [DOI] [PubMed] [Google Scholar]
- 270.Minaiyan M., Dastanian Z., Zolfaghari B., Talebi A. Assessing the Anti-Colitis Properties of Aqueous and Hydroalcoholic Extracts of Pinus eldarica in Rats with Acetic Acid-Induced Colitis. Res. J. Pharmacogn. 2024;11:61–70. [Google Scholar]
- 271.Dong W.-R., Li Y.-Y., Liu T.-T., Zhou G., Chen Y.-X. Ethyl acetate extract of Terminalia chebula alleviates DSS-induced ulcerative colitis in C57BL/6 mice. Front. Pharmacol. 2023;14:1229772. doi: 10.3389/fphar.2023.1229772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kim Y.-M., Kim H.-Y., Jang J.-T., Hong S. Preventive Effect of Ecklonia cava Extract on DSS-Induced Colitis by Elevating Intestinal Barrier Function and Improving Pathogenic Inflammation. Molecules. 2023;28:8099. doi: 10.3390/molecules28248099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vezza T., Molina-Tijeras J.A., Rodríguez-Nogales A., Garrido-Mesa J., Cádiz-Gurrea M.d.l.L., Segura-Carretero A., González-Tejero M.R., Rodríguez-Cabezas M.E., Gálvez J., Algieri F. The Antioxidant Properties of Salvia verbenaca Extract Contribute to Its Intestinal Antiinflammatory Effects in Experimental Colitis in Rats. Antioxidants. 2023;12:2071. doi: 10.3390/antiox12122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Da Silva V.C., Guerra G.C.B., Araújo D.F.D.S., De Araújo E.R., De Araújo A.A., Dantas-Medeiros R., Zanatta A.C., Da Silva I.L.G., De Araújo Júnior R.F., Esposito D., et al. Chemopreventive and immunomodulatory effects of phenolic-rich extract of Commiphora leptophloeos against inflammatory bowel disease: Preclinical evidence. J. Ethnopharmacol. 2024;328:118025. doi: 10.1016/j.jep.2024.118025. [DOI] [PubMed] [Google Scholar]
- 275.Feng Y., Chen S., Song Y., Liu S., Duan Y., Cai M., Kong T., Zhang H. A novel Sagittaria sagittifolia L. polysaccharides mitigate DSS-induced colitis via modulation of gut microbiota and MAPK/NF-κB signaling pathways. Int. J. Biol. Macromol. 2024;254:127835. doi: 10.1016/j.ijbiomac.2023.127835. [DOI] [PubMed] [Google Scholar]
- 276.Feng Z., Ye W., Feng L. Bioactives and metabolites of Tetrastigma hemsleyanum root extract alleviate DSS-induced ulcerative colitis by targeting the SYK protein in the B cell receptor signaling pathway. J. Ethnopharmacol. 2024;322:117563. doi: 10.1016/j.jep.2023.117563. [DOI] [PubMed] [Google Scholar]
- 277.Gülada B., Cam M.E., Yüksel M., Akakın D., Taşkın T., Emre G., Şener G., Karakoyun B. Gilaburu (Viburnum opulus L.) fruit extract has potential therapeutic and prophylactic role in a rat model of acetic acid-induced oxidant colonic damage. J. Ethnopharmacol. 2024;322:117624. doi: 10.1016/j.jep.2023.117624. [DOI] [PubMed] [Google Scholar]
- 278.Hui H., Wang Z., Zhao X., Xu L., Yin L., Wang F., Qu L., Peng J. Gut microbiome-based thiamine metabolism contributes to the protective effect of one acidic polysaccharide from Selaginella uncinata (Desv.) Spring against inflammatory bowel disease. J. Pharm. Anal. 2024;14:177–195. doi: 10.1016/j.jpha.2023.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li W., Wang Y., Zhang Y., Fan Y., Liu J., Zhu K., Jiang S., Duan J.l. Lizhong decoction ameliorates ulcerative colitis by inhibiting ferroptosis of enterocytes via the Nrf2/SLC7A11/GPX4 pathway. J. Ethnopharmacol. 2024;326:117966. doi: 10.1016/j.jep.2024.117966. [DOI] [PubMed] [Google Scholar]
- 280.Liu Y., Wu J., Tan L., Li Z., Gao P., He S., Wang Q., Tang D., Wang C., Wang F., et al. (-)-Syringaresinol attenuates ulcerative colitis by improving intestinal epithelial barrier function and inhibiting inflammatory responses. Phytomedicine. 2024;124:155292. doi: 10.1016/j.phymed.2023.155292. [DOI] [PubMed] [Google Scholar]
- 281.Shah B., Solanki N. Aegeline attenuates TNBS-induced colitis by suppressing the -NF-κB -mediated NLRP3 inflammasome pathway in mice. Inflammopharmacology. 2024;32:2589–2599. doi: 10.1007/s10787-024-01493-0. [DOI] [PubMed] [Google Scholar]
- 282.Sun X., Jin X., Wang L., Lin Z., Feng H., Zhan C., Liu X., Cheng G. Fraxetin ameliorates symptoms of dextran sulphate sodium-induced colitis in mice. Heliyon. 2024;10:e23295. doi: 10.1016/j.heliyon.2023.e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zang Z., Li L., Yang M., Zhang H., Naeem A., Wu Z., Zheng Q., Song Y., Tao L., Wan Z., et al. Study on the ameliorative effect of honeysuckle on DSS-induced ulcerative colitis in mice. J. Ethnopharmacol. 2024;325:117776. doi: 10.1016/j.jep.2024.117776. [DOI] [PubMed] [Google Scholar]
- 284.Cazarin C.B.B., Rodriguez-Nogales A., Algieri F., Utrilla M.P., Rodríguez-Cabezas M.E., Garrido-Mesa J., Guerra-Hernández E., Braga P.A.C., Reys F.G.R., Maróstica M.R., Jr., et al. Intestinal anti-inflammatory effects of Passiflora edulis peel in the dextran sodium sulphate model of mouse colitis. J. Funct. Foods. 2016;26:565–576. doi: 10.1016/j.jff.2016.08.020. [DOI] [Google Scholar]
- 285.Shao X., Sun C., Tang X., Zhang X., Han D., Liang S., Qu R., Hui X., Shan Y., Hu L., et al. Anti-Inflammatory and Intestinal Microbiota Modulation Properties of Jinxiang Garlic (Allium sativum L.) Polysaccharides toward Dextran Sodium Sulfate-Induced Colitis. J. Agric. Food Chem. 2020;68:12295–12309. doi: 10.1021/acs.jafc.0c04773. [DOI] [PubMed] [Google Scholar]
- 286.Elhennawy M.G., Abdelaleem E.A., Zaki A.A., Mohamed W.R. Cinnamaldehyde and hesperetin attenuate TNBS-induced ulcerative colitis in rats through modulation of the JAk2/STAT3/SOCS3 pathway. J. Biochem. Mol. Toxicol. 2021;35:e22730. doi: 10.1002/jbt.22730. [DOI] [PubMed] [Google Scholar]
- 287.Siracusa R., Fusco R., Peritore A.F., Cordaro M., D’Amico R., Genovese T., Gugliandolo E., Crupi R., Smeriglio A., Mandalari G., et al. The Antioxidant and Anti-Inflammatory Properties of Anacardium occidentale L. Cashew Nuts in a Mouse Model of Colitis. Nutrients. 2020;12:834. doi: 10.3390/nu12030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Prakash A.N., Prasad N., Puppala E.R., Panda S.R., Jain S., Ravichandiran V., Singh M., Naidu V.G.M. Loganic acid protects against ulcerative colitis by inhibiting TLR4/NF-κB mediated inflammation and activating the SIRT1/Nrf2 anti-oxidant responses in-vitro and in-vivo. Int. Immunopharmacol. 2023;122:110585. doi: 10.1016/j.intimp.2023.110585. [DOI] [PubMed] [Google Scholar]
- 289.Chen Z., Wang H., Tan L., Liu X. Protective Effects of Four Structurally Distinct Sanshools Ameliorate Dextran Sodium Sulfate-Induced Ulcerative Colitis by Restoring Intestinal Barrier Function and Modulating the Gut Microbiota. Antioxidants. 2024;13:153. doi: 10.3390/antiox13020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ouyang Q., Li X., Liang Y., Liu R. Sea Buckthorn Polysaccharide Ameliorates Colitis. Nutrients. 2024;16:1280. doi: 10.3390/nu16091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu B., Lin Q., Yang T., Zeng L., Shi L., Chen Y., Luo F. Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct. 2015;6:3454–3463. doi: 10.1039/C5FO00563A. [DOI] [PubMed] [Google Scholar]
- 292.Pervin M., Hasnat A., Lim J.-H., Lee Y.-M., Kim E.O., Um B.-H., Lim B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016;28:103–113. doi: 10.1016/j.jnutbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 293.Karakoyun B., Ertaş B., Yüksel M., Akakın D., Çevik Ú., Şener G. Ameliorative effects of riboflavin on acetic acid-induced colonic injury in rats. Clin. Exp. Pharmacol. Physiol. 2017;45:563–572. doi: 10.1111/1440-1681.12894. [DOI] [PubMed] [Google Scholar]
- 294.Araújo D.F.D.S., Guerra G.C.B., Júnior R.F.D.A., de Araújo A.A., de Assis P.O.A., de Medeiros A.N., de Sousa Y.R.F., Pintado M.M.E., Gálvez J., Queiroga R.C.R.D.E. Goat whey ameliorates intestinal inflammation on acetic acid-induced colitis in rats. J. Dairy Sci. 2016;99:9383–9394. doi: 10.3168/jds.2016-10930. [DOI] [PubMed] [Google Scholar]
- 295.Balaha M., Kandeel S., Elwan W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016;146:40–51. doi: 10.1016/j.lfs.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 296.Choi K.-C., Cho S.-W., Lee J.-C. Red bean extracts protect rats against intestinal inflammatory damage. Food Sci. Biotechnol. 2016;25:349–353. doi: 10.1007/s10068-016-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Colares J.R., Schemitt E.G., Hartmann R.M., Moura R.M., Morgan-Martins M.I., Fillmann H.S., Fillmann L., Marroni N.P. Effect of lecithin on oxidative stress in an experimental model of rats colitis induced by acetic acid. J. Coloproctology. 2016;36:97–103. doi: 10.1016/j.jcol.2016.03.002. [DOI] [Google Scholar]
- 298.Ewees M.G., Messiha B.A.S., Abo-Saif A.A., El-Latif H.A. Is Coenzyme Q10 Effective in Protection against Ulcerative Colitis? An Experimental Study in Rats. Biol. Pharm. Bull. 2016;39:159–166. doi: 10.1248/bpb.b16-00124. [DOI] [PubMed] [Google Scholar]
- 299.Khodir A.E., Atef H., Said E., ElKashef H.A., Salem H.A. Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacology. 2017;25:119–135. doi: 10.1007/s10787-016-0305-0. [DOI] [PubMed] [Google Scholar]
- 300.Nooh H.Z., Nour-Eldien N.M. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochem. 2016;118:588–595. doi: 10.1016/j.acthis.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 301.Palla A.H., Iqbal N.T., Minhas K., Gilani A.-H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 2016;38:153–166. doi: 10.1016/j.intimp.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 302.Shi L., Lin Q., Yang T., Nie Y., Li X., Liu B., Shen J., Liang Y., Tang Y., Luo F. Oral administration of Lentinus edodes β-glucans ameliorates DSS-induced ulcerative colitis in mice via MAPK-Elk-1 and MAPK-PPARγpathways. Food Function. 2016;7:4614–4627. doi: 10.1039/C6FO01043A. [DOI] [PubMed] [Google Scholar]
- 303.Kaur R., Thakur S., Rastogi P., Kaushal N. Resolution of Cox mediated inflammation by Se supplementation in mouse experimental model of colitis. PLoS ONE. 2018;13:e0201356. doi: 10.1371/journal.pone.0201356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shi C., Yue F., Shi F., Qin Q., Wang L., Wang G., Mu L., Liu D., Li Y., Yu T., et al. Selenium-Containing Amino Acids Protect Dextran Sulfate Sodium-Induced Colitis via Ameliorating Oxidative Stress and Intestinal Inflammation. J. Inflamm. Res. 2021;14:85–95. doi: 10.2147/JIR.S288412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li T., Zhu K., Wang L., Dong Y., Huang J. Stabilization by Chaperone GroEL in Biogenic Selenium Nanoparticles Produced from Bifidobacterium animalis H15 for the Treatment of DSS-Induced Colitis. ACS Appl. Mater. Interfaces. 2024;16:13439–13452. doi: 10.1021/acsami.3c16340. [DOI] [PubMed] [Google Scholar]
- 306.Lee W.T., Tung Y.T., Wu C.C., Tu P.S., Yen G.C. Camellia Oil (Camellia oleifera Abel.) Modifies the Composition of Gut Microbiota and Alleviates Acetic Acid-Induced Colitis in Rats. J. Agric. Food Chem. 2018;66:7384–7392. doi: 10.1021/acs.jafc.8b02166. [DOI] [PubMed] [Google Scholar]
- 307.Maghool F., Keshavarzi Z., Nurmohammadi F., Majlesi S., Maghool F. Protective effects of walnut extract against oxidative damage in acetic acid-induced experimental colitis rats. Physiol. Pharmacol. 2019;36:2096–2103. [Google Scholar]
- 308.Salamatian M., Mohammadi V., Froushani S.M.A. Ameliorative effects of aqueous cinnamon extract on ulcerative colitis in rats. Physiol. Pharmacol. 2019;23:140–149. [Google Scholar]
- 309.Kim M.S., Kim Y.D., Kang S., Kwon O., Shin J.-H., Kim J.Y. Cinnamon(Cinnamomum japonicum) subcritical water extract suppresses gut damage induced by dextran sodium sulfate in mouse colitis model. J. Funct. Foods. 2021;87:104775. doi: 10.1016/j.jff.2021.104775. [DOI] [Google Scholar]
- 310.Saw T.Y., Malik N.A., Lim K.P., Teo C.W.L., Wong E.S.M., Kong S.C., Fong C.W., Petkov J., Yap W.N. Oral Supplementation of Tocotrienol-Rich Fraction Alleviates Severity of Ulcerative Colitis in Mice. J. Nutr. Sci. Vitaminol. 2019;65:318–327. doi: 10.3177/jnsv.65.318. [DOI] [PubMed] [Google Scholar]
- 311.Sharma M., Kaur R., Kaushik K., Kaushal N. Redox modulatory protective effects of omega-3 fatty acids rich fish oil against experimental colitis. Toxicol. Mech. Methods. 2019;29:244–254. doi: 10.1080/15376516.2018.1553220. [DOI] [PubMed] [Google Scholar]
- 312.El Mahdy R.N., Nader M.A., Helal M.G., Abu-Risha S.E., Abdelmageed M.E. Eicosapentaenoic acid mitigates ulcerative colitis-induced by acetic acid through modulation of NF-κB and TGF-α/ EGFR signaling pathways. Life Sci. 2023;327:121820. doi: 10.1016/j.lfs.2023.121820. [DOI] [PubMed] [Google Scholar]
- 313.Kwon S.-H., Kothari D., Jung H.-I., Lim J.-M., Kim W.-L., Kwon H.-C., Han S.-G., Seo S.-M., Choi Y.-K., Kim S.-K., et al. Noni juice-fortified yogurt mitigates dextran sodium sulfate-induced colitis in mice through the modulation of inflammatory cytokines. J. Funct. Foods. 2021;86:104652. doi: 10.1016/j.jff.2021.104652. [DOI] [Google Scholar]
- 314.Deng Z., Cui C., Wang Y., Ni J., Zheng L., Wei H.K., Peng J. FSGHF3 and peptides, prepared from fish skin gelatin, exert a protective effect on DSS-induced colitis via the Nrf2 pathway. Food Funct. 2020;11:414–423. doi: 10.1039/C9FO02165E. [DOI] [PubMed] [Google Scholar]
- 315.Li P., Xiao N., Zeng L., Xiao J., Huang J., Xu Y., Chen Y., Ren Y., Du B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr. Polym. 2020;250:116958. doi: 10.1016/j.carbpol.2020.116958. [DOI] [PubMed] [Google Scholar]
- 316.Ganapathy A.S., Saha K., Wang A., Arumugam P., Dharmaprakash V., Yochum G., Koltun W., Nighot M., Perdew G., Thompson T.A. Alpha-tocopherylquinone differentially modulates claudins to enhance intestinal epithelial tight junction barrier via AhR and Nrf2 pathways. Cell Rep. 2023;42:112705. doi: 10.1016/j.celrep.2023.112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Zhu L., Song Y., Liu H., Wu M., Gong H., Lan H., Zheng X. Gut microbiota regulation and anti-inflammatory effect of β-carotene in dextran sulfate sodium-stimulated ulcerative colitis in rats. J. Food Sci. 2021;86:2118–2130. doi: 10.1111/1750-3841.15684. [DOI] [PubMed] [Google Scholar]
- 318.Wang F., Yuan M., Shao C., Ji N., Zhang H., Li C. Momordica charantia-Derived Extracellular Vesicles Provide Antioxidant Protection in Ulcerative Colitis. Molecules. 2023;28:6182. doi: 10.3390/molecules28176182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Prabha S., Tamoli S., Raghavamenon A.C., Manu K.A. Virgin Coconut Oil Alleviates Dextran Sulphate-Induced Inflammatory Bowel Disease and Modulates Inflammation and Immune Response in Mice. J. Am. Nutr. Assoc. 2024;43:61–71. doi: 10.1080/27697061.2023.2266742. [DOI] [PubMed] [Google Scholar]
- 320.Wu M., Wang Q., Li X., Yu S., Zhao F., Wu X., Fan L., Liu X., Zhao Q., He X., et al. Gut microbiota-derived 5-hydroxyindoleacetic acid from pumpkin polysaccharides supplementation alleviates colitis via MAPKs-PPARγ/NF-κB inhibition. Int. J. Biol. Macromol. 2024;264:130385. doi: 10.1016/j.ijbiomac.2024.130385. [DOI] [PubMed] [Google Scholar]
- 321.Yoon J.W., Ahn S.I., Jhoo J.W., Kim G.Y. Antioxidant Activity of Yogurt Fermented at Low Temperature and Its Anti-inflammatory Effect on DSS-induced Colitis in Mice. Food Sci. Anim. Resour. 2019;39:162–176. doi: 10.5851/kosfa.2019.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Chen C.-L., Hsu P.-Y., Pan T.-M. Therapeutic effects of Lactobacillus paracasei subsp. paracasei NTU 101 powder on dextran sulfate sodium-induced colitis in mice. J. Food Drug Anal. 2018;27:83–92. doi: 10.1016/j.jfda.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Choi S.-H., Lee S.-H., Kim M.G., Lee H.J., Kim G.-B. Lactobacillus plantarum CAU1055 ameliorates inflammation in lipopolysaccharide-induced RAW264.7 cells and a dextran sulfate sodium-induced colitis animal model. J. Dairy Sci. 2019;102:6718–6725. doi: 10.3168/jds.2018-16197. [DOI] [PubMed] [Google Scholar]
- 324.Catinean A., Neag M.A., Krishnan K., Muntean D.M., Bocsan C.I., Pop R.M., Mitre A.O., Melincovici C.S., Buzoianu A.D. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients. 2020;12:3607. doi: 10.3390/nu12123607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Din A.U., Hassan A., Zhu Y., Zhang K., Wang Y., Li T., Wang Y., Wang G. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J. Nutr. Biochem. 2020;79:108353. doi: 10.1016/j.jnutbio.2020.108353. [DOI] [PubMed] [Google Scholar]
- 326.Hu T., Wang H., Xiang C., Mu J., Zhao X. Preventive Effect of Lactobacillus acidophilus XY27 on DSS-Induced Ulcerative Colitis in Mice. Drug Des. Dev. Ther. 2020;14:5645–5657. doi: 10.2147/DDDT.S284422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cordeiro B.F., Alves J.L., Belo G.A., Oliveira E.R., Braga M.P., da Silva S.H., Lemos L., Guimarães J.T., Silva R., Rocha R.S., et al. Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model. Front. Microbiol. 2021;12:623920. doi: 10.3389/fmicb.2021.623920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Gao H., Li Y., Sun J., Xu H., Wang M., Zuo X., Fu Q., Guo Y., Chen Z., Zhang P. Saccharomyces boulardii Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating NF-κB and Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2021;2021:1622375. doi: 10.1155/2021/1622375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shi J., Xie Q., Yue Y., Chen Q., Zhao L., Evivie S.E., Li B., Huo G. Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice. Food Funct. 2021;12:5130–5143. doi: 10.1039/D1FO00317H. [DOI] [PubMed] [Google Scholar]
- 330.Jeong Y., Kim D.H., Lee K.W. Homeostasis effects of fermented Maillard reaction products by Lactobacillus gasseri 4M13 in dextran sulfate sodium-induced colitis mice. J. Sci. Food Agric. 2022;102:434–444. doi: 10.1002/jsfa.11374. [DOI] [PubMed] [Google Scholar]
- 331.Kangwan N., Kongkarnka S., Boonkerd N., Unban K., Shetty K., Khanongnuch C. Protective Effect of Probiotics Isolated from Traditional Fermented Tea Leaves (Miang) from Northern Thailand and Role of Synbiotics in Ameliorating Experimental Ulcerative Colitis in Mice. Nutrients. 2022;14:227. doi: 10.3390/nu14010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Liu Q., Jian W., Wang L., Yang S., Niu Y., Xie S.J., Hayer K., Chen K., Zhang Y., Guo Y., et al. Alleviation of DSS-induced colitis in mice by a new-isolated Lactobacillus acidophilus C4. Front. Microbiol. 2023;14:11. doi: 10.3389/fmicb.2023.1137701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang H., Zhang X., Kou X., Zhai Z., Hao Y. A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients. 2023;15:4993. doi: 10.3390/nu15234993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Ma X.W., Hu Y.C., Li X., Zheng X.T., Wang Y.T., Zhang J.M., Fu C., Geng F. Periplaneta americana Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Rats by Keap1/Nrf-2 Activation, Intestinal Barrier Function, and Gut Microbiota Regulation. Front. Pharmacol. 2018;9:944. doi: 10.3389/fphar.2018.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Peng S., Shen L., Yu X., Wu J., Zha L., Xia Y., Luo H. miR-200a attenuated oxidative stress, inflammation, and apoptosis in dextran sulfate sodium-induced colitis through activation of Nrf2. Front. Immunol. 2023;14:1196065. doi: 10.3389/fimmu.2023.1196065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wu J., Zhang Z., Wu Q., Zhang L., Chen Z., Zhao H., Wu X., Zhao Y., Zhang C., Ge J., et al. Antioxidative effect of Periplaneta americana extract on dextran sulfate sodium-induced ulcerative colitis through activation of the Nrf2 signal. Pharm. Biol. 2023;61:949–962. doi: 10.1080/13880209.2023.2220351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Abd-Ellatieff H.A., Georg K., Abourawash A.-R.A., Ghazy E.W., Samak D.H., Goda W.M. Aspergillus awamori: Potential antioxidant, anti-inflammatory, and anti-apoptotic activities in acetic acid-induced ulcerative colitis in rats. Inflammopharmacology. 2024;32:2541–2553. doi: 10.1007/s10787-024-01489-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wang R., Luo Y., Lu Y., Wang D., Wang T., Pu W., Wang Y. Maggot Extracts Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis via the Activation of Nrf2. Oxidative Med. Cell. Longev. 2019;2019:4703253. doi: 10.1155/2019/4703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zbakh H., Talero E., Avila J., Alcaide A., de los Reyes C., Zubia E., Motilva V. The Algal Meroterpene 11-Hydroxy-1′-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs. 2016;14:149. doi: 10.3390/md14080149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Garcia F.A.O., Sales-Campos H., Yuen V.G., Machado J.R., Viana G.S.B., Oliveira C.J.F., McNeill J.H. Arthrospira (Spirulina) platensis Attenuates Dextran Sulfate Sodium-induced Colitis in Mice by Suppressing Key Pro-inflammatory Cytokines. Korean J. Gastroenterol. 2020;76:150–158. doi: 10.4166/kjg.2020.76.3.150. [DOI] [PubMed] [Google Scholar]
- 341.Morsy M.A., Gupta S., Nair A.B., Venugopala K.N., Greish K., El-Daly M. Protective Effect of Spirulina platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients. 2019;11:2309. doi: 10.3390/nu11102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Hua Z., Hui L., Haihua W. Potential protective effects of the water-soluble Chinese propolis on experimental ulcerative colitis. J. Tradit. Chin. Med. 2023;43:925–933. doi: 10.19852/j.cnki.jtcm.20230727.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Lu K., Liu L., Lin P., Dong X., Ni L., Che H., Xie W. Saccharina japonica Ethanol Extract Ameliorates Dextran Sulfate Sodium-Induced Colitis via Reshaping Intestinal Microenvironment and Alleviating Inflammatory Response. Foods. 2023;12:1671. doi: 10.3390/foods12081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zizzo M.G., Caldara G., Bellanca A., Nuzzo D., Di Carlo M., Scoglio S., Serio R. AphaMax®, an Aphanizomenon Flos-Aquae Aqueous Extract, Exerts Intestinal Protective Effects in Experimental Colitis in Rats. Nutrients. 2020;12:3635. doi: 10.3390/nu12123635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Xie J., Liu L., Li H., Che H., Xie W. Ink melanin from Sepiapharaonis ameliorates colitis in mice via reducing oxidative stress, andprotecting the intestinal mucosal barrier. Food Res. Int. 2022;151:110888. doi: 10.1016/j.foodres.2021.110888. [DOI] [PubMed] [Google Scholar]
- 346.Xiang X.-W., Zhou X.-L., Wang R., Shu C.-H., Zhou Y.-F., Ying X.-G., Zheng B. Protective Effect of Tuna Bioactive Peptide on Dextran Sulfate Sodium-Induced Colitis in Mice. Mar. Drugs. 2021;19:127. doi: 10.3390/md19030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Guo H.-X., Wang B.-B., Wu H.-Y., Feng H.-Y., Zhang H.-Y., Gao W., Yuan B. Turtle peptide and its derivative peptide ameliorated DSS-induced ulcerative colitis by inhibiting inflammation and modulating the composition of the gut microbiota. Int. Immunopharmacol. 2024;132:112024. doi: 10.1016/j.intimp.2024.112024. [DOI] [PubMed] [Google Scholar]
- 348.Ávila-Román J., Talero E., Rodríguez-Luna A., García-Mauriño S., Motilva V. Anti-inflammatory effects of an oxylipin-containing lyophilised biomass from a microalga in a murine recurrent colitis model. Br. J. Nutr. 2016;116:2044–2052. doi: 10.1017/S0007114516004189. [DOI] [PubMed] [Google Scholar]
- 349.Nemoto M., Kuda T., Eda M., Yamakawa H., Takahashi H., Kimura B. Protective Effects of Mekabu Aqueous Solution Fermented by Lactobacillus plantarum Sanriku-SU7 on Human Enterocyte-Like HT-29-luc Cells and DSS-Induced Murine IBD Model. Probiotics Antimicrob. Proteins. 2017;9:48–55. doi: 10.1007/s12602-016-9226-x. [DOI] [PubMed] [Google Scholar]
- 350.Mendonça E.L.S.S., Xavier J.A., Fragoso M.B.T., Silva M.O., Escodro P.B., Oliveira A.C.M., Tucci P., Saso L., Goulart M.O.F. E-Stilbenes: General Chemical and Biological Aspects, Potential Pharmacological Activity Based on the Nrf2 Pathway. Pharmaceuticals. 2024;17:232. doi: 10.3390/ph17020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Alzoghaibi M.A., Al Mofleh I.A., Al-Jebreen A.M. Lipid peroxides in patients with inflammatory bowel disease. Saudi J. Gastroenterol. 2007;13:187–190. doi: 10.4103/1319-3767.36750. [DOI] [PubMed] [Google Scholar]
- 352.Day R.M., Suzuki Y.J. Cell proliferation, reactive oxygen and cellular glutathione. Dose-Response. 2006;3:425–442. doi: 10.2203/dose-response.003.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 354.Chen Z., Su Z., Pang W., Huang Y., Lin J., Ding Z., Wu C.L., Li X.H., Luo Y.Z., Qin L.Q., et al. Antioxidant status of serum bilirubin and uric acid in patients with polymyositis and dermatomyositis. Int. J. Neurosci. 2017;127:617–623. doi: 10.1080/00207454.2016.1220380. [DOI] [PubMed] [Google Scholar]
- 355.Bin P., Huang R., Zhou X. Oxidation Resistance of the Sulfur Amino Acids: Methionine and Cysteine. BioMed Res. Int. 2017;2017:9584932. doi: 10.1155/2017/9584932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Sies H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021;41:101867. doi: 10.1016/j.redox.2021.101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Niki E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016;595:19–24. doi: 10.1016/j.abb.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 358.Bienertova-Vasku J., Lenart P., Scheringer M. Eustress and Distress: Neither Good Nor Bad, but Rather the Same? BioEssays. 2020;42:e1900238. doi: 10.1002/bies.201900238. [DOI] [PubMed] [Google Scholar]
- 359.Zizzo M.G., Caldara G., Bellanca A., Nuzzo D., Di Carlo M., Serio R. Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology. 2019;27:349–359. doi: 10.1007/s10787-018-0506-9. [DOI] [PubMed] [Google Scholar]
- 360.Feng P., Li Q., Liu L., Wang S., Wu Z., Tao Y., Huang P., Wang P. Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022;23:3832. doi: 10.3390/ijms23073832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Crespo I., San-Miguel B., Mauriz J.L., de Urbina J.J.O., Almar M., Tuñón M.J., González-Gallego J. Protective effect of protocatechuic acid on TNBS-induced colitis in mice is associated with modulation of the SphK/S1P signaling pathway. Nutrients. 2017;9:288. doi: 10.3390/nu9030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zhang L., Xue H., Zhao G., Qiao C., Sun X., Pang C., Zhang D. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol. Med. Rep. 2019;19:3053–3060. doi: 10.3892/mmr.2019.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Abhari B.M., Farokhnezhad Afshar P., Alimoradzadeh R., Mirmiranpour H. Comparing the effect of including omega-3 to treatment regimen in elderly patients with ulcerative colitis with placebo: A randomized clinical trial. Immunopathol. Persa. 2020;6:e10. doi: 10.15171/ipp.2020.10. [DOI] [Google Scholar]
- 364.Geerling B.J., Badart-Smook A., van Deursen C., van Houwelingen A.C., Russel M., Stockbrügger R.W., Brummer R.J. Nutritional supplementation with n-3 fatty acids and antioxidants in patients with Crohn’s disease in remission: Effects on antioxidant status and fatty acid profile. Inflamm. Bowel Dis. 2000;6:77–84. doi: 10.1097/00054725-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 365.Martins A.S., Araújo O.R., Gomes A.D., Araujo F.L., Oliveira Junior J., Vasconcelos J.K., Rodrigues Junior J.I., Cerqueira I.T., Lins Neto M.A.F., Bueno N.B., et al. Effect of Curcumin Plus Piperine on Redox Imbalance, Fecal Calprotectin and Cytokine Levels in Inflammatory Bowel Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pharmaceuticals. 2024;17:849. doi: 10.3390/ph17070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Moradi S., Bagheri R., Amirian P., Zarpoosh M., Cheraghloo N., Wong A., Zobeiri M., Entezari M.H. Effects of Spirulina supplementation in patients with ulcerative colitis: A double-blind, placebo-controlled randomized trial. BMC Complement. Med. Ther. 2024;24:109. doi: 10.1186/s12906-024-04400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Nematgorgani S., Agah S., Shidfar F., Gohari M., Faghihi A. Effects of Urtica dioica leaf extract on inflammation, oxidative stress, ESR, blood cell count and quality of life in patients with inflammatory bowel disease. J. Herb. Med. 2017;9:32–41. doi: 10.1016/j.hermed.2017.05.002. [DOI] [Google Scholar]
- 368.Samsamikor M., Daryani N.E., Asl P.R., Hekmatdoost A. Resveratrol Supplementation and Oxidative/Anti-Oxidative Status in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med Res. 2016;47:304–309. doi: 10.1016/j.arcmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 369.Samsami-Kor M., Daryani N.E., Asl P.R., Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 370.Tahvilian N., Masoodi M., Kashani A.F., Vafa M., Aryaeian N., Heydarian A., Moradi N., Farsi F. Effects of saffron supplementation on oxidative/antioxidant status and severity of disease in ulcerative colitis patients: A randomized, double-blind, placebo-controlled study. Phytotherapy Res. 2021;35:946–953. doi: 10.1002/ptr.6848. [DOI] [PubMed] [Google Scholar]
- 371.Mulder T.P.J., Veer A.V.D.S., Verspaget H.W., Griffioen G., Peña A.S., Janssens A.R., Lamers C.B. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 1994;9:472–477. doi: 10.1111/j.1440-1746.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 372.Koláček M., Muchová J., Dvořáková M., Paduchová Z., Žitňanová I., Čierna I., Országhová Z., Székyová D., Jajcaiová-Zedníčková N., Kovács L., et al. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn’s disease--a pilot study. Free. Radic. Res. 2013;47:624–634. doi: 10.3109/10715762.2013.807508. [DOI] [PubMed] [Google Scholar]
- 373.Barbosa D.S., Cecchini R., El Kadri M.Z., Rodriguez M.A., Burini R.C., Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837–842. doi: 10.1016/S0899-9007(03)00162-X. [DOI] [PubMed] [Google Scholar]
- 374.Karimi Karimi S., Tabataba-Vakili S., Yari Z., Alborzi F., Hedayati M., Ebrahimi-Daryani N., Hekmatdoost A. The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J. 2019;18:16. doi: 10.1186/s12937-019-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Nikkhah-Bodaghi M., Darabi Z., Agah S., Hekmatdoost A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytotherapy Res. 2019;33:1027–1032. doi: 10.1002/ptr.6296. [DOI] [PubMed] [Google Scholar]
- 376.Nikkhah-Bodaghi M., Maleki I., Agah S., Hekmatdoost A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement. Ther. Med. 2019;43:1–6. doi: 10.1016/j.ctim.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 377.Papada E., Forbes A., Amerikanou C., Torović L., Kalogeropoulos N., Tzavara C., Triantafillidis J.K., Kaliora A.C. Antioxidative Efficacy of a Pistacia Lentiscus Supplement and Its Effect on the Plasma Amino Acid Profile in Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Nutrients. 2018;10:1779. doi: 10.3390/nu10111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Silvestrini A., Meucci E., Ricerca B.M., Mancini A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023;24:10978. doi: 10.3390/ijms241310978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Aghdassi E., Wendland B.E., Steinhart A.H., Wolman S.L., Jeejeebhoy K., Allard J.P. Antioxidant vitamin supplementation in Crohn’s disease decreases oxidative stress. A randomized controlled trial. Am. J. Gastroenterol. 2003;98:348–353. doi: 10.1111/j.1572-0241.2003.07226.x. [DOI] [PubMed] [Google Scholar]
- 380.Akobeng A.K., Richmond K., Miller V., Thomas A.G. Effect of exclusive enteral nutritional treatment on plasma antioxidant concentrations in childhood Crohn’s disease. Clin. Nutr. 2007;26:51–56. doi: 10.1016/j.clnu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 381.Morshedzadeh N., Shahrokh S., Aghdaei H.A., Pourhoseingholi M.A., Chaleshi V., Hekmatdoost A., Karimi S., Zali M.R., Mirmiran P. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complement. Ther. Med. 2019;46:36–43. doi: 10.1016/j.ctim.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 382.Khazdouz M., Daryani N.E., Cheraghpour M., Alborzi F., Hasani M., Ghavami S.B., Shidfar F. The effect of selenium supplementation on disease activity and immune-inflammatory biomarkers in patients with mild-to-moderate ulcerative colitis: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2023;62:3125–3134. doi: 10.1007/s00394-023-03214-9. [DOI] [PubMed] [Google Scholar]
- 383.Trebble T.M., Arden N.K., A Wootton S., Calder P.C., Mullee M.A., Fine D.R., Stroud M.A. Fish oil and antioxidants alter the composition and function of circulating mononuclear cells in Crohn disease. Am. J. Clin. Nutr. 2004;80:1137–1144. doi: 10.1093/ajcn/80.5.1137. [DOI] [PubMed] [Google Scholar]
- 384.von Martels J.Z.H., Bourgonje A.R., Klaassen M.A.Y., A A Alkhalifah H., Sadabad M.S., Vila A.V., Gacesa R., Gabriëls R.Y., Steinert R.E., Jansen B.H., et al. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP study] J. Crohn’s Colitis. 2019;14:595–607. doi: 10.1093/ecco-jcc/jjz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Heydarian A., Kashani A.H.F., Masoodi M., Aryaeian N., Vafa M., Tahvilian N., Hosseini A.F., Fallah S., Moradi N., Farsi F. Effects of saffron supplementation on serum inflammatory markers and quality of life in patients with ulcerative colitis: A double blind randomized controlled clinical trial. J. Herb. Med. 2022;36:100593. doi: 10.1016/j.hermed.2022.100593. [DOI] [Google Scholar]
- 386.Kaliora A.C., Stathopoulou M.G., Triantafillidis J.K., Dedoussis G.V., Andrikopoulos N.K. Chios mastic treatment of patients with active Crohn’s disease. World. J. Gastroenterol. 2007;13:748–753. doi: 10.3748/wjg.v13.i5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Therkelsen S.P., Hetland G., Lyberg T., Lygren I., Johnson E. Cytokine Levels After Consumption of a Medicinal Agaricus blazei Murill-Based Mushroom Extract, AndoSan™, in Patients with Crohn’s Disease and Ulcerative Colitis in a Randomized Single-Blinded Placebo-Controlled Study. Scand. J. Immunol. 2016;84:323–331. doi: 10.1111/sji.12476. [DOI] [PubMed] [Google Scholar]
- 388.Alemany-Cosme E., Sáez-González E., Moret I., Mateos B., Iborra M., Nos P., Sandoval J., Beltrán B. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants. 2021;10:64. doi: 10.3390/antiox10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rogler G., Singh A., Kavanaugh A., Rubin D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118–1132. doi: 10.1053/j.gastro.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.da Paz Martins A.S., de Andrade K.Q., de Araujo O.R.P., da Conceicao G.C.M., da Silva Gomes A., Goulart M.O.F., Moura F.A. Extraintestinal Manifestations in Induced Colitis: Controversial Effects of N-Acetylcysteine on Colon, Liver, and Kidney. Oxidative Med. Cell. Longev. 2023;2023:8811463. doi: 10.1155/2023/8811463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Duryee M.J., Ahmad R., Eichele D.D., Hunter C.D., Mitra A., Talmon G.A., Singh S., Smith L.M., Rosen M.J., Dhawan P., et al. Identification of Immunoglobulin G Autoantibody Against Malondialdehyde-Acetaldehyde Adducts as a Novel Serological Biomarker for Ulcerative Colitis. Clin. Transl. Gastroenterol. 2022;13:e00469. doi: 10.14309/ctg.0000000000000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Bouzid D., Gargouri B., Mansour R., Amouri A., Tahri N., Lassoued S., Masmoudi H. Oxidative stress markers in intestinal mucosa of Tunisian inflammatory bowel disease patients. Saudi J. Gastroenterol. 2013;19:131–135. doi: 10.4103/1319-3767.111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Marrocco I., Altieri F., Peluso I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017;2017:6501046. doi: 10.1155/2017/6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Menzel A., Samouda H., Dohet F., Loap S., Ellulu M.S., Bohn T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice-Which to Use Regarding Disease Outcomes? Antioxidants. 2021;10:414. doi: 10.3390/antiox10030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chandra M., Panchatcharam M., Miriyala S. Biomarkers in ROS and Role of Isoprostanes in Oxidative Stress. In: Rizwan A., editor. Free Radicals and Diseases. IntechOpen; Rijeka, Croatia: 2016. Chapter 7. [Google Scholar]
- 396.Iborra M., Beltrán B., Nos P. Noninvasive Testing for Mucosal Inflammation in Inflammatory Bowel Disease. Gastrointest. Endosc. Clin. North Am. 2016;26:641–656. doi: 10.1016/j.giec.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 397.Cracowski J.-L., Bonaz B., Bessard G., Bessard J., Anglade C., Fournet J. Increased urinary F2-isoprostanes in patients with Crohn’s disease. Am. J. Gastroenterol. 2002;97:99–103. doi: 10.1111/j.1572-0241.2002.05427.x. [DOI] [PubMed] [Google Scholar]
- 398.Cai Z., Yan L.J. Protein Oxidative Modifications: Beneficial Roles in Disease and Health. J. Biochem. Pharmacol. Res. 2013;1:15–26. [PMC free article] [PubMed] [Google Scholar]
- 399.Cadet J., Davies K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017;107:2–12. doi: 10.1016/j.freeradbiomed.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Wiseman H., Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Pt 13Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Juan C.A., de la Lastra J.M.P., Plou F.J., Pérez-Lebeña E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021;22:4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Yun B.H., Guo J., Bellamri M., Turesky R.J. DNA adducts: Formation, biological effects, and new biospecimens for mass spectrometric measurements in humans. Mass Spectrom. Rev. 2020;39:55–82. doi: 10.1002/mas.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Andrés C.M.C., de la Lastra J.M.P., Juan C.A., Plou F.J., Pérez-Lebeña E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023;24:15240. doi: 10.3390/ijms242015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Foppa C., Rizkala T., Repici A., Hassan C., Spinelli A. Microbiota and IBD: Current knowledge and future perspectives. Dig. Liver Dis. 2024;56:911–922. doi: 10.1016/j.dld.2023.11.015. [DOI] [PubMed] [Google Scholar]
- 405.Li L., Peng P., Ding N., Jia W., Huang C., Tang Y. Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease? Antioxidants. 2023;12:967. doi: 10.3390/antiox12040967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Park C., Cha H.-J., Lee H., Kim G.-Y., Choi Y.H. The regulation of the TLR4/NF-κB and Nrf2/HO-1 signaling pathways is involved in the inhibition of lipopolysaccharide-induced inflammation and oxidative reactions by morroniside in RAW 264.7 macrophages. Arch. Biochem. Biophys. 2021;706:108926. doi: 10.1016/j.abb.2021.108926. [DOI] [PubMed] [Google Scholar]
- 407.Wang L., Zhang P., Li C., Xu F., Chen J. A polysaccharide from Rosa roxburghii Tratt fruit attenuates high-fat diet-induced intestinal barrier dysfunction and inflammation in mice by modulating the gut microbiota. Food Funct. 2022;13:530–547. doi: 10.1039/D1FO03190B. [DOI] [PubMed] [Google Scholar]
- 408.Cheng J., Liu D., Huang Y., Chen L., Li Y., Yang Z., Fu S., Hu G. Phlorizin Mitigates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota and Inhibiting Ferroptosis. J. Agric. Food Chem. 2023;71:16043–16056. doi: 10.1021/acs.jafc.3c01497. [DOI] [PubMed] [Google Scholar]
- 409.Huang X.X., Hu J.T., Zhang H., Li J., Zhu X., Liu Y.Y., Liang Y., Mei Y. Clostridium butyricum and Chitooligosaccharides in Synbiotic Combination Ameliorate Symptoms in a DSS-Induced Ulcerative Colitis Mouse Model by Modulating Gut Microbiota and Enhancing Intestinal Barrier Function. Microbiol. Spectr. 2023;11:19. doi: 10.1128/spectrum.04370-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wang T., Wang P., Yin L., Wang X., Shan Y., Yi Y.L., Zhou Y., Liu B., Wang X., Lü X. Dietary Lactiplantibacillus plantarum KX041 attenuates colitis-associated tumorigenesis and modulates gut microbiota. Food Sci. Hum. Wellness. 2023;12:1626–1636. doi: 10.1016/j.fshw.2023.02.012. [DOI] [Google Scholar]
- 411.Li Y., Liu J., Shi X., Li S., Zhang H., Zhang L., Huang X., Liu S., Wang W., Tian L., et al. Casein-quaternary chitosan complexes induced the soft assembly of egg white peptide and curcumin for ulcerative colitis alleviation. Int. J. Biol. Macromol. 2024;269:132107. doi: 10.1016/j.ijbiomac.2024.132107. [DOI] [PubMed] [Google Scholar]
- 412.Chang Y., Wu X., Lu S., Du J., Long Y., Zhu Y., Qin H. Engineered procyanidin-Fe nanoparticle alleviates intestinal inflammation through scavenging ROS and altering gut microbiome in colitis mice. Front. Chem. 2023;11:1089775. doi: 10.3389/fchem.2023.1089775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Singh G., Brim H., Haileselassie Y., Varma S., Habtezion A., Rashid M., Sinha S.R., Ashktorab H. Microbiomic and Metabolomic Analyses Unveil the Protective Effect of Saffron in a Mouse Colitis Model. Curr. Issues Mol. Biol. 2023;45:5558–5574. doi: 10.3390/cimb45070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Chen J., Pan M., Wang J., Zhang M., Feng M., Chai X., Zhang Q., Sun Y. Hydroxysafflor yellow A protects against colitis in mice by suppressing pyroptosis via inhibiting HK1/NLRP3/GSDMD and modulating gut microbiota. Toxicol. Appl. Pharmacol. 2023;467:116494. doi: 10.1016/j.taap.2023.116494. [DOI] [PubMed] [Google Scholar]
- 415.Khalil N.A., ALFaris N.A., ALTamimi J.Z., Mohamed Ahmed I.A. Anti-inflammatory effects of bay laurel (Laurus nobilis L.) towards the gut microbiome in dextran sodium sulfate induced colitis animal models. Food Sci. Nutr. 2024;12:2650–2660. doi: 10.1002/fsn3.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Shi K., Yu F., Li A., Wang Y.-J., Sun W.-C. The protective effect of Okanin on Colitis induced by dextran sulfate sodium in mice. Food Biosci. 2024;57:103527. doi: 10.1016/j.fbio.2023.103527. [DOI] [Google Scholar]
- 417.Song J., Chen Y., Lv Z., Taoerdahong H., Li G., Li J., Zhao X., Jin X., Chang J. Structural characterization of a polysaccharide from Alhagi honey and its protective effect against inflammatory bowel disease by modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2024;259:128937. doi: 10.1016/j.ijbiomac.2023.128937. [DOI] [PubMed] [Google Scholar]
- 418.Wu J., Wei Z., Cheng P., Qian C., Xu F., Yang Y., Wang A., Chen W., Sun Z., Lu Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10:10665–10679. doi: 10.7150/thno.43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Wang X., Shen C., Wang X., Tang J., Wu Z., Huang Y., Shao W., Geng K., Xie H., Pu Z., et al. Schisandrin protects against ulcerative colitis by inhibiting the SGK1/NLRP3 signaling pathway and reshaping gut microbiota in mice. Chin. Med. 2023;18:112. doi: 10.1186/s13020-023-00815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Jin D.X., He J.F., Zhang K.Q., Luo X.G., Zhang T.C. EtOAc extract of H. attenuatum Choisy inhibits inflammation by suppressing the NF-κB and MAPK pathways and modulating the gut microbiota. Phytomedicine. 2019;57:292–304. doi: 10.1016/j.phymed.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 421.Liu W., Zhang Y., Qiu B., Fan S., Ding H., Liu Z. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Sci. Rep. 2018;8:14916. doi: 10.1038/s41598-018-33092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liu K.Y., Nakatsu C.H., Jones-Hall Y., Kozik A., Jiang Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021;163:180–189. doi: 10.1016/j.freeradbiomed.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 423.Wang T., Tian J., Su W., Yang F., Yin J., Jiang Q., Li Y., Yao K., Li T., Yin Y. Effect of Ornithine α-Ketoglutarate on Intestinal Microbiota and Serum Inflammatory Cytokines in Dextran Sulfate Sodium Induced Colitis. Nutrients. 2023;15:2476. doi: 10.3390/nu15112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Miyashita A., Xia Y., Kuda T., Yamamoto M., Nakamura A., Takahashi H. Effects of Sichuan pepper (huājiāo) powder on disease activity and caecal microbiota of dextran sodium sulphate-induced inflammatory bowel disease mouse model. Mol. Biol. Rep. 2024;51:126. doi: 10.1007/s11033-023-09103-y. [DOI] [PubMed] [Google Scholar]
- 425.Zou Q., Zhang X., Liu X., Li Y., Tan Q., Dan Q., Yuan T., Liu X., Liu R.H., Liu Z. Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Function. 2020;11:6666–6679. doi: 10.1039/D0FO01162B. [DOI] [PubMed] [Google Scholar]
- 426.Shin M.-Y., Yong C.-C., Oh S. Regulatory Effect of Lactobacillus brevis Bmb6 on Gut Barrier Functions in Experimental Colitis. Foods. 2020;9:864. doi: 10.3390/foods9070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Im Y., Wang Q., Park J., Lee H., Kim H. Sargassum horneri Extract Ameliorates DSS-Induced Colitis through Modulation of mTOR Axis and Intestinal Microbiota. Appl. Sci. 2023;13:1742. doi: 10.3390/app13031742. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is an unpublished study that is not undergoing the submission process in any other scientific journal. All data are privately accessible.