Abstract
Phytochemical investigations of Melia azedarach fruits have led to the isolation of a novel tirucallane triterpenoid (1), four new limonoids (2–5), and four known limonoids (6–9). Their structures were clarified by comprehensive spectroscopic and spectrometric analyses. The anti-inflammatory activities of isolated compounds were assessed in vitro. Compound 2 exhibited the most potent anti-inflammatory effect, with an IC50 value of 22.04 μM. Additionally, compound 2 attenuated LPS-induced reactive oxygen species (ROS) production and reduced the levels of inflammatory mediators IL-6 and TNF-α. A mechanistic study revealed that limonoid 2 suppresses the expression of iNOS and JAK2 and is implicated in the modulation of the NF-κB signaling cascade, which reveals its anti-inflammatory actions.
Keywords: Melia azedarach, meliaceae, limonoids, anti-inflammatory, NF-κB
1. Introduction
Reactive oxygen species (ROS), natural by-products of oxygen metabolism, are critical in cell signaling and homeostasis [1]. However, excessive ROS generation during various infections and pathological states can lead to protein and nucleic acid oxidation, affecting toxic inflammatory effects on cellular structures [2]. These highly reactive, electron-deficient radicals cause oxidative damage to cellular membranes, DNA, and proteins. Persistent oxidative injury activates intracellular signaling cascades, leading to a chronic systemic inflammatory response, exacerbating conditions such as cardiovascular disorders and cancer [3,4].
Inflammation is a significant risk factor for numerous disorders, with macrophages playing a vital role as primary immune cells defending against pathogens, such as bacteria and viruses [5]. During inflammation, macrophages overproduce inducible nitric oxide synthase and pro-inflammatory factors such as IL-6 and TNF-α [6]. Excessive production of these mediators can worsen conditions such as allergies, autoimmune diseases, cancers, and metabolic syndromes [7,8]. The nuclear factor-κB (NF-κB) pathway mediates the induction of NO, IL-6, TNF-α, and other pro-inflammatory cytokines in mononuclear/macrophages, contributing to the amplification and spread of inflammatory responses [9].
Lipopolysaccharides (LPS), components of the outer cell walls of Gram-negative bacteria, trigger host inflammatory responses by increasing the production of chemokines, cytokines, and pro-inflammatory [10] mediators. LPS-exposed macrophages stimulate cytokine and chemokine production during microbial infections, triggering further inflammatory events. Therefore, suppressing macrophage activation by LPS represents a crucial strategy in targeting inflammatory diseases. Regulation of inflammatory mediators like NF-κB, JAK2, and iNOS, as well as pro-inflammatory factors like NO, IL-6, and TNF-α, could serve as potential therapeutic approaches to treat inflammatory damage [11,12,13,14].
Melia azedarach L. (Meliaceae) is native to China and other Southeast Asian countries [15]. The bark and fruit of Melia azedarach are traditionally used for insecticidal, analgesic, and dermatological applications [16]. Various constituents, including limonene, triterpenes, and steroids [17,18,19,20,21,22,23,24], have been separated from different parts of the plant, with limonenes demonstrating notable antibacterial [25], cytotoxic [26], food repellent [27], and insecticidal activities [28]. Despite these properties, the anti-inflammatory effects and mechanisms of M. azedarach remain poorly understood.
This study utilized LPS-stimulated macrophages to investigate the anti-inflammatory properties of compounds isolated from M. azedarach fruit. A new tirucallane triterpenoid (1) and four new limonoids (2–5), along with four known limonoids (6–9), were isolated and characterized. Compound 2 exhibited the most significant inhibitory effect on nitric oxide production, as demonstrated by the Griess assay. Further cellular assays targeting TNF-α, IL-6, ROS, NF-κB, eNOS, and JAK2 signaling pathways elucidated the anti-inflammatory potential of compound 2, indicating its action primarily through the NF-κB and JAK2 pathways.
2. Material and Methods
2.1. General Experimental Procedure
CD spectra were acquired using a Chirascan spectropolarimeter (Applied Photophysics, Surrey, UK). Optical rotation was determined with a JASCO P-1020 rotameter (Tokyo, Japan). Nuclear Magnetic Resonance spectra were recorded on a Bruker ARX-600 spectrometer (Rheinstetten, Germany). HR-ESI-MS was performed using an Agilent 6530B TOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). Compounds werepurified using HPLC with a C18 column (YMCPackODSA, Waters Company, Milford, MA, USA).
2.2. Plant Materials
The fruits of Melia azedarach were gathered in November 2021 from Minhou County, Fuzhou City, Fujian Province, China. The plant specimens were authenticated by Professor Dongmei Shi from Fujian Medical University, and the sample voucher (No. 20211112) has been stored in the School of Pharmacy at the same university.
2.3. Extraction and Separation
Approximately 23.7 kg of dried fruits of M. azedarach were extracted with MeOH three times weekly. After concentrating the combined filtrates, a total of 1.8 kg of crude extract was obtained. This extract was subsequently dissolved in water and sequentially partitioned with hexane, CH2Cl2, EtOAc, and n-butanol to fractionate the various chemical constituents.
The CH2Cl2 fraction (0.9 kg) was fractionated over silica gel columns, PE–ethyl acetate (10:1 to 0:2) to afford night-major portions (Fr. 1-9). Fr. 1 (9.8 g) was isolated on an ODS gel column (40%, 60%, 80%, and 100% MeOH/H2O), followed by Sephadex LH-20 (MeOH-CH2Cl2) and HPLC (CH3CN/H2O) to give compound 1 (15.6 mg). Fr.3 (25.0 g) was partitioned via ODS columns (40%, 60%, 80%, and 100% MeOH/H2O) and subsequently purified using SephadexLH-20 (MeOH-CH2Cl2) and HPLC (CH3CN/H2O) to provide compounds 2 (21.0 mg), 6 (25.7 mg), and 7 (25.7 mg). Fr.4 (12.0 g) was isolated using an ODS gel column (40:60 to 100:0, MeOH/H2O), purified using SephadexLH-20 (90:10, MeOH-CH2Cl2), and fractionated by HPLC (58:42, MeOH/H2O) to give compounds 8 (9.4 mg) and 9 (7.9 mg). Fr.5 (20.0 g) was isolated using an ODS gel column (30:70 to 100:0, MeOH/H2O) and four fractions (Fr. 5-1 to 5-4). Separation of Fr. 5-2 (6.5 g) on a SephadexLH-20 column (MeOH), followed by HPLC (58:42, MeOH/H2O), afforded compounds 3 (8.5 mg) and 4 (5.7 mg). Fr. 5-3 (8.0 g) was isolated using Sephadex LH-20 (90:10, MeOH-CH2Cl2) and HPLC (CH3CN/H2O, 47:53) to obtain compound 5 (11.1 mg).
Compound 1. Colorless powder; [α]25D: +439° (c0.10, MeOH); IR (KBr)νmax: 3444, 2951, 1705, 1625, 1450, 1384, 1142 cm−1; NMR data: see Table 1 and Table 2, Figures S3–S8; HRESIMS m/z 511.3690 [M + Na]+ (Figure S9) (calc. for C30H48O5Na+ as 511.3394).
Table 1.
1H NMR (600 MHz) data of compounds 1–5 (CDCl3).
| Proton | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.49(m) | 3.59(t,2.8) | 3.86(t,3.9) | 4.86(m) | 3.56(s) |
| 2.01(m) | |||||
| 2 | 2.26(dt,11.2,3.2) | 2.02(dd,2.8,15.1) | 1.09(dd,2.0,15.1) | 1.06(dd,1.5,10.9) | 2.15(m) |
| 2.78(dt,14.7,3.8) | 2.34(dd,3.2,15.1) | 2.28(dd,2.3,15.1) | 2.38(dd,2.8,10.9) | 2.19(m) | |
| 3 | 5.08(t,2.7) | 4.94(t,4.1) | 4.89(t,4.1) | 4.86(t,2.5) | |
| 5 | 1.75(m) | 2.67(d,12.6) | 2.45(d,12.1) | 2.66(d,4.8) | 2.76(d,12.7) |
| 6 | 2.11(m) | 4.28(d,12.6) | 4.17(d,12.1) | 4.16(d,12.2) | 4.01(dd,12.5,3.0) |
| 7 | 5.34(t,3.1) | 5.90(d,3.0) | 4.19(d,2.8) | 4.22(d,3.3) | 5.68(d,2.8) |
| 9 | 2.32(m) | 3.58(dd,5.5,7.3) | 2.64(dd,5.5,6.9) | 2.94(dd,3.6,4.4) | 2.98(d,7.5) |
| 11 | 1.61(m) | 2.54(m) | 2.28(m) | 2.28(m) | 1.72(m) |
| 2.34(m) | 2.03(m) | 2.08(m) | 1.78(m) | ||
| 12 | 1.60(m) | 4.87(m) | 3.85(m) | 5.94(s) | |
| 2.03(m) | |||||
| 15 | 1.62(m) | 5.73(t,2.5) | 5.66(t,3.7) | 5.64(t,3.5) | 4.95(7.4) |
| 16 | 1.51(m) | 2.34(m) | 2.45(m) | 2.38(m) | 1.77(m) |
| 1.91(m) | 2.43(m) | 2.64(m) | 2.51(m) | 2.19(m) | |
| 17 | 1.85(m) | 3.46(m) | 2.90(m) | 2.94(m) | 3.36(m) |
| 18 | 0.84(s) | 0.97(s) | 0.95(s) | 0.91(s) | 1.79(s) |
| 19 | 1.03(s) | 0.98(s) | 1.00(s) | 1.03(s) | 0.89(s) |
| 20 | 2.03(m) | ||||
| 21 | 5.48(d,2.2) | 7.25(t,1.6) | 5.88(s) | 6.09(s) | |
| 22 | 2.02(m) | 6.48(d,1.6) | 5.85(s) | 6.85(d,1.2) | 5.82(m) |
| 1.12(m) | |||||
| 23 | 4.66(d,5.1) | 7.27(s) | 6.43(d,1.8) | ||
| 24 | 3.37(d,5.2) | ||||
| 26 | 1.18(s) | ||||
| 27 | 1.17(s) | ||||
| 28 | 1.07(s) | 3.18(d,7.8) | 3.62(d,7.6) | 3.62(d,7.6) | 3.47(d,7.6) |
| 3.46(d,7.8) | 4.12(d,7.6) | 4.11(d,8.0) | 3.56(d,7.6) | ||
| 29 | 1.14(s) | 1.17(s) | 1.15(s) | 1.15(s) | 1.12(s) |
| 30 | 1.06(s) | 1.18(s) | 1.09(s) | 1.09(s) | 1.46(s) |
| 12-OCH3 | 3.37(s) | ||||
| 1-OAC | 1.83(s) | ||||
| 3-OAC | 1.82(s) | 2.02(s) | |||
| 12-OAC | 2.03(s) | ||||
| 2’ | 6.40(d,15.9) | 6.03(d,17.9) | |||
| 3’ | 8.05(dd,7.6,1.3) | 7.72(d,16.0) | 7.71(d,15.9) | 6.88(qd,7.0,1.5) | |
| 4’ | 7.39(t,7.6) | 1.73(d,7.0) | |||
| 5’ | 7.53(t,7.5) | 7.48(dd,7.2,1.7) | 7.49(dd,4.4,1.9) | 1.78(s) | |
| 6’ | 7.39(t,7.6) | 7.38(t,4.9) | 7.39(t,6.9) | ||
| 7’ | 8.05(dd, 7.6,1.3) | 7.40(t,4.1) | 7.40(t,8.1) | ||
| 8’ | 7.38(t,4.9) | 7.39(t,6.9) | |||
| 9’ | 7.48(dd,7.2,1.7) | 7.49(dd,4.4,1.9) |
Table 2.
13C NMR (150 MHz) data of compounds 1–5 (CDCl3).
| Carbon | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.6 | 71.8 | 71.1 | 77.2 | 70.6 |
| 2 | 35.1 | 30.3 | 25.1 | 24.5 | 29.1 |
| 3 | 217.0 | 73.6 | 73.1 | 72.4 | 72.2 |
| 4 | 47.7 | 42.5 | 43.9 | 43.9 | 42.4 |
| 5 | 52.5 | 40.2 | 38.7 | 38.7 | 39.0 |
| 6 | 24.5 | 72.9 | 73.7 | 73.9 | 72.5 |
| 7 | 118.2 | 74.7 | 74.1 | 74.0 | 74.6 |
| 8 | 145.7 | 44.5 | 39.7 | 39.8 | 45.4 |
| 9 | 48.0 | 36.6 | 34.3 | 34.8 | 36.4 |
| 10 | 35.2 | 40.3 | 45.2 | 45.2 | 41.2 |
| 11 | 17.9 | 34.1 | 30.5 | 30.4 | 30.5 |
| 12 | 31.6 | 213.8 | 77.9 | 71.2 | 97.8 |
| 13 | 43.6 | 61.5 | 52.5 | 51.7 | 140.3 |
| 14 | 50.9 | 154.1 | 156.1 | 157.1 | 146.1 |
| 15 | 34.4 | 123.8 | 122.5 | 122.2 | 77.9 |
| 16 | 27.6 | 34.2 | 35.5 | 35.8 | 35.8 |
| 17 | 48.5 | 42.9 | 51.2 | 48.9 | 49.3 |
| 18 | 23.3 | 19.4 | 15.5 | 15.1 | 16.5 |
| 19 | 12.8 | 15.6 | 16.1 | 15.5 | 16.7 |
| 20 | 47.1 | 124.6 | 169.1 | 137.5 | _ a |
| 21 | 101.8 | 142.4 | 99.1 | 171.4 | 98.7 |
| 22 | 33.6 | 112.6 | 120.4 | 146.5 | 118.7 |
| 23 | 76.7 | 141.0 | 170.9 | 96.5 | 170.8 |
| 24 | 86.5 | ||||
| 25 | 71.1 | ||||
| 26 | 25.9 | ||||
| 27 | 25.7 | ||||
| 28 | 24.6 | 78.1 | 78.4 | 78.4 | 78.1 |
| 29 | 21.7 | 18.8 | 20.2 | 20.2 | 19.0 |
| 30 | 27.4 | 25.8 | 26.7 | 26.9 | 20.7 |
| 12-OCH3 | 55.2 | ||||
| 1-OAC | 170.8 | ||||
| 21.2 | |||||
| 3-OAC | 169.2 | 170.7 | |||
| 20.9 | 21.3 | ||||
| 12-OAC | 170.9 | ||||
| 21.5 | |||||
| 1’ | 165.1 | 165.4 | 165.4 | 166.7 | |
| 2’ | 130.5 | 117.0 | 117.2 | 128.6 | |
| 3’ | 129.6 | 146.8 | 144.8 | 137.5 | |
| 4’ | 128.6 | 133.8 | 134.1 | 14.6 | |
| 5’ | 133.2 | 128.4 | 128.5 | 12.2 | |
| 6’ | 128.6 | 129.2 | 129.2 | ||
| 7’ | 129.6 | 131. | 130.9 | ||
| 8’ | 129.2 | 129.2 | |||
| 9’ | 128.4 | 128.5 |
a Signal not clearly observable from 1D and 2D NMR.
Compound 2. Colorless powder; [α]25D: +1° (c0.10, MeOH); IR (KBr) νmax: 3446, 2923, 1714, 1629, 1384, 1270, 1144 cm−1; NMR data (Table 1 and Table 2, Figures S10–S15); HRESIMS m/z 589.2812 [M + H]+ (Figure S16) (calc. for C35H41O8 as 589.2796).
Compound 3. Amorphous powder; [α]21D: +59.4° (c0.20, MeOH); IR (KBr) νmax: 3443, 2923, 1696, 1629, 1460, 1384, 1247 cm−1; NMR data (Table 1 and Table 2, Figures S17–S22); HRESIMS m/z 671.2797 [M + Na]+ (Figure S23) (calc. for C37H44O10Na+ as 671.2827).
Compound 4. Amorphous powder; [α]21D: +69.3° (c0.20, MeOH); IR (KBr) νmax: 3438, 2975, 1700, 1629, 1461, 1384, 1256, 1082 cm−1; NMR data (Table 1 and Table 2, Figures S24–S29); HRESIMS m/z 671.2873 [M + Na]+ (Figure S30) (calc. for C37H44O10Na+ as 671.2827).
Compound 5. Colorless powder; [α]21D: −59.4° (c0.20, MeOH); IR (KBr) νmax: 3452, 2928, 1738, 1634, 1384, 1251, 1049 cm−1; NMR data (Table 1 and Table 2, Figures S31–S36); HRESIMS m/z 653.2935 [M + Na]+ (Figure S37) (calc. for C34H46NaO11 as 653.2932).
2.4. Cell Culture
Mouse macrophages RAW264.7 were procured from the Shanghai Model Culture Preservation Centre, Chinese Academy of Sciences. The cells were incubated on DMEM media enriched by 10% FBS and incubated in a CO2 incubator at 37 °C with 5% humidity.
2.5. Cell Viability Test
To evaluate cell viability, all isolated compounds from the fruit of M. azedarach were tested using the SRB assay in an LPS-mediated RAW264.7 cell model [29]. RAW264.7 macrophages were seeded into 96-well plates and cultured for 24 h prior to treatment. Limonoids 1–9 were dissolved in DMSO, diluted six-fold with fresh medium, and administered to each well for 24 h. Absorbance was measured at 515 nm using an ELISA reader (BioTek, Winooski, VT, USA).
2.6. Anti-Inflammatory Effect Test
The anti-inflammatory activity of isolated compounds was assessed by measuring NO production in macrophages. Cells were prepared as described in the cell viability assay. Limonoids 1–9 were treated with the cells for 4 h, followed by a 24 h LPS treatment. NO production in the cell supernatant was determined using the Griess reaction [30]. An equal volume of cell culture supernatant was combined with the Griess reagent, and the absorbance was determined at 540 nm to calculate the IC50 values.
2.7. Flow Cytometry Test
Cells were pre-treated and seeded in a 6-well plate at a density of 2 × 105 cells in each well and cultivated for 24 h. To detect ROS levels, DCFH-DA was added to the medium (20 min, 1 μM) after the incubation period with limonoid 2 [31]. Cells were then washed three times with serum-free medium, and fluorescence signals were collected using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
2.8. Cytokine Detection
Cytokine levels of TNF-α and IL-6 in limonoid 2-treated samples were quantified using enzyme-assisted immunosorbent assays [32]. RAW264.7 cells were incubated in 96-well plates for 24 h. After a 4 h pre-treatment period, cells were stimulated with LPS at a concentration of 2.5 µg/mL for an additional 24 h. The concentrations of IL-6 and TNF-α in the supernatant were then determined with a commercially available ELISA kit.
2.9. Western Blotting Analysis
RAW264.7 macrophages were pre-treated with limonoid 2 for 4 h and subsequently mediated by 2.5 µg/mL LPS for 24 h. Cells were lysed using a buffer containing a protease inhibitor cocktail, and total protein was quantified with BCA protein assay kits. The protein was isolated by 10% SDS-PAGE and transferred to a PVDF membrane. Membranes were stopped for 1 h at room temperature with 5% skimmed milk and 0.1% Tween-20 in triple-buffered saline. Overnight culture was conducted at 4 °C using primary antibodies (1:1000 dilution) targeting p65, p-IκBα, p-IKK, p-JAK2, p-STAT3, iNOS, eNOS, and β-actin (Table S1). After washing the membrane with TBST, it was incubated with HRP-labeled secondary antibody for 30 min at room temperature. Immunoreactive bands were detected with the ECL test kit (Supplementary S1).
2.10. Immunofluorescence Assay
Immunofluorescence staining was performed to assess whether limonoid 2 affects the nuclear translocation of the p65 subunit. Macrophages were seeded on confocal dishes and incubated overnight. Following the incubation period with limonoid 2, cells were stained with monoclonal primary antibodies and fluorescently labeled secondary antibodies diluted to 1:500 and 1:100, respectively (Table S1) [33]. A Leica TCS SP5 laser confocal microscope (Leica, Wetzlar, Germany) was used for imaging, and image processing was performed using ImageJ software v1.53 (Supplementary S2).
2.11. Statistical Analyses
Data are presented as mean ± standard error. The band intensities of the Western blots were quantified using Image Lab 3.0 software. For the calculation of IC50, we put the data through the non-linear fitting process of Prism 8 software to obtain the result. All statistical analyses, including t-tests or one-way ANOVA to evaluate significant differences, were performed using Prism 8 software. Statistical significance was taken as p < 0.05.
3. Results
3.1. Chemical Structural Determination of Compounds 1–5
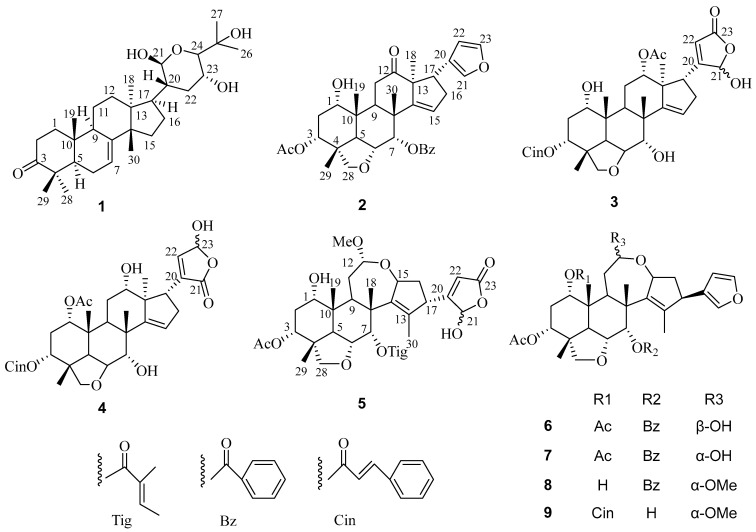
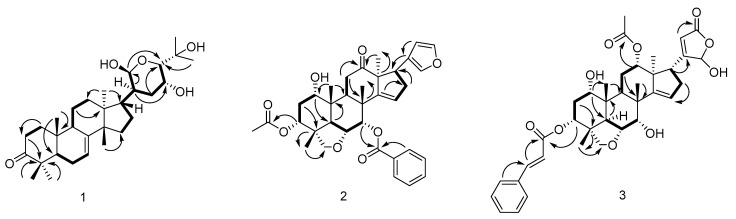
Compound 1 was separated as a white powder. HRESIMS of compound 1 revealed a conjugated molecular ion peak at m/z 511.3690 [M + Na]+, consistent with the calculated mass of 511.3394 and confirming the molecular formula C30H48O5. The IR spectroscopy displayed absorption bands characterized by hydroxyl (3444 cm−1) and carbonyl (1705 cm−1) moieties. The 1H- and 13C-NMR spectra exhibited signals typical of tirucallane triterpenoids [34]. Comparison with the IR and NMR spectra of bourjotinolone A [35] revealed a close similarity, with the notable difference of an additional hydroxyl group at the C-21 position. This modification was further confirmed through comprehensive analysis using 13C NMR and DEPT spectra, where the oxymethine moiety was detected at δC 101.8 (C-21). Complete assignment of all proton and carbon resonances for compound 1 was accomplished with HMQC, HMBC, and COSY experiments. Specifically, the HMBC correlations from proton H-20 to carbon C-21 (δC 101.8) provided definitive evidence of the hydroxyl group at the C-21 position (Figure 1). The HMBC correlation of H-21 (δH 5.48) to C-24 (δC 86.5), C-20 (δC 47.1); H-20 (2.03, m, 1H) to C-22 (δC 33.6), C-23 (δC 76.7); H-24 (δH 3.37, 1H, d, J = 5.2 Hz)/C-25 (71.1), C-23 (76.7) also demonstrates the existence of a six-membered cycle (Figure 2). In addition, the HMBC correlation of H-17 (δH 1.85, 1H, m) and C-20 (δC 47.1), C-21 (δC 101.8), and C-15 (δC 34.4) established a link between the tetracyclic portion and the hydroxyl ring, and the relative conformations of 1 were established based on biological genetic concerns and interpretations of NOE data. The core structure of compound 1, comprising the basic tertiary rings A, B, C, and D, is conformationally identical to those of bourjotinolone A, sharing identical backbones. The NOE correlation of H3-30 with H-11 and H3-30 with H3-19 indicate that these protons are coplanar and assume a β-orientation, aligning with previously reported data for bourjotinolone A. Additionally, interactions between H-5 with H3-28 and H-20 with H-21 establish the α-orientations of H-5, H-21, H3-28, and H-20 (Figure S1). The absolute configuration of compound 1 was verified through comparative experiments and ECD calculations. A systematic conformational search, geometry optimization, and TDDFT/ECD analysis using Gaussian16 [36,37] software confirmed this configuration. The theoretical ECD spectrum of compound 1 matched the experimental (Figure S2) data, allowing the absolute configurations to be precisely defined as 5R, 9R, 10R, 13S, 14S, 17R, 20S, 21R, 23R, and 24S. Thus, compound 1 is confidently identified as 21β-hydroxy bourjotinolone A.
Figure 1.
The chemical structures of compounds 1–9.
Figure 2.
Partial 1H-1H COSY and HMBC correlation for compounds 1–5.
Compound 2 showed an [M + H]+ ion peak in the positive ion mode of HRESIMS at m/z 589.2812 (C35H41O8, calcd589.2796), which corresponds to the molecular formula C35H40O8. The IR spectra of compound 2 displayed characteristic absorption bands for hydroxyl (3446 cm−1), ester carbonyl (C=O at 1714 cm⁻1), additional carbonyl (1629 cm−1), and furan (807 cm−1) moieties. The 1H and 13C NMR data of 2 revealed the existence of four tertiary Me moieties (δH 0.97, 0.98, 1.17, and 1.18), one Ac Me moiety (δH 1.82), one benzoyl moiety (δH 8.05, dd7.6, 1.3; 7.39, t7.6; 7.53, t7.5), one CH2-O group (δH 3.18, d7.8, and 3.46, d7.8; δC 78.1), three CH-O moieties (δH 3.59, t 2.8; 5.08, t 2.7; and 5.90, d 3.0), one vinyl CH moiety (δH 5.73, t 2.5; δC 123.8), one carbonyl group (δC213.8), and a β-substituted furan ring (δH 7.25, t1.6; 6.48, d1.6; 7.27, s; δC 124.6, 142.4, 112.6, and 141.0) [38], indicating that 2 shares a nimbidinin-type backbone structure [39] with Ac and benzoyl moieties at C-3 and C-7, respectively (Figure 1). HMBC correlation of H-3 (δH 5.08, t 2.7) with AcO-3 (δC169.2), of H-7 (δH 5.90, d 3.0) to C-1′ (δC 165.1), and of H-9 (δH 3.58, dd 5.5,7.3) and H-11(δH 2.54, m, and 2.34, m), and of Me-18 (δH 0.97) with C-12 (δC 213.8), and the NOE correlation of the 1Hsignal of H-3 (δH 5.08, t 2.7), Me-29 (δH 1.17), Me-19 (δH 0.98), Me-30 (δH 1.18), and H-7, and of Me-30 and H-17 (δH 3.46, m) of 2 supported the proposed structure (Figure 2). The relative configurations of compound 2 were ascertained using NOESY correlations alongside a comparison of the NMR data with that of nimbidinin (Figure S1). The computed ECD spectra (Figure S2) align well with experimental observations, confirming the absolute configurations of 1S, 3R, 4R, 5R, 6R, 7S, 8R, 9S, 10R, 13S, and 17R. As a result, the structure of 2 has been elucidated as 3-acetyl-7-benzoylnimbidinin.
Compound 3 was separated as an amorphous powder. HRESIMS analysis of compound 3 displayed a [M + Na]+ peak at m/z 671.2797, which is in close agreement with the calculated value (671.2827) for the molecular formula C37H44O10. The IR spectroscopy indicated the existence of OH at 3443 cm−1, C=O at 1696 cm−1, and double bond (1629 cm−1) moieties. The 1H NMR and COSY spectra displayed similarities to those of compound 14 [40] with features including four methyl groups (δH 0.95, s, 3H; δH 1.00, s, 3H; δH 1.15, s, 3H; and δH 1.09, s, 3H), an acetyl methyl (δH 2.03, s, 3H), and a cinnamoyl (δH 7.48–7.38, m, 5H; δH 7.72, d, J = 16.0, H-3′; δH 6.40, d, J = 16.0, H-2′) group. The distinction between compound 3 and compound 14 is notable in the E-ring, where compound 3 shows two broad singlets at 5.88 (H-21) and 5.85 (H-22) for a γ-hydroxylbutyrolactone [19], in contrast to the furan chain signals found in compound 14 [40]. The 13C NMR spectra of 3 confirmed the presence of hemiketal carbon (δC 99.1, C-21) and α, β-unsaturated lactone (δC 169.0, C-20; 120.4, C-22 and 170.9, C-23) signals, aligning with the spectroscopic data for γ-hydroxybutyrolactone units observed in related structures [41] (Figure 1). HMBC interactions between H2-16 and C-20; H-17 to C-20, C-21, and C-22; H-21 to C-22 and C-23; and H-22 to C-21 and C-23 supported the identification of a hydroxybutyrolactone structure linked to C-17, akin to that found in munronolide [40]. The NMR data indicate that the distinction between compound 3 and munronolide is primarily in the C-12 AcO and C-3 cinnamoyl groups. The AcO group is linked to C-12 via HMBC correlations from H-12 (δH 4.87, m) to the AcO moiety (δC 170.9). The cinnamoyl group is attached to C-3 of compound 3 through an HMBC correlation from H-3 (δH 4.18) to C-1′ (δC 165.4) (Figure 2). The relative configuration of compound 3 was determined using ROESY experiments (Figure S1). The absolute configuration of 1S, 3R, 4R, 5R, 6R, 7S, 8R, 9S, 10R, 12S, 13S, and 17R for compound 3 was established by comparing experimental and computed ECD data (Figure S2). Based on this evidence, compound 3 was identified as a new malonolide derivative named 1,3-deacetyl-3-O-cinnamoyl munronolide 12-acetate, with cinnamoyl and AcO groups at C-3 and C-12, respectively.
Compound 4, as shown by HRESIMS, has the same molecular formula as compound 3, C37H44O10. The NMR spectra of compound 4 closely resemble those of compound 3, except for the absence of the 21-hydroxybut-20(22)-en-21,23-γ-lactone ring signal found in compound 3. Instead, signals indicating a 23-hydroxybut-20(22)-en-21,23-γ-lactone cycle at the C-17 position are observed, evidenced by 1H (δH 6.43(H-23) and 6.85(H-22) and 13C [δC 137.5(C-20), 171.4(C-21), 146.5(C-22), and 96.5(C-23)]) data [19] (Figure 1). The HMBC cross-correlation of H-17 (δH 2.94) to C-20 and C-21, and H-22 (δH 6.85) to C-17, C-20, C-21, and C-23, and the NOE correlation of the 1H- signal of H-7β (δH 4.22) with H-16β (δH 2.38), and H-16β with H-17β of 4 demonstrated that the γ-lactone ring is situated at C-17 and oriented to α. The AcO group is positioned at C-1 of compound 4, as indicated by HMBC correlations from H-1 (δH 4.86) to the AcO group (δC 170.8). The OH group is linked to C-12, confirmed by the C-12/12-OH cross-peak in the HMBC spectrum (Figure 2). The relative configurations of compound 4 were assessed from the NOESY data and compared with the NMR data for compounds 3 and 4 (Figure S1). The absolute configurations of (1S, 3R, 4R, 5R, 6R, 7S, 8R, 9S, 10R, 12S, 13S, and 17R) for compound 4 were determined by the similarity of the computed ECD spectra (Figure S2). Consequently, the structure of compound 4 was elucidated as 3-deacetyl-3-O-cinnamoyl-12-hydroxy-17 (4-hydroxy-2-buten-4-olide-2-yl) munronolideand named munronolide A.
Compound 5 was characterized as an amorphous powder with a molecular formula C34H46O11 evidenced by (HRESIMS) with an ionization of m/z 653.2935 [M + Na]+, calculated for C34H46NaO11 as 653.2932. This corresponds to a degree of unsaturation of 12. The 1H-NMR spectra displayed signals for four methyl groups (δH 1.79, 1.46, 1.12, 0.89), a methoxy group (δH 3.37), an acetyl group (δH 2.02), and a Tig group (δH 6.88, 1.78, and 1.73). The 13C-NMR spectra revealed three ester groups (δC 170.8, 170.7, and 166.7) and carbons associated with two double bonds (δC 146.1, 140.3, 137.5, and 128.6). A proton singlet at δH 5.90 and 5.82, along with carbon resonances at δC 170.8, 118.7, and 98.7, indicate a 21-hydroxybutenolide unit within the structure of 5, as compared to meliazedalide A [42]. The presence of a methoxyl moiety at the C-12 position instead of a hydroxyl moiety, as in meliazedalide A (Figure 1), is corroborated by HMBC cross-peaks from H-OMe, H2-11, and H-9 to C-12, confirmed by a downfield chemical shift to δC 97.8 (ΔδC +6.0 ppm) for C-12 (Figure 2). NOESY spectra of compound 5 and melazadine A reveal similar relative conformations (Figure S1). Based on computed ECD curves, the absolute configurations of compound 5 are proposed as (1S, 3R, 4R, 5R, 6R, 7S, 8R, 9S, 10R, 12S, 15S, and 17R) (Figure S2). Consequently, compound 5 was identified as 12-α-methylmeliazedalide A (Figure 1).
By comparing the measured 1H-NMR, 13C-NMR, and MS data with those reported in the literature, known limonoids were identified as 12β-hydroxynimbolinin A (6) [39], nimbolinin A (7) [39], nimbolinin D (8) [39], and nimbolinin C (9) [43] (Figure 1).
3.2. Effects of Isolate on Nitrite Levels in Macrophages
Previous studies have documented the use of M. azedarach fruit as a traditional remedy for various conditions. However, detailed pharmacological studies on its anti-inflammatory effects and mechanisms are limited. This study explored the inhibitory effects of the bioactive components isolated from M. azedarach on macrophage-mediated inflammation. Key inflammatory factors like TNF-α, IL-6, and NO either inhibit or promote inflammatory responses through multiple pathways. In this investigation, along with one new tirucallane triterpenoid (1), four new limonoids (2–5) and four known limonoids (6–9) were identified. These compounds were characterized as 21β-hydroxy bourjutinolone A (1), 3-acetyl-7-benzoylnimbidinin (2), 1,3-deacetyl-3-O-cinnamoyl munronolide 12-acetate (3), Munronolide A (4), and 12-α-methylmeliazedalide A (5), respectively.
The anti-inflammatory activities of constituents 1–9 were assessed by measuring nitrite levels in macrophages. Nitrites, which can either inhibit or promote inflammation, activate NF-κB; this transcription factor then spurs the liberation of pro-inflammatory cytokines like IL-6 and TNF-α, thereby accelerating the inflammatory process [44]. Inhibition of nitrite synthesis may thus mitigate inflammation. In this study, the effects of a new tirucallane triterpenoid (1), four new limonoids (2–5), and four known limonoids (6–9) on nitrite levels in LPS-mediated macrophages were assessed using the Griess method (Table 3). Limonoids 1, 2, and 5 showed potent anti-inflammatory effects. Pre-treatment with these limonoids significantly reduced nitrite production induced by LPS, with limonoid 2 demonstrating the strongest inhibition, exhibiting an IC50 value of 22.04 μM. However, the specific anti-inflammatory mechanism of limonoid 2 requires further exploration. Additionally, cytotoxicity tests using the SRB assay revealed that limonoids 1–9 were non-toxic to macrophages at concentrations up to 40 μM.
Table 3.
Anti-inflammatory activity for compounds 1–9 in vitro.
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | 26.85 ± 1.56 | 6 | 64.95 ± 3.09 |
| 2 | 22.04 ± 0.96 | 7 | 64.95 ± 3.09 |
| 3 | >100 | 8 | 50.80 ± 4.06 |
| 4 | >100 | 9 | >100 |
| 5 | 24.06 ± 1.92 | Indomethacin a | 37.06 ± 2.56 |
a Positive control.
3.3. Inhibition of the Inflammatory Response Through Limonoid 2
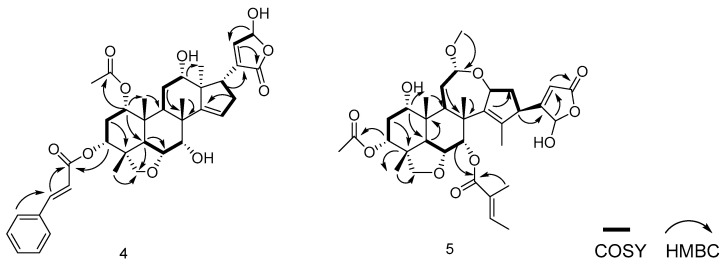
The expressions of inflammatory mediators like TNF-α and IL-6 are mainly regulated by NF-κB, which is a crucial component of the inflammatory process [9]. ROS also play an important role in inflammation, particularly in the LPS-mediated generation of the pro-inflammatory agents TNF-α and IL-6 and activation of the NF-κB cascade [45,46,47]. TNF-α and IL-6 are significant markers of inflammation in LPS-induced macrophage activation. ROS are crucial signaling molecules in the inflammatory process [45]. As depicted in Figure 3A,B, the flow cytometry results demonstrated a significant increase in ROS production in macrophages following LPS treatment. In contrast, limonoid 2 inhibited ROS production in a dose-dependent manner (Figure 3A,B). Furthermore, the levels of IL-6 and TNF-α were also elevated in macrophages [48,49] following LPS stimulation. However, limonoid 2 significantly decreased the levels of these cell factors in LPS-stimulated macrophages (Figure 3C,D), indicating its potential as an effective anti-inflammatory agent.
Figure 3.
Compound 2 reduced the LPS-mediated pro-inflammatory response of RAW264.7 cells. (A) RAW264.7 cells were preconditioned by compound 2 (15, 30 μM) for 4 h and subsequently incubated with LPS for 24 h. Cells were marked by DCFH-DA for 20 min and measured by flow cytometry. (B) Statistical analysis of the fluorescence intensity of reactive oxygen species (ROS). (C,D) The cells were preconditioned by compound 2 (15, 30 μM) for 4 h and then incubated with LPS for 24 h. The supernatant was gathered, and the pro-inflammatory factors TNF-α and IL-6 were detected using an ELISA kit. * p < 0.05, ** p < 0.01, compared with the LPS group alone.
3.4. Exploring the Role of Limonoid 2 in the NF-κB Cascade
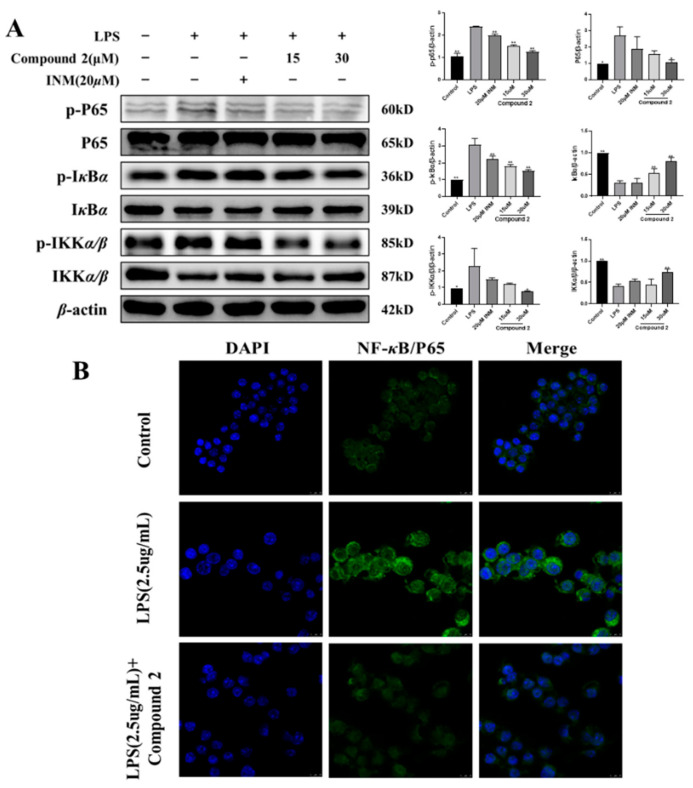
Transcription factor-NF-κB plays a crucial role in LPS-mediated inflammatory procedures [47]. It is known to regulate various inflammatory mediators like IL-6, TNF-α, iNOS, and JAK2 [50]. The NF-κB signaling cascade is also pivotal in developing chronic infectious diseases. Studies have shown that in macrophages activated by LPS, NF-κB is sequestered in the cytoplasm until the upregulation of IKKα/β, which is crucial for NF-κB activation and promotes the phosphorylation and subsequent degradation of IκB [51]. It is probable that NF-κB p65 is phosphorylated on dephosphorylated IκB and translocated to the nucleus, thereby enhancing the release of pro-inflammatory factors and accelerating inflammatory damage [49]. We hypothesize that the NF-κB cascade may be implicated in the anti-inflammatory effects of limonoid 2. Our current research indicates that the expressions of p65, p-IKKα, p-IKKβ, and p-IκBα/β in LPS-stimulated macrophages were elevated but were significantly reduced by limonoid 2 (Figure 4A). Additionally, immunofluorescence staining revealed that limonoid 2 inhibited the nuclear translocation of NF-κB p65 (Figure 4B), suggesting that the NF-κB cascade contributes to the anti-inflammatory action of limonoid 2.
Figure 4.
The NF-κB pathway is implicated in the anti-inflammatory process of limonoid 2. (A) RAW264.7 cells were pre-treated by limonoid 2 (15, 30 μM) for 4 h and then mediated by LPS for 24 h. The expression of proteins p65, p-p65, IκBα, p-IκBα, IKKα/β, and p-IKKα/β was measured by Western blotting. (B) RAW264.7 cells were pre-treated by limonoid 2 for 4 h and then treated with LPS for 24 h. Translocation of p65 was detected by immunofluorescence, as outlined in the Methods sections. Primary anti-NF-κBp65 (1:500), secondary anti-fluorescein-coupled Goat Anti-Rabbit IgG (H + L) (1:100). * p < 0.05, ** p < 0.01, compared with the LPS group alone. The scale bar represents 10 μm.
3.5. Exploring the Role of Limonoid 2 in iNOS and JAK2 Cascades
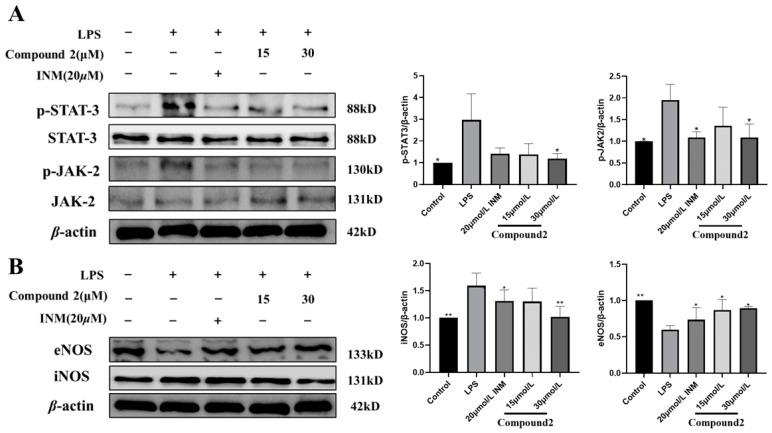
To determine the impact of limonoid 2 on the activation of JAK2 and STAT3, we analyzed the level of p-JAK2 and p-STAT3 in both cytoplasmic and nuclear extracts via Western blotting. Results depicted in Figure 5A show that limonoid 2 partially inhibited the translocation of p-JAK2 and p-STAT3, indicating that limonoid 2 reduces LPS-induced JAK2 activity by blocking p-JAK2/STAT3 in the cytoplasm. Consequently, the anti-inflammatory properties of limonoid 2 may stem from its modulation of the JAK2/STAT3 signaling pathway. In this study, limonoid 2 preconditioning decreased iNOS levels and increased eNOS protein expression in a dose-dependent manner in LPS-mediated macrophages (Figure 5B). These findings suggest that limonoid 2 exhibits anti-inflammatory and antioxidant effects in LPS-mediated macrophages by suppressing the induction of JAK2 and eNOS.
Figure 5.
JAK2 and iNOS cascades are implicated in the anti-inflammatory process of limonoid 2. (A) RAW264.7 cells were pre-treated by limonoid 2 (15, 30 μM) for 4 h and then treated with LPS for 24 h. Protein expression of p-STAT-3, STAT-3, p-JAK-2, and JAK-2 was measured by Western blotting. (B) RAW264.7 cells were pre-treated by limonoid 2 (15, 30 μM) for 4 h and then treated with LPS for 24 h. The expression of eNOS and iNOS proteins was detected by Western blotting. * p < 0.05, ** p < 0.01, compared with the LPS group alone.
4. Discussions
Limonoids, derived from triterpenes, are unique, structurally complex, and highly oxidized phytochemicals known for their extensive rearrangements. Inflammation, the body’s natural response to infections and chronic diseases, can escalate into various health complications, including cancer. The anti-inflammatory activities of limonoid compounds have, therefore, been targeted to identify potential therapeutic agents for inflammation management. Beyond their structural diversity, the biological activities of limonoids have garnered significant attention due to their broad spectrum of biological properties, including insecticidal, antimicrobial, antimycotic, antimalarial, cytotoxic, antiviral, and anti-inflammatory effects. Research into anti-inflammatory limonins is crucial for developing new chemical entities to treat inflammatory and immune disorders.
In this study, a new tirucallane triterpenoid (1), four novel limonoids (2–5), and four known limonoids (6–9) were separated from the fruits of M. azedarach. The structural analysis of isolated compounds was primarily conducted using HRMS and NMR data. Stereochemical localizations were mainly determined through rule-of-thumb-based 1H NMR and 1H NMR homonuclear decoupling experiments. Among the isolates, tirucallane triterpenoid (1) and limonoids 2 and 5 exhibited potent anti-inflammatory activities against LPS-induced macrophages. Notably, limonoid 2 demonstrated superior inhibition of nitrite production with an IC50 value of 22.04 μM. The anti-inflammatory and antioxidative mechanisms of limonoid 2 were further investigated, revealing that this compound modulates the NF-κB and JAK2 signaling pathways.
Free radicals, like ROS and reactive nitrogen species (RNS), are unstable molecules that lack an electron and seek stability by accepting electrons or hydrogen atoms. Certain compounds, known for their high antioxidant activity, can donate electrons or hydrogen, thereby stabilizing various free radicals and ROS effectively due to their resonance stability [52]. In this context, various limonoids exhibit remarkable antioxidant activity by supplying electrons or hydrogen atoms to stabilize free radicals [53]. In the current study, limonoid 2 inhibited ROS generation in a dose-dependent manner in LPS-mediated RAW264.7 cells, suggesting that limonoid 2 possesses both anti-inflammatory and antioxidant properties through the modulation of ROS generation.
Macrophages, integral components of the immune system, initiate immune responses through the secretion of NO and factors in reaction to outside stimuli or by phagocytosis to eliminate foreign substances. Prolonged reactions may lead to chronic inflammation, contributing to various diseases [54]. In this study, limonoid 2 demonstrated significant antioxidant activity and played a crucial anti-inflammatory role by inhibiting the generation of NO, inflammatory factors, and ROS.
NO is synthesized by nitric oxide synthase (NOS). Inducible NOS (iNOS) is vital in the inflammatory response [55]. NO produced intracellularly can react with ROS to form RNS, which induce oxidative injury to macromolecules and accelerate inflammation-mediated cellular injury by impeding mitochondrial function [56,57]. Previous research has shown that materials with strong antioxidant activity can utilize ROS to suppress NO production [58]. This study shows that limonoid 2, due to its potent antioxidant capabilities, may inhibit NO production via an LPS-mediated reduction in ROS generation.
Activated macrophages secrete TNF-α, IL-6, and IL-1β and are typical inflammatory cytokines. They can activate other immune cells or enhance macrophage activation through autocrine effects [59]. Overproduction of these cytokines can promote apoptosis and lead to tissue injury [60]. Limonoid 2 reduced the production of these cytokines in LPS-stimulated macrophages, exhibiting anti-inflammatory activity.
The nuclear transcription factor NF-κB is recognized for its pivotal role in modulating the expressions of different pro-inflammatory markers [61]. Thus, inhibiting NF-κB activation is central to the pharmacological mechanism of anti-inflammatory agents [62]. In response to inflammatory stresses like LPS, NF-κB activation leads to IκB-α phosphorylation and degradation and nuclear translocation of NF-κB p65 protein. Translocated p65 interacts with NF-κB bonding sites to activate the transcription of anti-inflammatory agents [63]. We demonstrated that treatment with limonoid 2 suppresses both the phosphorylation of IκB-α and the nuclear translocation of NF-κB p65 proteins.
Pro-inflammatory factors, like iNOS, eNOS, JAK2, and STAT3, play crucial roles in various inflammation models due to their regulatory effects [64,65,66]. Studies have shown that in these models, the overproduction of NO, which enhances iNOS expression, can subsequently influence the expression of JAK2. Consequently, iNOS and JAK2 are identified as potential targets for inflammation mitigation [55]. In this study, limonoid 2 was observed to diminish LPS-mediated JAK2 activity by inhibiting the phosphorylation of JAK2/STAT3 in the cytoplasm. Preconditioning with limonoid 2 reduced iNOS and increased eNOS protein expression in macrophages. These outcomes demonstrate that limonoid 2 inhibits JAK2 phosphorylation, thereby preventing macrophage activation in response to extracellular stimuli. These results indicate that the eNOS and NF-κB pathways might underpin the anti-inflammatory activities of limonoid 2.
This study had limitations. First, the effects of limonoid 2 observed after 4 h may not reflect its effects at different points. In future research, we can compare the differences in therapeutic effects of limonoid 2 with different durations of its action. Second, the impact of limonoid 2 on ROS production has not been investigated, so in vivo animal studies are also required to validate the mechanism of action further. Consequently, future studies should address these limitations to better understand its antioxidant and anti-inflammatory effects.
5. Conclusions
This study examined the active components and anti-inflammatory mechanisms of M. azedarach from chemical and pharmacological perspectives, identifying nine constituents from M. azedarach fruits, including one novel tirucallane triterpenoid (1), four new limonoids (2–5), and four known limonoids (6–9). Limonoid 2 exhibited significant anti-inflammatory effects by reducing nitrite levels, modulating ROS synthesis, and decreasing IL-6 and TNF-α. The eNOS and NF-κB pathways are integral to the anti-inflammatory effects of limonoid 2. Collectively, these findings suggested that limonoid 2 considerably diminishes inflammation. Future in vivo studies are needed to confirm these properties of limonoid 2 and elucidate its potential impact on combating inflammation and oxidative stress.
Abbreviations
Interleukin-6 (IL-6); Tumor Necrosis Factor-α (TNF-α); Nitric Oxide (NO); Inducible Nitric Oxide Synthase (iNOS); Janus Kinases 2 (JAK2); Nuclear Factor Kappa-B (NF-κB); Endothelial Nitric Oxide Synthase (eNOS); NF-kappa-B Inhibitor Alpha (IKB-α); Inhibitor of Kappa B KinaseAlpha/Beta (IKKα/β); Fetal Bovine Serum (FBS); Sulforhodamine B (SRB); Lipopolysaccharide (LPS).
Supplementary Materials
The following supplementary data can be downloaded at https://www.mdpi.com/article/10.3390/antiox13111338/s1. Figures S1–S37: ROESY correlations, ECD, NMR, and HRMS spectra of new compounds 1–5. Table S1. Information about the chemical reagents used in the experiment. Supplementary S1. Detailed procedures for Western blotting. Supplementary S2. Detailed procedures for Immunofluorescence.
Author Contributions
Conceptualization, Y.-H.Z.; methodology, B.L. and Z.-W.C.; investigation, F.C. and J.C.; resources, Z.-T.L.; data curation, X.-H.M. and H.-Y.L.; writing—original draft preparation, F.C. and X.-H.M.; writing—review and editing, Y.-H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was supported by the Fujian Natural Science Foundation (2020J01619, 2023J01550) and Fujian Medical University Key Laboratory of Natural Medicine Pharmacology (FJNMP202202).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J.Y., Peng S.Y., Cheng Y.B., Wang C.L., Farooqi A.A., Yu T.J., Hou M.F., Wang S.C., Yen C.H., Chan L.P., et al. Ethyl acetate extract of Nepenthes adrianii x clipeata induces antiproliferation, apoptosis, and DNA damage against oral cancer cells through oxidative stress. Environ. Toxicol. 2019;34:891–901. doi: 10.1002/tox.22748. [DOI] [PubMed] [Google Scholar]
- 4.Wang C., Schuller Levis G.B., Lee E.B., Levis W.R., Lee D.W., Kim B.S., Park S.Y., Park E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Int. Immunopharmacol. 2004;4:1039–1049. doi: 10.1016/j.intimp.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Pemmari A., Paukkeri E.L., Hämäläinen M., Leppänen T., Korhonen R., Moilanen E. MKP-1 promotes anti-inflammatory M(IL-4/IL-13) macrophage phenotype and mediates the anti-inflammatory effects of glucocorticoids. Basic Clin. Pharmacol. Toxicol. 2019;124:404–415. doi: 10.1111/bcpt.13163. [DOI] [PubMed] [Google Scholar]
- 6.Flórez-Fernández N., Rodríguez-Coello A., Latire T., Bourgougnon N., Torres M.D., Buján M., Muíños A., Muiños A., Meijide-Faílde R., Blanco F.J., et al. Anti-inflammatory potential of ulvan. Pt 4Int. J. Biol. Macromol. 2023;253:126936. doi: 10.1016/j.ijbiomac.2023.126936. [DOI] [PubMed] [Google Scholar]
- 7.Hermanns H.M., Wohlfahrt J., Mais C., Hergovits S., Jahn D., Geier A. Endocytosis of pro-inflammatory cytokine receptors and its relevance for signal transduction. Biol. Chem. 2016;397:695–708. doi: 10.1515/hsz-2015-0277. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y., Yu C.Y., Deng W.M. The role of pro-inflammatory cytokines in lipid metabolism of metabolic diseases. Int. Rev. Immunol. 2019;38:249–266. doi: 10.1080/08830185.2019.1645138. [DOI] [PubMed] [Google Scholar]
- 9.Campos D.C.O., Costa A.S., Luz P.B., Soares P.M.G., Alencar N.M.N., Oliveira H.D. Morindacitrifolia lipid transfer protein 1 exhibits anti-inflammatory activity by modulation of pro- and anti-inflammatory cytokines. Int. J. Biol. Macromol. 2017;103:1121–1129. doi: 10.1016/j.ijbiomac.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 10.Noailles A., Maneu V., Campello L., Lax P., Cuenca N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018;9:350. doi: 10.1038/s41419-018-0355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin W.B., Dong X., Kim Y.S., Park J.S., Kim S.J., Go E.A., Kim E.K., Park P.J. Anti-inflammatory Effects of Batillariamultiformis Water Extracts via NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Cells. Adv. Exp. Med. Biol. 2019;1155:1001–1014. doi: 10.1007/978-981-13-8023-5_83. [DOI] [PubMed] [Google Scholar]
- 12.Diao J., Chi Z., Guo Z., Zhang L. Mung Bean Protein Hydrolysate Modulates the Immune Response Through NF-κB Pathway in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Food Sci. 2019;84:2652–2657. doi: 10.1111/1750-3841.14691. [DOI] [PubMed] [Google Scholar]
- 13.Jeong Y.H., Oh Y.C., Cho W.K., Yim N.H., Ma J.Y. Hoveniae Semen Seu Fructus Ethanol Extract Exhibits Anti-Inflammatory Activity via MAPK, AP-1, and STAT Signaling Pathways in LPS-Stimulated RAW 264.7 and Mouse Peritoneal Macrophages. Mediators Inflamm. 2019;2019:9184769. doi: 10.1155/2019/9184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim T.W., Shin J.S., Chung K.S., Lee Y.G., Baek N.I., Lee K.T. Anti-Inflammatory Mechanisms of Koreanaside A, a Lignan Isolated from the Flower of Forsythia koreana, against LPS-Induced Macrophage Activation and DSS-Induced Colitis Mice: The Crucial Role of AP-1, NF-κB, and JAK/STAT Signaling. Cells. 2019;8:1163. doi: 10.3390/cells8101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahoor M., Ahmed M., Naz S., Ayaz M. Cytotoxic, antibacterial and antioxidant activities of extracts of the bark of Melia azedarach (China Berry) Nat. Prod. Res. 2015;29:1170–1172. doi: 10.1080/14786419.2014.982649. [DOI] [PubMed] [Google Scholar]
- 16.Descalzo A.M., Coto C. Inhibición del virus de pseudorrabia (Suid herpesvirus 1) poracción de un antiviral aislado de hojas de Melia azedarach [Inhibition of the pseudorabies virus (Suis herpesvirus 1) by an antiviral agent isolated from the leaves of Melia azedarach] Rev. Argent. Microbiol. 1989;21:133–140. (In Spanish) [PubMed] [Google Scholar]
- 17.D’Ambrosio M., Guerriero A. Degraded limonoids from Melia azedarach and biogenetic implications. Phytochemistry. 2002;60:419–424. doi: 10.1016/S0031-9422(02)00107-3. [DOI] [PubMed] [Google Scholar]
- 18.Su Z.S., Yang S.P., Zhang S., Dong L., Yue J.M. Meliarachins A-K: Eleven limonoids from the twigs and leaves of Melia azedarach. Helv. Chim. Acta. 2011;94:1515–1526. doi: 10.1002/hlca.201000444. [DOI] [Google Scholar]
- 19.Akihisa T., Pan X., Nakamura Y., Kikuchi T., Takahashi N., Matsumoto M., Ogihara E., Fukatsu M., Koike K., Tokuda H. Limonoids from the fruits of Melia azedarach and their cytotoxic activities. Phytochemistry. 2013;89:59–70. doi: 10.1016/j.phytochem.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Pan X., Matsumoto M., Nakamura Y., Kikuchi T., Zhang J., Ukiya M., Suzuki T., Koike K., Akihisa R., Akihisa T. Three new and other limonoids from the hexane extract of Melia azedarach fruits and their cytotoxic activities. Chem. Biodivers. 2014;11:987–1000. doi: 10.1002/cbdv.201400052. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F., Ma X.H., Li Z.J., Li W., Zheng W.M., Wang Z.B., Zeng X.M., Sun K.H., Zhang Y.H. Four New Tirucallane Triterpenoids from the Fruits of Melia azedarach and Their Cytotoxic Activities. Chem. Biodivers. 2016;13:1738–1746. doi: 10.1002/cbdv.201600149. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Gao Y., Li X., Sun X., Wang Z., Wang H., Nie R., Yu W., Zhou Y. Coniferyl Aldehyde Inhibits the Inflammatory Effects of Leptomeningeal Cells by Suppressing the JAK2 Signaling. BioMed Res. Int. 2020;2020:4616308. doi: 10.1155/2020/4616308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S.N., Huang L., Ma R.J., Yang M.F., Wei B.F., Song H.Z., Wang H.S., Tan Q.G. Chemical constituents from the barks of Melia azedarach and their PTP1B inhibitory activity. Nat. Prod. Res. 2021;35:4442–4447. doi: 10.1080/14786419.2020.1729146. [DOI] [PubMed] [Google Scholar]
- 24.Song M., Luo H.J., Li Z.W., Qiu L., Zhao Y.X., He C.W., Zhang X.Q., Ye W.C., Lin L.G., Zhang Q.W. Limonoids from the roots of Melia azedarach and their anti-inflammatory activity. Phytochemistry. 2023;216:113869. doi: 10.1016/j.phytochem.2023.113869. [DOI] [PubMed] [Google Scholar]
- 25.Hieu T.T., Thuy P.T., Duc D.X. Isolation and Bioactivities of Limonoids from Meliaceae Family: A Review. Curr. Org. Chem. 2022;26:1359–1430. doi: 10.2174/1385272827666221017141255. [DOI] [Google Scholar]
- 26.Zhou H., Hamazaki A., Fontana J.D., Takahashi H., Wandscheer C.B., Fukuyama Y. Cytotoxic limonoids from Brazilian Melia azedarach. Chem. Pharm. Bull. 2005;53:1362–1365. doi: 10.1248/cpb.53.1362. [DOI] [PubMed] [Google Scholar]
- 27.Huang R.C., Okamura H., Iwagawa T., Nakatani M. The Structures of Azedarachins, Limonoid Antifeedants from ChineseMeliaazedarachLinn. Bull. Chem. Soc. Jpn. 1994;67:2468–2472. doi: 10.1246/bcsj.67.2468. [DOI] [Google Scholar]
- 28.Nakatani M., Chun Huang R., Okamura H., Naoki H., Iwagawa T. Limonoid antifeedants from chinese Melia azedarach. Phytochemistry. 1994;36:39–41. doi: 10.1016/S0031-9422(00)97008-0. [DOI] [Google Scholar]
- 29.Vajrabhaya L.O., Korsuwannawong S. Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. J. Anal. Sci. Technol. 2018;9:15. doi: 10.1186/s40543-018-0146-0. [DOI] [Google Scholar]
- 30.Zhang L., Chen J., Liang R., Liu C., Chen M., Chen J. Synergistic Anti-Inflammatory Effects of Lipophilic Grape Seed Proanthocyanidin and Camellia Oil Combination in LPS-Stimulated RAW264.7 Cells. Antioxidants. 2022;11:289. doi: 10.3390/antiox11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robles V., Riesco M.F., Martínez-Vázquez J.M., Valcarce D.G. Flow Cytometry and Confocal Microscopy for ROS Evaluation in Fish and Human Spermatozoa. Methods Mol. Biol. 2021;2202:93–102. doi: 10.1007/978-1-0716-0896-8_8. [DOI] [PubMed] [Google Scholar]
- 32.Kim S.H., Kang I.C. Induction of TNF-α by Filifactoralocis in THP-1 macrophagic cells. Arch. Oral Biol. 2023;155:105806. doi: 10.1016/j.archoralbio.2023.105806. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang J., Hong Y., Wan Y., He X., Geng B., Yang X., Xiang J., Cai J., Zeng Z., Liu Z., et al. PVB exerts anti-inflammatory effects by inhibiting the activation of MAPK and NF-κB signaling pathways and ROS generation in neutrophils. Int. Immunopharmacol. 2024;126:111271. doi: 10.1016/j.intimp.2023.111271. [DOI] [PubMed] [Google Scholar]
- 34.Vieira I.J., Azevedo Ode A., de Souza J.J., Braz-Filho R., Gonçalves Mdos S., de Araújo M.F. Hirtinone, a Novel cycloartane-type triterpene and other compounds from Trichiliahirta L. (Meliaceae) Molecules. 2013;18:2589–2597. doi: 10.3390/molecules18032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolad S.D., Hoffmann J.J., Cole J.R., Tempesta M.S., Bates R.B. Constituents of Trichilia hispida (Meliaceae). 2. A new triterpenoid, hispidone, and bourjotinolone A. J. Org. Chem. 1980;45:3132–3135. doi: 10.1021/jo01303a044. [DOI] [Google Scholar]
- 36.Pescitelli G., Bruhn T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality. 2016;28:466–474. doi: 10.1002/chir.22600. [DOI] [PubMed] [Google Scholar]
- 37.Pescitelli G. ECD exciton chirality method today: A modern tool for determining absolute configurations. Chirality. 2022;34:333–363. doi: 10.1002/chir.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manosroi A., Kitdamrongtham W., Ishii K., Shinozaki T., Tachi Y., Takagi M., Ebina K., Zhang J., Manosroi J., Akihisa R., et al. Limonoids from Azadirachta indica var. siamensis extracts and their cytotoxic and melanogenesis-inhibitory activities. Chem. Biodivers. 2014;11:505–531. doi: 10.1002/cbdv.201300406. [DOI] [PubMed] [Google Scholar]
- 39.Li S., Li Y., Xu R., Kong L.Y., Luo J. New meliacarpin-type (C-seco) and C-ring intact limonoids from the fruits of Melia toosendan. Fitoterapia. 2020;144:104605. doi: 10.1016/j.fitote.2020.104605. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H.P., Bao G.H., Wang H.B., Qin G.W. Two new limonoids from Munroniahenryi. Nat. Prod. Res. 2004;18:415–419. doi: 10.1080/14786410310001620547. [DOI] [PubMed] [Google Scholar]
- 41.Park S., Nhiem N.X., Subedi L., Oh I., Kim J.Y., Kim S.Y., Kim S.H. Isolation of bioactive limonoids from the fruits of Melia azedarach. J. Asian Nat. Prod. Res. 2020;22:830–838. doi: 10.1080/10286020.2019.1666826. [DOI] [PubMed] [Google Scholar]
- 42.Qiu L., Heng L., Xu R., Luo J., Li Y. Two new nimbolinin- and trichilin-class limonoids isolated from the fruits of Melia azedarach. Chin. J. Nat. Med. 2019;17:227–230. doi: 10.1016/S1875-5364(19)30025-1. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q., Shi Y., Liu X.T., Liang J.Y., Ip N.Y., Min Z.D. Minor limonoids from Melia toosendan and their antibacterial activity. Planta Med. 2007;73:1298–1303. doi: 10.1055/s-2007-981618. [DOI] [PubMed] [Google Scholar]
- 44.Brasier A.R. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrestha A., Shrestha A., Park P., Lee E. Hydroxyl-and Halogen-containing Chalcones for the Inhibition of LPS-stimulated ROS Production in RAW 264.7 Macrophages: Design, Synthesis and Structure–Activity Relationship Study. Bull. Korean Chem. Soc. 2019;40:729–734. doi: 10.1002/bkcs.11813. [DOI] [Google Scholar]
- 46.Zong L., Zhang J., Dai L., Liu J., Yang Y., Xie J., Luo X. The Anti-Inflammatory Properties of Rhododendron molle Leaf Extract in LPS-Induced RAW264.7. Chem. Biodivers. 2020;17:e2000477. doi: 10.1002/cbdv.202000477. [DOI] [PubMed] [Google Scholar]
- 47.Yu S., Chen X., Xiu M., He F., Xing J., Min D., Guo F. The regulation of Jmjd3 upon the expression of NF-κB downstream inflammatory genes in LPS activated vascular endothelial cells. Biochem. Biophys. Res. Commun. 2017;485:62–68. doi: 10.1016/j.bbrc.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Dunkhunthod B., Talabnin C., Murphy M., Thumanu K., Sittisart P., Hengpratom T., Eumkeb G. Intracellular ROS Scavenging and Anti-Inflammatory Activities of Oroxylum indicum Kurz (L.) Extract in LPS plus IFN-γ-Activated RAW264.7 Macrophages. Evid. Based Complement. Altern. Med. 2020;2020:7436920. doi: 10.1155/2020/7436920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H., Pan Z., Ma X., Cui J., Gao J., Miao Q., Zhu Z., Chen X., Su S. ROCK inhibitor fasudil reduces the expression of inflammatory factors in LPS-induced rat pulmonary microvascular endothelial cells via ROS/NF-κB pathway. BMC Pharmacol. Toxicol. 2022;23:24. doi: 10.1186/s40360-022-00565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh G., Bhatti R., Mannan R., Singh D., Kesavan A., Singh P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology. 2019;27:949–960. doi: 10.1007/s10787-018-0486-9. [DOI] [PubMed] [Google Scholar]
- 51.Wu H., Liu H., Zhao X., Zheng Y., Liu B., Zhang L., Gao C. IKIP Negatively Regulates NF-κB Activation and Inflammation through Inhibition of IKKα/β Phosphorylation. J. Immunol. 2020;204:418–427. doi: 10.4049/jimmunol.1900626. [DOI] [PubMed] [Google Scholar]
- 52.Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- 53.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 54.Fujiwara N., Kobayashi K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 55.Laroux F.S., Pavlick K.P., Hines I.N., Kawachi S., Harada H., Bharwani S., Hoffman J., Grisham M.B. Role of nitric oxide in inflammation. Acta Physiol. Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 56.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxinitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaushal G.P., Chandrashekar K., Juncos L.A. Molecular interaction between reactive oxygen species and autophage in kidney disease. Int. J. Mol. Sci. 2019;20:3791. doi: 10.3390/ijms20153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandoval-Acuna C., Ferreira J., Speisky H. Polyphenola and mitochondria: An update on their increasingly emerging ROS scavenging independent action. Arch. Biochem. Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 59.Samard T.A., Moore K.A., Spirstein A., Billet S., Allchrone A., Poole S., Bonventre J.V., Woolf C.J. Interleukin-1-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 60.Dinarello C.A. Proinflammatory Cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 61.Lawrence T., Gilroy D.W., Colville-Nash P.R., Willoughby D.A. Possible new role for NF-kB in the resolution of inflammation. Nat. Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- 62.Lewis A.J., Mamming A.M. New targets for anti-inflammatory drugs. Curr. Opin. Chem. Biol. 1999;3:489–494. doi: 10.1016/S1367-5931(99)80071-4. [DOI] [PubMed] [Google Scholar]
- 63.Neumann M., Naumann M. BeyondIkBs: Alternative regulation of NF-kB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 64.Yang X., He G., Hao Y., Chen C., Li M., Wang Y., Zhang G., Yu Z. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J. Neuroinflamm. 2010;7:54. doi: 10.1186/1742-2094-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebru E., Kang E.H., Damte D., Lee J.S., Jang S.H., Kim M.H., Cheng H., Park S.C. The role of Janus kinase 2 (JAK2) activation in pneumococcal EstAprotein-induced inflammatory response in RAW 264.7 macrophages. Microb. Pathog. 2011;51:297–303. doi: 10.1016/j.micpath.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Huang F.M., Chang Y.C., Lee S.S., Yang M.L., Kuan Y.H. Expression of pro-inflammatory cytokines and mediators induced by Bisphenol A via ERK-NFκB and JAK1/2-STAT3 pathways in macrophages. Environ. Toxicol. 2019;34:486–494. doi: 10.1002/tox.22702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are included in the article.