Simple Summary
Kobuviruses (KoVs) possess a wide host range and have been associated with enteric disease in several host species. On screening of 38 stool samples collected from healthy (n = 21) and diarrheic (n = 17) animals, using a pan-KoV RT-PCR, viral RNA was detected in 10/38 (26.3%) samples. Six strains (including two Aichivirus D strains) were from animals with enteric signs. Applying whole genome sequencing, the Aichivirus D strains (ITA/2019/572-1 and ITA/2020/30-2) showed a close relatedness with a Chinese Aichivirus D strain and a possible recombinant nature. Understanding the genetic diversity of KoVs in animals will be useful in improving diagnostics and filling epidemiological gaps.
Keywords: Kobuvirus, cattle, recombination, metagenomics, Aichivirus
Abstract
Kobuviruses (KoVs) are a group of small, non-enveloped RNA viruses classified in the genus Kobuvirus within the Picornaviridae family, comprising Aichivirus species A to F. KoVs have been identified in humans and several mammals, including domestic ungulates. This study investigated the presence of KoVs in a collection of bovine stool samples (n = 38) obtained from animals with enteritis or without clinical signs. By RT-PCR screening, KoV RNA was detected in 10/38 animals (26.3%). Six of the ten positive animals had enteric signs. On sequence analysis of the amplicons, eight strains were related to species Aichivirus B, commonly identified in cattle. In contrast, two strains (ITA/2019/572-1 and ITA/2020/bovine/30-2), displayed the highest nt identity (up to 97.1%) to cattle, yak, and goat Aichivirus D strains. On whole genome analysis, strains ITA/2019/572-1 and ITA/2020/30-2 showed 88.9% nt identity to each other and 87.8–90.3% nt to the bovine kobuvirus strain CHN/2021/ON730709 identified in China. Interestingly these three Aichivirus D strains showed a recombinant makeup, clustering with D1 genotype in the capsid region and with D2 genotype in the non-structural genes. These findings suggest that Aichivirus D KoVs are common components of livestock virome. Understanding the genetic diversity of KoVs in animals will be useful to improve the diagnostics and gather epidemiological data.
1. Introduction
Viral enteric infections may profoundly impact the health and productivity of cattle herds, chiefly affecting young calves, and often leading to fatal consequences [1]. The viruses frequently associated with bovine enteric disease are rotavirus A, coronavirus, bovine viral diarrhea virus, and astroviruses [2,3]. However, other viruses have been detected as common components of the bovine enteric virome, including kobuviruses (KoVs) [4].
KoVs are a group of small, non-enveloped RNA viruses classified in the genus Kobuvirus within the family Picornaviridae [5]. They possess a single-stranded, positive-sense RNA genome approximately 8.2–8.4 kilobases in length. The viral genome is enclosed in a capsid comprising 60 identical subunits. The RNA of KoVs contains 5′ and 3′ untranslated regions (UTRs) with a single large open reading frame (ORF) encoding for a polyprotein of 2436–2437 amino acids (aa) that undergoes protease processing to yield a leader protein (L), three structural viral proteins (VP0, VP3, and VP1) and seven non-structural proteins (NSPs) (2A-2C and 3A-3D). The VP1 capsid protein is the most variable structure and mainly elicits an immune response [6].
KoVs were first discovered in 1989 from an outbreak of gastroenteritis in human patients in Aichi Prefecture, Japan [7]. Subsequently, similar viruses have been reported in a multitude of animal hosts. Based on the genetic diversity of the complete polyprotein, VP0-VP3-VP1, 2C, and 3CD regions, KoVs are currently classified into six species, formerly known as Aichivirus (AiV) A to F, and 20 genetic types indicated with numbers [8].
Kobuvirus aichi, known as AiV A, has been found in several host species including humans (A1) [9], dogs (A2) [10], rodents (A3, A6–A10) [11], domestic cats (A4) [12], and birds (A5) [13]. Kobuvirus bejaponia, known as AiV B, has been found mostly in cattle (B1) [14], but it is also present in mustelids (B2) [15] and sheep (B3) [16]. Kobuvirus cebes, known as AiV C, is associated with goats (C2) [17] and pigs (C1) [18], while Kobuvirus dekago, or AiV D, has recently been discovered in Japanese black cattle [19]. Kobuvirus femyomini, or AiV F, has been identified in chiropterans (types F1 and F2) [20] and Kobuvirus ecuni, or AiV E, in rabbits (E1) [21].
Several studies have investigated the role of KoVs in enteric diseases of cattle. Bovine KoV (strain U-1) was first identified as a laboratory cell culture contaminant in 2003 in Japan [14]. Bovine KoVs have been subsequently reported in studies worldwide in animals with and without enteric signs in different age groups [22]. Epidemiological information from studies in several countries has been summarized in a review on bovine kobuviruses [22]. Overall, the incidence of kobuvirus in animals with enteric signs is reported to be 5.3–66.6%, whilst the incidence of kobuvirus in asymptomatic animals is 4.9–25.0%. On sequence analysis, the various KoV strains in cattle have shown genetic diversification, with several lineages, yet are all classified in the unique species AiV B1 [22]. In 2016, KoVs distantly related to AiV B1 were identified in Japan [19]. Based on the genome sequence, the prototype KoV strains (JPN/2014/Kago-1-22 and JPN/2015/Kago-2-24) have been classified into a novel species (AiV D) and proposed as two different genotypes, D1 and D2, respectively. Using specific primers, similar KoVs have been detected, respectively, in 16.9% and 10.4% of animals with enteritis from the same geographical area, but not in animals from other prefectures [8]. AiV D viruses have been subsequently reported in studies in China in yak (Bos grunneris) [23] and small ruminants [24]. In this study, we report the identification and characterization of AiV D in cattle in Italy.
2. Materials and Methods
2.1. Samples Collection
A convenience sampling collection was used in the study. Sample collection covered a period of 3 months, spanning from October 2019 to January 2020. A total of 6 farms were sampled in Taranto (5, Apulia region) and Cosenza (1, Calabria region), Italy, ranging in size from 50 to 500 head of cattle. In detail, 38 stool samples were collected either from animals showing enteric signs (n = 17) (acute diarrhea, weight loss, anorexia) or from healthy calves (n = 21). The age ranged from 20 days to 96 months old (Supplementary Table S1). The number of samples per farm varied from 2 to 16, sampling a least one healthy animal for each with enteritis.
2.2. Sample Preparation and Nucleic Acid Extraction
For sample preparation, the NetoVir protocol was used [25]. Briefly, stool samples were aliquoted and diluted with sterile PBS to create a 10% suspension. The homogenization was performed by Qiagen TissueLyser (QiagenTM, Hilden, Germany) with a frequency of 25/s, followed by centrifugation at 16,000× g for 3 min. The extraction of nucleic acids was performed using the Indispin Pathogen DNA/RNA Mini Kit (Indical®, Leipzig, Germany) from 400 μL of the supernatants, according to the manufacturer’s instructions. Finally, nucleic acid was eluted (100 μL) and stored at −80 °C until later use.
2.3. RT-PCR Screening for Kobuvirus
A pan-KoV set of primers UNIV-kobu-F/UNIV-kobu-R was used for reverse transcription-polymerase chain reaction (RT-PCR). The primers are designed to amplify a 217-bp region of the viral RNA-dependent RNA polymerase complex (RdRp) of all known KoV species [26]. The amplicons were run on a 1.5% agarose gel containing a fluorescent dye (GelRed® Nucleic Acid Gel Stain; Biotium, Fremont, CA, USA) at 90 V for 50 min and visualized on a Gel Doc imaging system (Bio-Rad Laboratories, Hercules, CA, USA). Specific amplicons of about 200 nt in length were visualized and excised from gel for purification (Invitrogen™, PureLink™ Quick Gel Extraction Kit) and sent for Sanger sequencing (Eurofins Genomics, Ebersberg, Germany).
2.4. Random Amplification and Illumina Sequencing
A modified version of the Whole Transcriptome Amplification (WTA) protocol (Sigma-Aldrich) was performed [27]. After a first step of denaturation at 95 °C for 2 min, the RNA was reverse transcribed using primers with a semi-degenerate 3′ end and a universal 5′ end (universal primers). The cDNA was amplified with a random PCR amplification for 17 cycles. The WTA PCR product was purified using the MSB® Spin PCRapace kit (Invitek Molecular, Berlin, Germany). WTA amplification product was quantified using Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Library preparation was performed using an adjusted protocol of the Nextera XT Library Preparation Kit (Illumina, San Diego, CA, USA). The size of the library was checked with an A2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) with a High Sensitivity DNA chip, to evaluate the length distribution of the obtained fragments. The samples were sequenced on an Illumina NextSeq 500 platform (2 × 150 bp paired end).
2.5. Whole Genome Sequencing of Kobuviruses
The complete genome sequence of KoVs was reconstructed using a primer walking strategy with multiple sets of primers designed in conserved regions. The 5′ and 3′ ends sequences were obtained by Rapid Amplification of complementary DNA End (RACE) protocols [28,29] using SuperScript-III Taq kit (Invitrogen™, Life Technologies, Milan, Italy) for reverse transcription LaTakara PCR kit (TaKaRa Bio Europe S.A.S, France) for amplification. The PCR products were run on 1.5% agarose gel and the bands of the expected size were excised and purified (PureLink™ Quick Gel Extraction Kit, Invitrogen™, Life Technologies, Milan, Italy) and sequenced with Sanger technology.
2.6. Sequence and Phylogenetic Analysis
Samples that tested positive by the pan-KoV RT-PCR were sequenced and analyzed to identify homologous hits in the NCBI database using the Basic Local Alignment Search Tool (BLAST) http://www.ncbi.nlm.nih.gov (accessed on 9 March 2024) with default parameters. Data obtained by Illumina sequencing were analyzed in parallel by the software package Geneious Prime version 2024.2 (Dotmatics, Germany) and the online software Genome Detective Virus Tool v 2.48 (GDVT) [30].
The KoV sequences were aligned with cognate KoV strains retrieved from the GenBank database (NCBI, accessed on 15 March 2024) using MAFFT v7.490 [31]. The best substitution model parameters for phylogenetic inference and assessment of selection pressure were predicted using “Find the best protein DNA/Protein Models” implemented in MEGA X version 11.0.13 [32]. Bootstrap replication was set to 1000 to assess the reliability of the inferred tree.
The software tools SimPlot v1.3.0 [33], RdP v5.58 [34], and Python package Recan v0.5 [35] with default parameters were used to assess potential recombination events in the genome sequences.
2.7. GenBank Sequence Submission
The nucleotide sequences of strains ITA/2019/572-1, ITA/2020/30-1 and ITA/2020/30-2 employed for phylogeny were deposited in the GenBank database under accession numbers PQ360972, PQ360973 and PQ360974 respectively.
2.8. Statistical Analysis
A Fisher’s exact test was used to find possible association between the presence of KoV and enteric signs in the 38 animals. Statistical calculations were performed using GraphPad Prism v8.1.2 program Intuitive Software for Science, San Diego, CA, USA. The statistical significance level was always set at 0.05.
3. Results
3.1. Pan-Kobuvirus RT-PCR Screening
On pan-KoV RT-PCR screening, 10 samples out of 38 (26.3%) tested positive. The samples were collected from three different meat farms located in Taranto (Apulia, Italy) and from a farm situated in Cosenza (Calabria, Italy). Of the 10 KoV-positive samples, 4 were from healthy animals, and 6 were from juveniles showing enteric signs.
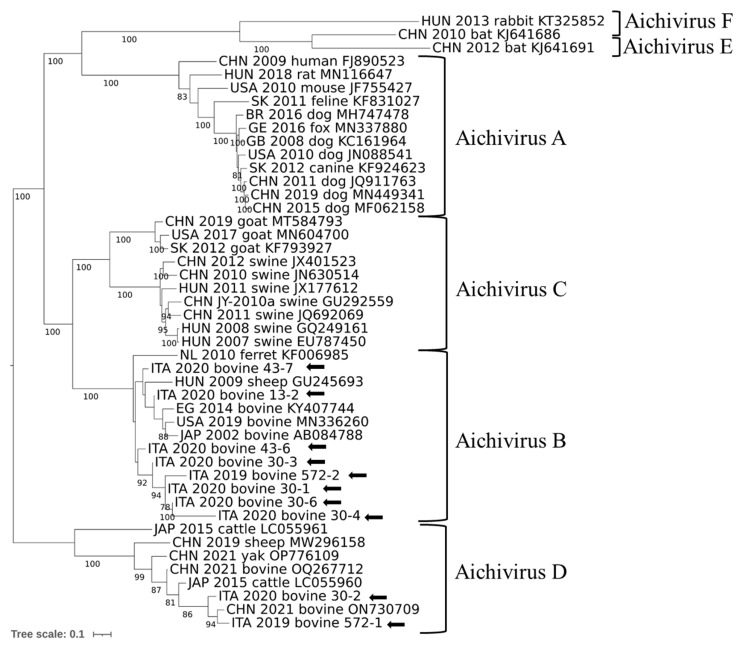
Interrogation of NCBI databases using the BLASTn tool (NCBI; accessed in March 2024) confirmed the specificity of the amplicons (nucleotide [nt] identity 94.2–97.7%). Identity among the 10 sequences obtained in this study ranged from 90.6% to 99.4%. A phylogenetic tree was generated based on the partial RdRp sequence (Figure 1). In the tree, most sequences were grouped with AiV B1 genotype strains, whilst two sequences were grouped with AiV D2 genotype.
Figure 1.
Phylogenetic reconstruction based on partial RdRp sequences obtained in this study (arrows) and reference sequences obtained from Genbank. Statistical support was determined using 1000 bootstrap replicates, with gamma distribution and invariant sites.
3.2. Full Genome Sequence Analysis and Amplification
The WTA protocol (Sigma-Aldrich) was used to generate libraries from the 38 samples and sequenced on an Illumina NextSeq 500 platform. Overall, sequencing yielded 0.40–3.85 GB of data for the 38 samples (mean = 2.52 GB). The number of reads obtained ranged between 589 and 849,166 (mean = 72,244.60, Median = 8782.12; St. Dev. = 164,041.21), with an average length of 50–134 bp. The FASTq data were analyzed using Genome Detective Virus Tool v 2.48 (GDVT). The sequencing reads were classified in various taxa, including CRESS DNA virus, parvovirus, porprismacovirus, rotavirus, picobirnavirus, torovirus, enterovirus, kobuvirus, parapoxvirus, and mastadenovirus. Overall, five samples (572/19–1, 572/19–2, 30/20–1, 30/20–2, and 30/20–4) contained reads classified at genus level as KoV, ranging from 189 to 43,063 reads (mean = 13,716.66; median = 1032.5).
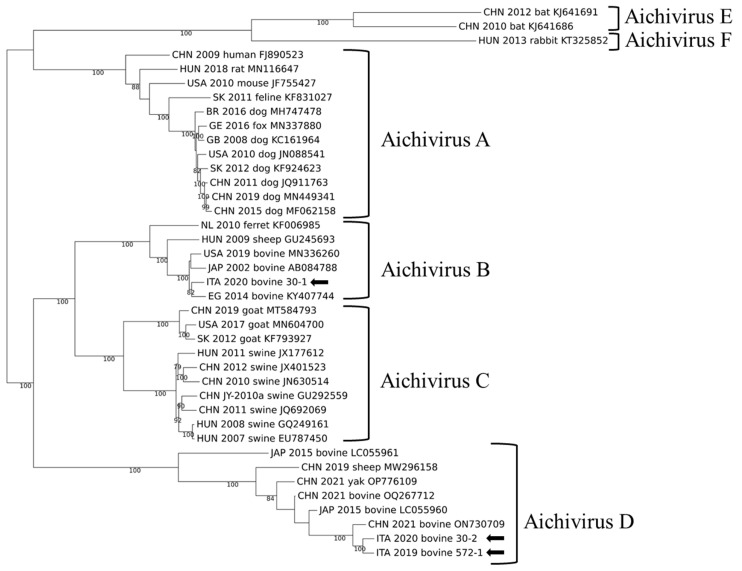
For three strains, genome coverage after Illumina sequencing was above 90% (572/19-1, 30/20-2, and 30/20-1), with a depth of coverage ranging between 30.7 and 688.0 × (mean = 425.66). The complete genome sequence of strains ITA/2019/572-1, ITA/2020/30-2, and ITA/2019/572-1 were obtained. In the complete genome-based phylogenetic analysis, strain ITA/2020/30-1 was characterized as AiV B1, while strains ITA/2019/572-1 and ITA/2020/30-2 were related to AiV D strains identified in cattle, yak, and sheep in Asia [19,23,24] (Figure 2).
Figure 2.
Phylogenetic reconstruction based on complete KoV genomes obtained in this study (arrows) and hit sequences obtained from GenBank. Statistical support was determined using 1000 bootstrap replicates, with gamma distribution and invariant sites.
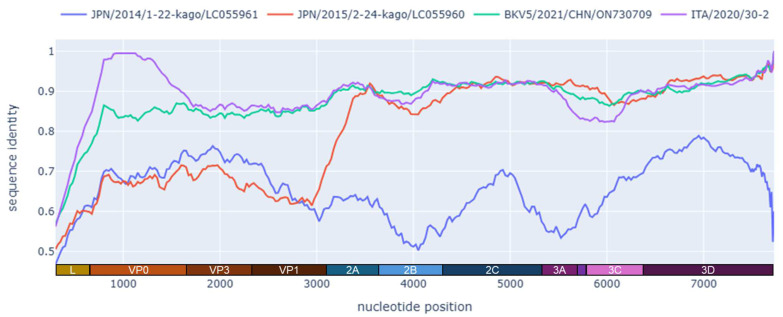
On pairwise sequence comparison, strains ITA/2019/572-1 and ITA/2020/30-2 displayed the highest genetic relatedness (87.8–90.3% nt identity) to a bovine KoV complete genome sequence (CHN/2021/BKV5, GenBank accession no. ON730709) detected in feces from cattle in China in 2021 (unpublished) (Figure 3), whilst nt identities to other AiV D strains were 77.5–80.7%. Strain ITA/2020/30-2 was correlated to other bovine KoVs (AiV B1), showing the highest nucleotide identity (91.6%) to a complete genome detected in the USA (IL35164, GenBank accession no. MN336260).
Figure 3.
Whole genome identity plot of strain ITA/2019/572-1 in comparison with strains ITA/2020/30-2, JAP/2015/2-24-kago/LC055960 (D2), JAP/2014/1-22-kago/LC055961 (D1), and BKV5/2021/CHN/ON730709. The plot was generated using the Python package Recan v0.5 with a window of 200 nt and a shift of 50 nt.
The genome coding sequences of strains ITA/2019/572-1 and ITA/2020/30-2, excluding the terminal UTR regions, were respectively 7509-nt and 7518-nt in length, with a single ORF encoding a predicted polyprotein of 2503 and 2506 aa, respectively. The genome layout of the polyprotein was similar to that of other members of the genus Kobuvirus comprising an L protein, three structural proteins (VP0, VP3, and VP1), and seven NSPs (2A to 2 C and 3A to 3D). The cleavage sites, predicted by sequence alignments and NCBI Conserved Domain Search, https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 2 September 2024), were Q/G (L/VP0), Q/H (VP0/VP3), Q/P (VP3/VP1), Q/C (VP1/2A), Q/G (2A/2B), QS (2B/2C), QG (2C/3A), QA (3A/3B), QG (3B/3C), and Q/S (3C/3D).
When comparing the aa sequences, the ICTV species demarcation criteria were considered [8]. In the 2C+3CD regions, the aa identity of ITA/2019/572-1 and ITA/2020/30-2 with AiV D2 reference strain (JPN/2015/Kago-2-24, LC055960) was 96.3% and 96.4%, respectively. However, in the P1 region (VP0, VP3, and VP1), the aa identity for strain JPN/2015/Kago-2-24 (LC055960, AiV D2) and JPN/2014/Kago 1-22 (LC055961, AiV D1) was significantly lower (61.3–66.1% and 62.4–67.9%, respectively), exceeding the 30% divergence threshold (Table 1).
Table 1.
Identity of strains CHN/2021/ON730709, ITA/2019/572-1 and ITA/2020/30-2 2C, 3CD, P1, and Polyprotein AA sequences with strains JPN/2014/LC055961 and JPN/2015/LC055960, considered as reference strains for AiV D1 and D2, respectively.
| 2C+3CD (aa) (>79% Identity) (<20% Divergence) |
P1 (aa) (>69% Identity) (<30% Divergence) |
Polyprotein (aa) (>69% Identity) (<30% Divrgence) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ON730709 | LC055960 (D2) | LC055961 (D1) | ON730709 | LC055960 (D2) | LC055961 (D1) | ON730709 | LC055960 (D2) | LC055961 (D1) | |
| ON730709 | x | 96.4 | 68.0 | x | 67.1 | 68.9 | x | 85.1 | 63.6 |
| ITA/2020/30-2 | 96.4 | 95.2 | 68.1 | 85.7 | 61.3 | 62.4 | 93.0 | 82.3 | 61.1 |
| ITA/2019/572-1 | 96.3 | 96.7 | 67.9 | 90.9 | 66.1 | 67.9 | 94.4 | 85.2 | 61.0 |
| D1 | 68.3 | x | 73.5 | x | 65.3 | x | |||
| D2 | x | 68.3 | x | 73.5 | x | 65.3 | |||
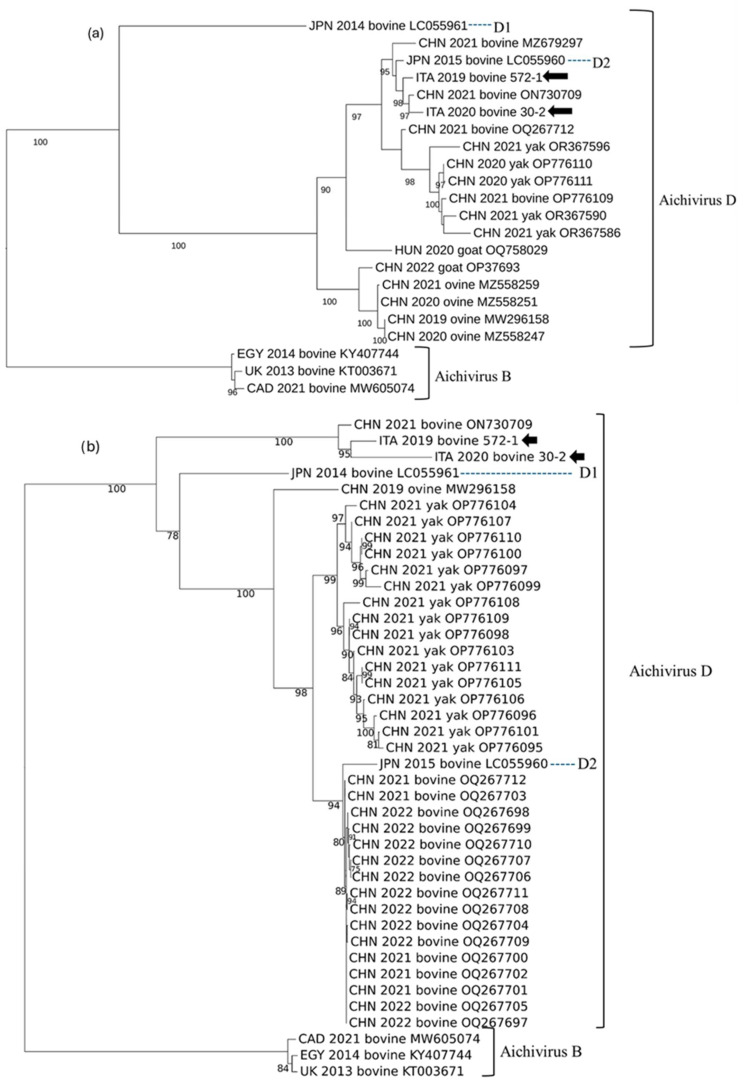
Trees based on the P1 and the seven NSPs (2A to 3D) were also generated (Figure 4). In the P1-based tree, the strains ITA/2019/572-1, ITA/2020/30-2, and CHN/2021/BKV5 (ON730709) segregated together, in a branch rooted with the D1 strain JPN/2014/Kago 1-22 (LC055961). In the tree based on the NSPs, the strains ITA/2019/572-1, ITA/2020/30-2, and CHN/2021/BKV5 (ON730709) segregated together with the D2 strain JPN/2015/Kago-2-24 (LC055960) (AiV D2).
Figure 4.
Phylogenetic reconstruction based on the NSPs (2A to 3D) (a) and P1 (VP0, VP3, VP1) (b) KoV sequences obtained in this study (arrows) and hit sequences obtained from GenBank. Statistical support was determined using 1000 bootstrap replicates, with gamma distribution and invariant sites.
Recombination analysis identified a possible recombination event affecting the P1 region, seemingly located near the junction of the VP1 and A2 genes (Figure 3).
3.3. Evaluation of Statistical Association
In the 38 animals, no significant association was found between RT-PCR positivity to KoV and the presence of enteric signs (p = 0.29).
4. Discussion
The epidemiology of KoVs has been the subject of studies in several animal species in recent years. Their possible association with enteric disease has been hypothesized in various animal hosts, including humans, domestic carnivores, and pigs [12,36,37]. In cattle, KoVs have been involved in multifactorial enteritis outbreaks in co-infection with other enteric pathogens [38,39,40] and have been detected in both healthy and sick animals [41,42]. A possible role of KoVs as enteric pathogens in cattle has been hypothesized in several studies, although this has not been demonstrated firmly [40,41,43,44]. However, experimental infection with a genotype D2 KoV strain isolated on VERO cells produced diarrhea and depression in 2-month-old yak calves. The clinical signs started 3 days after infection and peaked at 6 days. Some of the infected animals were euthanized on day 8 post-infection and gross pathological lesions were observed in the duodenum and jejunum, while the virus was also detected in most internal organs, indicating systemic infection [23].
In this study, using a pan-KoV RT-PCR, an overall positivity rate of 26.3% (10/38) was observed among the animals sampled from six farms. KoV RNA was detected in five out of six farms. Six of the ten KoV-positive animals exhibited enteric signs. Since we screened a small number of animals after a convenience sampling, the epidemiological value of our findings is limited. Most (80%, 8/10) bovine KoV strains detected in our study were related to bovine AiV B1, a species/genotype commonly reported in cattle [42,43]. However, two strains, ITA/2019/572-1 and ITA/2020/30-2, were more related genetically to AiV D strains detected in ruminants in Asia [23]. The highest aa identity was to the bovine strain CHN/2021/BKV5 (ON730709) and it was 93.0–94.4% in the polyprotein, 85.7–90.9% in the P1, and 96.4–96.3% in the 2C+3CD. The sequences were also compared to the prototype Japanese D1 and D2 strains. In the 2C+3CD region, there was a high identity (95.2–96.7% aa) to the genotype D2 strain JPN/2015/LC055960 and a lower identity (67.9–68.1% aa) to the D1 strain JPN/2014/LC055961. In the functional P1 region (VP0-VP3-VP1), there was a low aa identity to both AiV D1 (62.4–67.9%) and D2 (61.3–66.1%), below the threshold established for species classification. Based on the ICTV criteria, KoVs of the same species display a divergence of <30%aa in the polyprotein, <30% aa in the P1, and <20% aa in the 2C+3CD. Accordingly, the strains ITA/2019/572-1, ITA/2020/30-2, and CHN/2021/BKV5 would not meet, strictly, the criteria for classification into the AiV D species, if considering only the P1 gene, challenging the ICTV classification criteria. By observing the identity plot (Figure 3), it was clear that from the 2A encoding gene onwards (regions P2/2A-2B-2C and P3/3A-3B-3C-3D), there was a high degree of conservation among the AiV D strains, while upstream, in the P1 region, a high genetic diversity was observed. Interestingly, in the P1-based tree, the strains ITA/2019/572-1, ITA/2020/30-2, and CHN/2021/BKV5 (ON730709) segregated together, in a branch rooted with the D1 strain JPN/2014/Kago 1-22 (LC055961), while in the tree based on the NSPs (i.e., P2 and P3 functional regions), the three strains segregated together with the D2 strain JPN/2015/Kago-2-24 (LC055960). These inconsistencies in the phylogenetic patterns could suggest a recombination event between a D2 KoV strain, and an, as of yet, uncharacterized KoV strain, distantly related to JPN/2014/Kago 1-22 (LC055961) (Figure 3).
Genetic recombination is a driving force in the evolution of single-stranded RNA viruses [45,46]. Picornaviruses are characterized by marked genetic variability, often due to intraspecies/intertypic recombination events, and, less frequently, recombination at the interspecies level [47]. These events seem to increase the adaptability and pathogenicity of viral strains [48,49,50,51]. In many picornavirus genera, recombination has been demonstrated [52,53,54], with the cross-over points being frequently located at the 3′ end of the P1 region that encodes for the structural proteins [55]. Recombination within the P1 region has been rarely reported, usually between viruses of the same serotype [56,57]. Regardless, the Italian strains ITA/2019/572-1, ITA/2020/30-2, and the Chinese strain CHN/2021/BKV5 (ON730709) retained a conserved genomic composition, based on our analyses, thus indicating that these viruses are circulating in cattle in distinct geographical areas. Likewise, recombination patterns and mosaic genome organization have been observed in human AiVs (Kobuvirus Aichi) [58,59]. The possibility of recombination events between human and animal picornaviruses has not been demonstrated, although interspecies transmission seems to have occurred on more occasions. Swine vesicular disease virus (SVDV) emerged around the 1960s worldwide and originated from a Coxsackievirus B5, a common human enterovirus [60]. In 1975, however, an SVDV (strain T75), likely originated from a human coxsackievirus B4, caused an epizootic in pigs in the Soviet Union, demonstrating that these events are not uncommon [61]. Cross-species transmission has been reported for kobuviruses in cattle, goats, and pigs, highlighting the complexity of the evolutionary pathways of kobuviruses in animals [51,62]. However, animal-like KoVs have not been identified in humans, thus far.
Based on our data and literature, bovine KoVs are genetically diverse, and this could challenge the attempts to understand their pathogenic role since different KoV strains could possess different virulence phenotypes [23]. Interestingly, the two AiV D strains identified in this study were detected in calves with enteritis. Experimental infections with B1 and D1 KoV types could help assess if all bovine KoVs possess this virulent phenotype. However, further studies are needed to assess the viability of bovine KoVs detected in stools. Likewise, epidemiological investigations using diagnostic tools with type-specific resolution could be helpful to investigate the pathogenetic role, in any, of bovine KoVs.
5. Conclusions
In this study, a high (26.3%) KoV prevalence was found in calves. Sequence analysis revealed a marked genetic diversity and a potential novel KoV type of recombinant origin. Gathering information on the genetic diversity of KoVs will help design more reliable diagnostic tools and improve our knowledge of these enteric viruses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14223315/s1, Table S1: Data, health conditions, and results of the animals sampled, from October 2019 to January 2020.
Author Contributions
Conceptualization, V.M. and J.M.; methodology, G.L. and V.M.; software, F.P., A.P. and B.D.M.; validation, G.D., K.B. and B.D.M.; formal analysis, F.P., A.P. and G.L.; investigation, F.P., F.C., G.D., A.C. and A.S.; data curation, F.P. and V.M.; writing—original draft preparation, F.P.; writing—review and editing, G.L., B.D.M., A.C., M.C., K.B., J.M. and V.M.; visualization F.C., G.L. and A.S.; supervision, G.L. and M.C.; funding acquisition, K.B. and V.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The project was based on the collection of fecal samples, using non-invasive procedures, since 2019, from cattle with and without gastroenteric signs of disease for the research of emerging viral pathogens. The lack of animal testing on liver or sacrificed animals for scientific purposes justified the exemption for ethical approval by the CESA-DiMeV (Prot. n. 4624-III/13).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by EU funding within the Ministero dell’Università e della ricercar (Rome, Italy) Piano Nazionale di Ripresa e Resilienza MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT). This work was also supported by the National Laboratory for Infectious Animal Diseases, Antimicrobial Resistance, Veterinary Public Health and Food Chain Safety, RRF-2.3.1-21-2022-00001. Additional support was obtained from the National Research, Development and Innovation Office, Hungary under grant RRF-2.3.1-21-2022–00010 (National Laboratory of Virology).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Castells M., Colina R. Viral Enteritis in Cattle: To Well Known Viruses and Beyond. Microbiol. Res. 2021;12:663–682. doi: 10.3390/microbiolres12030048. [DOI] [Google Scholar]
- 2.Gomez D.E., Weese J.S. Viral Enteritis in Calves. Can. Vet. J. 2017;58:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Ren Y., Dai G., Li X., Xiang Y., Zhang J., Jiang Y., Jiang S., Hou X., Zhu Z., et al. Genetic Characterization and Clinical Characteristics of Bovine Viral Diarrhea Viruses in Cattle Herds of Heilongjiang Province, China. Iran. J. Vet. Res. 2022;23:69–73. doi: 10.22099/IJVR.2021.38650.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khamrin P., Maneekarn N., Okitsu S., Ushijima H. Epidemiology of Human and Animal Kobuviruses. Virusdisease. 2014;25:195–200. doi: 10.1007/s13337-014-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King A., Brown F., Christian P., Hovi T., Knowles N., Lemon S., Minor P., Palmenberg A., Skern T., Stanway G., et al. Family Picornaviridae. Academic Press; Cambridge, MA, USA: 2000. Seventh Report of the International Committee for the Taxonomy of Viruses. [Google Scholar]
- 6.Reuter G., Boros Á., Pankovics P. Kobuviruses—A Comprehensive Review. Rev. Med. Virol. 2011;21:32–41. doi: 10.1002/rmv.677. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita T., Kobayashi S., Sakac K., Nakata S., Chiba S., Ishihara Y., Isomura S. Isolation of Cytopathic Small Round Viruses with BS-C-l Cells from Patients with Gastroenteritis. J. Infect. Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- 8.Zell R., Delwart E., Gorbalenya A.E., Hovi T., King A.M.Q., Knowles N.J., Lindberg A.M., Pallansch M.A., Palmenberg A.C., Reuter G., et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. General. Virol. 2017;98:2421–2422. doi: 10.1099/jgv.0.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergallo M., Galliano I., Montanari P., Rassu M., Daprà V. Aichivirus in Children with Diarrhea in Northern Italy. Intervirology. 2017;60:196–200. doi: 10.1159/000487051. [DOI] [PubMed] [Google Scholar]
- 10.Charoenkul K., Thaw Y.N., Phyu E.M., Jairak W., Nasamran C., Chamsai E., Chaiyawong S., Amonsin A. First Detection and Genetic Characterization of Canine Bufavirus in Domestic Dogs, Thailand. Sci. Rep. 2024;14:4773. doi: 10.1038/s41598-024-54914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You F.-F., Zhang M.-Y., He H., He W.-Q., Li Y.-Z., Chen Q. Kobuviruses Carried by Rattus Norvegicus in Guangdong, China. BMC Microbiol. 2020;20:94. doi: 10.1186/s12866-020-01767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho Y.-Y., Lim S.-I., Kim Y.K., Song J.-Y., Lee J.-B., An D.-J. Molecular Characterization of the Full Kobuvirus Genome in a Cat. Genome Announc. 2014;2:10–1128. doi: 10.1128/genomeA.00420-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankovics P., Boros Á., Kiss T., Reuter G. Identification and Complete Genome Analysis of Kobuvirus in Faecal Samples of European Roller (Coracias garrulus): For the First Time in a Bird. Arch. Virol. 2015;160:345–351. doi: 10.1007/s00705-014-2228-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita T., Ito M., Kabashima Y., Tsuzuki H., Fujiura A., Sakae K. Isolation and Characterization of a New Species of Kobuvirus Associated with Cattle. J. General. Virol. 2003;84:3069–3077. doi: 10.1099/vir.0.19266-0. [DOI] [PubMed] [Google Scholar]
- 15.Smits S.L., Raj V.S., Oduber M.D., Schapendonk C.M.E., Bodewes R., Provacia L., Stittelaar K.J., Osterhaus A.D.M.E., Haagmans B.L. Metagenomic Analysis of the Ferret Fecal Viral Flora. PLoS ONE. 2013;8:e71595. doi: 10.1371/journal.pone.0071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter G., Boros Á., Pankovics P., Egyed L. Kobuvirus in Domestic Sheep, Hungary. Emerg. Infect. Dis. 2010;16:869–870. doi: 10.3201/eid1605.091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oem J.-K., Lee M.-H., Lee K.-K., An D.-J. Novel Kobuvirus Species Identified from Black Goat with Diarrhea. Vet. Microbiol. 2014;172:563–567. doi: 10.1016/j.vetmic.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Nantel-Fortier N., Lachapelle V., Letellier A., L’Homme Y., Brassard J. Kobuvirus Shedding Dynamics in a Swine Production System and Their Association with Diarrhea. Vet. Microbiol. 2019;235:319–326. doi: 10.1016/j.vetmic.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Otomaru K., Naoi Y., Haga K., Omatsu T., Uto T., Koizumi M., Masuda T., Yamasato H., Takai H., Aoki H., et al. Detection of Novel Kobu-like Viruses in Japanese Black Cattle in Japan. J. Vet. Med. Sci. 2016;78:321–324. doi: 10.1292/jvms.15-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., Yang L., Ren X., He G., Zhang J., Yang J., Qian Z., Dong J., Sun L., Zhu Y., et al. Deciphering the Bat Virome Catalog to Better Understand the Ecological Diversity of Bat Viruses and the Bat Origin of Emerging Infectious Diseases. ISME J. 2016;10:609–620. doi: 10.1038/ismej.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankovics P., Boros Á., Bíró H., Horváth K.B., Phan T.G., Delwart E., Reuter G. Novel Picornavirus in Domestic Rabbits (Oryctolagus cuniculus var. domestica) Infect. Genet. Evol. 2016;37:117–122. doi: 10.1016/j.meegid.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao L., Chen C., Bailey K., Wang L. Bovine Kobuvirus—A Comprehensive Review. Transbound. Emerg. Dis. 2021;68:1886–1894. doi: 10.1111/tbed.13909. [DOI] [PubMed] [Google Scholar]
- 23.Yan N., Yue H., Liu Q., Wang G., Tang C., Liao M. Isolation and Characteristics of a Novel Aichivirus D from Yak. Microbiol. Spectr. 2023;11:e00099-23. doi: 10.1128/spectrum.00099-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abi K., Yu Z., Jing Z.Z., Tang C. Identification of a Novel Aichivirus D in Sheep. Infect. Genet. Evol. 2021;91:104810. doi: 10.1016/j.meegid.2021.104810. [DOI] [PubMed] [Google Scholar]
- 25.Conceição-Neto N., Yinda K.C., Van Ranst M., Matthijnssens J. Human Virome: Methods and Protocols. Humana Press; New York, NY, USA: 2018. NetoVIR: Modular Approach to Customize Sample Preparation Procedures for Viral Metagenomics; pp. 85–95. [DOI] [PubMed] [Google Scholar]
- 26.Reuter G., Egyed L. Bovine Kobuvirus in Europe. Emerg. Infect. Dis. 2009;15:822–823. doi: 10.3201/eid1505.081427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korfhage C., Fricke E., Meier A. Whole-Transcriptome Amplification of Single Cells for Next-Generation Sequencing. Curr. Protoc. Mol. Biol. 2015;111:7–20. doi: 10.1002/0471142727.mb0720s111. [DOI] [PubMed] [Google Scholar]
- 28.Scotto-Lavino E., Du G., Frohman M.A. 5′ End CDNA Amplification Using Classic RACE. Nat. Protoc. 2006;1:2555–2562. doi: 10.1038/nprot.2006.480. [DOI] [PubMed] [Google Scholar]
- 29.Scotto-Lavino E., Du G., Frohman M.A. 3′ End CDNA Amplification Using Classic RACE. Nat. Protoc. 2006;1:2742–2745. doi: 10.1038/nprot.2006.481. [DOI] [PubMed] [Google Scholar]
- 30.Vilsker M., Moosa Y., Nooij S., Fonseca V., Ghysens Y., Dumon K., Pauwels R., Alcantara L.C., Vanden Eynden E., Vandamme A.-M., et al. Genome Detective: An Automated System for Virus Identification from High-Throughput Sequencing Data. Bioinformatics. 2019;35:871–873. doi: 10.1093/bioinformatics/bty695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katoh K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lole K.S., Bollinger R.C., Paranjape R.S., Gadkari D., Kulkarni S.S., Novak N.G., Ingersoll R., Sheppard H.W., Ray S.C. Full-Length Human Immunodeficiency Virus Type 1 Genomes from Subtype C-Infected Seroconverters in India, with Evidence of Intersubtype Recombination. J. Virol. 1999;73:152–160. doi: 10.1128/JVI.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin D.P., Varsani A., Roumagnac P., Botha G., Maslamoney S., Schwab T., Kelz Z., Kumar V., Murrell B. RDP5: A Computer Program for Analyzing Recombination in, and Removing Signals of Recombination from, Nucleotide Sequence Datasets. Virus Evol. 2021;7:veaa087. doi: 10.1093/ve/veaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babin Y. Recan: Python Tool for Analysis of Recombination Events in Viral Genomes. J. Open Source Softw. 2020;5:2014. doi: 10.21105/joss.02014. [DOI] [Google Scholar]
- 36.Kapoor A., Simmonds P., Dubovi E.J., Qaisar N., Henriquez J.A., Medina J., Shields S., Lipkin W.I. Characterization of a Canine Homolog of Human Aichivirus. J. Virol. 2011;85:11520–11525. doi: 10.1128/JVI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuter G., Boldizsár Á., Pankovics P. Complete Nucleotide and Amino Acid Sequences and Genetic Organization of Porcine Kobuvirus, a Member of a New Species in the Genus Kobuvirus, Family Picornaviridae. Arch. Virol. 2009;154:101–108. doi: 10.1007/s00705-008-0288-2. [DOI] [PubMed] [Google Scholar]
- 38.Dall Agnol A.M., Lorenzetti E., Leme R.A., Ladeia W.A., Mainardi R.M., Bernardi A., Headley S.A., Freire R.L., Pereira U.P., Alfieri A.F., et al. Severe Outbreak of Bovine Neonatal Diarrhea in a Dairy Calf Rearing Unit with Multifactorial Etiology. Braz. J. Microbiol. 2021;52:2547–2553. doi: 10.1007/s42770-021-00565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamed F.F., Mansour S.M.G., Orabi A., El-Araby I.E., Ng T.F.F., Mor S.K., Goyal S.M. Detection and Genetic Characterization of Bovine Kobuvirus from Calves in Egypt. Arch. Virol. 2018;163:1439–1447. doi: 10.1007/s00705-018-3758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savard C., Ariel O., Fredrickson R., Wang L., Broes A. Detection and Genome Characterization of Bovine Kobuvirus (BKV) in Faecal Samples from Diarrhoeic Calves in Quebec, Canada. Transbound. Emerg. Dis. 2022;69:1649–1655. doi: 10.1111/tbed.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeoung H.-Y., Lim J.-A., Jeong W., Oem J.-K., An D.-J. Three Clusters of Bovine Kobuvirus Isolated in Korea, 2008–2010. Virus Genes. 2011;42:402–406. doi: 10.1007/s11262-011-0593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Martino B., Di Profio F., Di Felice E., Ceci C., Pistilli M.G., Marsilio F. Molecular Detection of Bovine Kobuviruses in Italy. Arch. Virol. 2012;157:2393–2396. doi: 10.1007/s00705-012-1439-z. [DOI] [PubMed] [Google Scholar]
- 43.Park S.-J., Kim H.-K., Song D.-S., Moon H.-J., Park B.-K. Molecular Detection and Genetic Characterization of Kobuviruses in Fecal Samples Collected from Diarrheic Cattle in Korea. Infect. Genet. Evol. 2011;11:1178–1182. doi: 10.1016/j.meegid.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Wang D., Gao H., Zhao L., Lv C., Dou W., Zhang X., Liu Y., Kang X., Guo K. Detection of the Dominant Pathogens in Diarrheal Calves of Ningxia, China in 2021–2022. Front. Vet. Sci. 2023;10:1155061. doi: 10.3389/fvets.2023.1155061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukashev A.N. Role of Recombination in Evolution of Enteroviruses. Rev. Med. Virol. 2005;15:157–167. doi: 10.1002/rmv.457. [DOI] [PubMed] [Google Scholar]
- 46.Lai M.M. RNA Recombination in Animal and Plant Viruses. Microbiol. Rev. 1992;56:61–79. doi: 10.1128/mr.56.1.61-79.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zell R. Picornaviridae—The Ever-Growing Virus Family. Arch. Virol. 2018;163:299–317. doi: 10.1007/s00705-017-3614-8. [DOI] [PubMed] [Google Scholar]
- 48.Combelas N., Holmblat B., Joffret M.-L., Colbère-Garapin F., Delpeyroux F. Recombination between Poliovirus and Coxsackie A Viruses of Species C: A Model of Viral Genetic Plasticity and Emergence. Viruses. 2011;3:1460–1484. doi: 10.3390/v3081460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McWilliam Leitch E.C., Cabrerizo M., Cardosa J., Harvala H., Ivanova O.E., Koike S., Kroes A.C.M., Lukashev A., Perera D., Roivainen M., et al. The Association of Recombination Events in the Founding and Emergence of Subgenogroup Evolutionary Lineages of Human Enterovirus 71. J. Virol. 2012;86:2676–2685. doi: 10.1128/JVI.06065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyriakopoulou Z., Pliaka V., Amoutzias G.D., Markoulatos P. Recombination among Human Non-Polio Enteroviruses: Implications for Epidemiology and Evolution. Virus Genes. 2015;50:177–188. doi: 10.1007/s11262-014-1152-y. [DOI] [PubMed] [Google Scholar]
- 51.Huang M., Gan J., Xu Z., Guo Y., Chen Z., Gao G.F., Liang H., Liu W.J. A Black Goat-Derived Novel Genotype of Aichi Virus C Blurs the Boundary between Caprine and Porcine Kobuviruses. Virology. 2023;585:215–221. doi: 10.1016/j.virol.2023.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Zhao X., Shi Y., Xia Y. Genome Analysis Revealed Novel Genotypes and Recombination of the Human Parechoviruses Prevalent in Children in Eastern China. Gut Pathog. 2016;8:52. doi: 10.1186/s13099-016-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krumbholz A., Egerer R., Braun H., Schmidtke M., Rimek D., Kroh C., Hennig B., Groth M., Sauerbrei A., Zell R. Analysis of an Echovirus 18 Outbreak in Thuringia, Germany: Insights into the Molecular Epidemiology and Evolution of Several Enterovirus Species B Members. Med. Microbiol. Immunol. 2016;205:471–483. doi: 10.1007/s00430-016-0464-z. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q.-Y., Sun Z.-H., Che Y.-L., Chen R.-J., Wu X.-M., Wu R.-J., Wang L.-B., Zhou L.-J. High Prevalence, Genetic Diversity, and Recombination of Porcine Sapelovirus in Pig Farms in Fujian, Southern China. Viruses. 2023;15:1751. doi: 10.3390/v15081751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukashev A.N., Lashkevich V.A., Ivanova O.E., Koroleva G.A., Hinkkanen A.E., Ilonen J. Recombination in Circulating Human Enterovirus B: Independent Evolution of Structural and Non-Structural Genome Regions. J. General. Virol. 2005;86:3281–3290. doi: 10.1099/vir.0.81264-0. [DOI] [PubMed] [Google Scholar]
- 56.Lin K.-H., Hwang K.-P., Ke G.-M., Wang C.-F., Ke L.-Y., Hsu Y.-T., Tung Y.-C., Chu P.-Y., Chen B.-H., Chen H.-L., et al. Evolution of EV71 Genogroup in Taiwan from 1998 to 2005: An Emerging of Subgenogroup C4 of EV71. J. Med. Virol. 2006;78:254–262. doi: 10.1002/jmv.20534. [DOI] [PubMed] [Google Scholar]
- 57.Huang S.-W., Hsu Y.-W., Smith D.J., Kiang D., Tsai H.-P., Lin K.-H., Wang S.-M., Liu C.-C., Su I.-J., Wang J.-R. Reemergence of Enterovirus 71 in 2008 in Taiwan: Dynamics of Genetic and Antigenic Evolution from 1998 to 2008. J. Clin. Microbiol. 2009;47:3653–3662. doi: 10.1128/JCM.00630-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han X., Zhang W., Xue Y., Shao S. Sequence Analysis Reveals Mosaic Genome of Aichi Virus. Virol. J. 2011;8:390. doi: 10.1186/1743-422X-8-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivadulla E., Romalde J.L. Correction to: A Comprehensive Review on Human Aichi Virus. Virol. Sin. 2021;36:342. doi: 10.1007/s12250-020-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang H.-W., Chu P.-H., Pan C.-H., Wang C.-F., Lin C.-C., Lu P.-L., Chen Y.-S., Shi Y.-Y., Su H.-J., Chou L.-C., et al. Evolutionary Histories of Coxsackievirus B5 and Swine Vesicular Disease Virus Reconstructed by Phylodynamic and Sequence Variation Analyses. Sci. Rep. 2018;8:8821. doi: 10.1038/s41598-018-27254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lomakina N.F., Yu Shustova E., Strizhakova O.M., Felix Drexler J., Lukashev A.N. Epizootic of Vesicular Disease in Pigs Caused by Coxsackievirus B4 in the Soviet Union in 1975. J. Gen. Virol. 2016;97:49–52. doi: 10.1099/jgv.0.000318. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y., Li J., Guo J., Pan Y., Tong X., Liu C., Wang D., Xu W., Shi Y., Ji Y., et al. Evolutionary Origin, Genetic Recombination, and Phylogeography of Porcine Kobuvirus. Viruses. 2023;15:240. doi: 10.3390/v15010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.