Abstract
Patient: Female, 35-year-old
Final Diagnosis: HELLP syndrome
Symptoms: Abdominal pain
Clinical Procedure: Cesarean section
Specialty: Critical Care Medicine • Obstetrics and Gynecology
Objective:
Rare coexistence of disease or pathology
Background:
Spontaneous hepatic hematoma and liver capsule rupture is a rare but severe complication of Hemolysis, Elevated Liver Enzyme, and Low Platelet (HELLP) syndrome, with a high mortality rate. We report a case of a pregnant woman with HELLP syndrome and liver subcapsular hematoma rupture that was diagnosed during surgery.
Case Report:
A 35-year-old woman with 34+1 weeks of pregnancy came to the emergency department due to abdominal pain for 4 days. She was diagnosed with HELLP syndrome after a blood test. She was transferred to the obstetrics department, and an emergency cesarean section was performed under general anesthesia, due to fetal distress. During the surgery, non-clotting blood was found flowing out during the suturing of the incision. We suspended the surgery and organized an emergency multidisciplinary consultation. Subcapsular liver hematoma was diagnosed after intraoperative ultrasound detection. Emergency upper abdominal laparotomy was performed, and a ruptured liver capsule and active bleeding were found. The liver capsule was sutured and blood products were infused before the patient was sent to the intensive care unit. She recovered and was discharged 12 days after surgery. No special discomfort was reported during the 30-day follow-up after surgery.
Conclusions:
Our case emphasizes that all parturients with abdominal pain and HELLP syndrome must be screened for spontaneous hepatic hematoma, and clinicians should pay attention to whether there is rupture of the liver capsule. Multidisciplinary consultations can increase the probability of successful rescue for such patients.
Key words: Cesarean Section, HELLP Syndrome, Liver, Pregnancy
Introduction
Hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome happens in about 1% of all pregnancies, but the rate is 10–20% in women with preeclampsia [1]. Among women with HELLP syndrome, spontaneous hepatic hematoma formation has been reported in 39% of the patients, and 0.5–2% of these progress to capsular rupture, which may cause maternal and fetal mortality in 17% and 38% of these cases, respectively [2]. Diagnosis of spontaneous hepatic hematoma and liver capsule rupture in this condition can be difficult because symptoms are usually nonspecific. Therefore, for pregnant women with HELLP syndrome, any suspected symptoms or signs of liver rupture should be given sufficient attention.
We report a case of a 35-year-old woman with HELLP syndrome who was suspected and finally diagnosed with spontaneous hepatic rupture during an emergency cesarean delivery. Multidisciplinary consultation and treatment led to a better clinical outcome in this case.
Case Report
A 35-year-old woman, G5P1A3, 34+1 weeks of pregnancy, was admitted to the emergency department due to upper abdominal pain that lasted for 4 days and worsened over the course of 6 hours. The parturient did not report elevated blood pressure during pregnancy, and no other symptoms, such as nausea and vomiting, had been experienced since the onset of the disease. On admission, her body temperature was 36.8°C, heart rate was 80 beats per minute, and blood pressure was 135/84 mmHg. The physical examination showed upper abdomen tenderness. The fetal heart rate was 150 beats per minute, and occasionally uterine contractions could be palpable.
Blood examination was conducted, and the results were as follows: platelets 56×109/L, red blood cells (RBC) 4.37×1012/L, hemoglobin 133 g/L, alanine aminotransferase (ALT) 90.43 U/L, aspartate aminotransferase (AST) 82.76 U/L, total bilirubin (TB) 22.42 µmol/L, direct bilirubin (DB) 6.05 µmol/L, amylase 38.14 U/L, lactate dehydrogenase (LDH) 315.46 U/L, D-dimer 6.63 mg/L. Emergency abdominal ultrasound did not detect any abnormal liver or gallbladder signs (Figure 1). Considering the diagnosis of HELLP syndrome and the occurrence of uterine contractions, the parturient was transferred to the obstetrics department, and immediate fetal heart rate monitoring and parturient electrocardiogram monitoring were performed. Shortly after admission to the obstetrics department, the Doppler monitor revealed slow fetal heart rate, and frequent fetal movement was observed. Due to the presence of HELLP syndrome in the parturient and fetal distress, the parturient was immediately sent to the operating room for cesarean section after obtaining informed consent.
Figure 1.
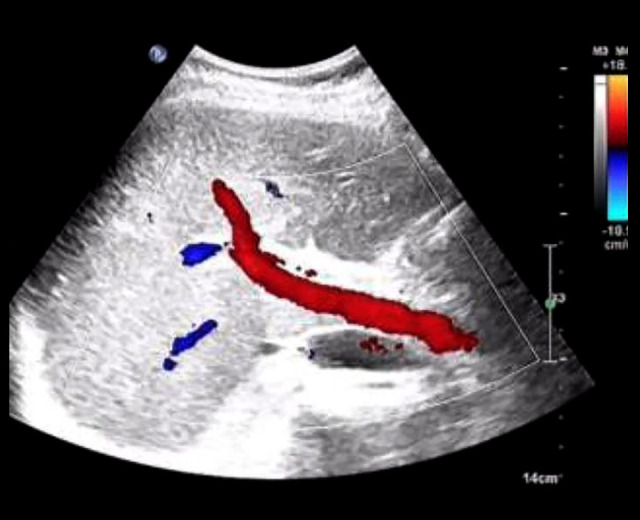
Liver ultrasound of the parturient in the emergency department. After the patient was admitted to the emergency department, we conducted an abdominal ultrasound examination. The results showed that the size and shape of the liver were normal, the liver surface was smooth, and the liver margin was sharp, which indicated that there was no subcapsular liver hematoma upon admission.
After entering the operating room, the parturient immediately underwent electrocardiogram monitoring, which revealed a blood pressure of 95/56 mmHg and a heart rate of 102 beats per minute. The parturient’s consciousness was slightly indifferent with no other discomforts. At the same time as deciding to perform the surgery, we immediately notified the transfusion department to prepare red blood cells, platelets, cryoprecipitate, and plasma, and the neonatologist arrived to prepare for the rescue of the newborn before the parturient was moved to the operating room. General anesthesia had been prepared and conducted. After the parturient’s consciousness disappeared, the cesarean section surgery was immediately initiated, and tracheal intubation was completed before breaking the amniotic sac. The fetus was successfully delivered 2 minutes after anesthesia with a postnatal weight of 1620 g. The fetus had no spontaneous breathing or crying, and tracheal intubation was performed immediately, alongside other emergency treatment. At 4 minutes, the fetus’ Apgar score was 4 points (1 point for heart rate, skin color, reaction, and muscle tone). After continuing with auxiliary ventilation and other rescue measures, the Apgar score reached 10 points at 10 minutes. The neonate was admitted to neonatology for further treatment.
Shortly after anesthesia induction, blood products were delivered to the operating room, and 2 units of platelets and 10 units of cryoprecipitate were immediately infused, alongside Ringer’s solution. The vital signs of the parturient were stable with no obvious abnormalities in the blood gas analysis after the delivery of the fetus. However, during cesarean section incision closure, the obstetrician found a significant amount of non-clotting blood flowing out of the incision. Considering the parturient’s preoperative upper abdominal pain and the presence of HELLP syndrome, there was a high suspicion of liver capsule rupture. Subsequently, the suturing was suspended, a multidisciplinary consultation process was immediately initiated, and physicians from ultrasound, critical care medicine, gastrointestinal surgery, hepatobiliary surgery, and hematology presented within a few minutes. The ultrasound physician immediately performed intraoperative ultrasound examination upon arrival and found hypoechoic sign around the liver, which was suspected to represent blood clots. The hematology physicians assisted with blood transfusion and coagulation treatment. The hepatobiliary and gastrointestinal surgeons assisted in determining the bleeding location. Then, the critical care physicians determined that the patient should be transferred to the ICU after surgery. An intravenous infusion of 1.0 g tranexamic acid was administered when the liver hematoma was detected by ultrasound. After obtaining informed consent from the parturient’s family, the hepatobiliary surgeon performed an upper abdominal incision. A diffuse, 12 cm-diameter subcapsular hematoma on the right liver was found, and the liver capsule on the hematoma had ruptured, with active bleeding. After suturing the ruptured hepatic capsule, the hepatobiliary surgeon compressed the hematoma with gauze until no active bleeding was observed. Subsequently, the capsule incision was covered with absorbable hemostatic gauze and compressed with a gelatin sponge. Considering the possibility of further bleeding after surgery, an abdominal drainage tube was placed. Ten units of cryoprecipitate were infused while suturing the liver capsule, and 1.5 units of red blood cells were used during the compression process. Besides the blood products, almost 3000 ml of Ringer’s solution was infused, and the urine output was approximately 500 ml during the surgery. After all the incisions in the upper abdomen and cesarean section were sutured, we applied pressure to the patient’s upper abdomen using an abdominal belt.
Postoperatively, the patient was transferred to the ICU for further monitoring and treatment. On the first day after surgery, a bedside ultrasound exam revealed hypoechoic material surrounding the liver and a small amount of fluid in the upper right abdominal cavity. One day after surgery, blood examination showed the following values: ALT 321.4 U/L, AST 335.0 U/L, TB 16.2 µmol/L, DB 9.1 µmol/L, HG 68.0 g/L, RBC 2.22×1012/L, PT 12.5 s, APTT 27.2 s, fibrinogen 4.06 g/L, and the volume of fluid drained from the abdominal drainage tube was 190 ml. In the ICU, the parturient received treatment with antibiotics, fibrinogen, tranexamic acid, infusion of red blood cells, fresh frozen plasma, and cryoprecipitate, as well as intravenous nutrition supplementation. On postoperative day 1, 26 hours after surgery, the tracheal intubation was removed, and the patient was transferred back to the obstetrics department. Subsequently, the patient’s liver function gradually improved. On the 7th day after surgery, blood laboratory values were as follows: ALT 118.4 U/L, AST 25.1 U/L, LDH 204.5 U/L, hemoglobin 93.0 g/L, RBC 3.19×1012/L. Computed tomography scan revealed subcapsular hematoma and gas accumulation (Figure 2). The volume of abdominal drainage fluid gradually decreased. Then, 11 days after surgery, there was no significant drainage fluid, and the tube was removed. The patient was discharged 12 days after surgery. Thirty days after surgery, the parturient did not report any special discomfort. Three months after surgery, a follow-up abdominal ultrasound revealed that the hematoma had been almost completely absorbed (Figure 3).
Figure 2.
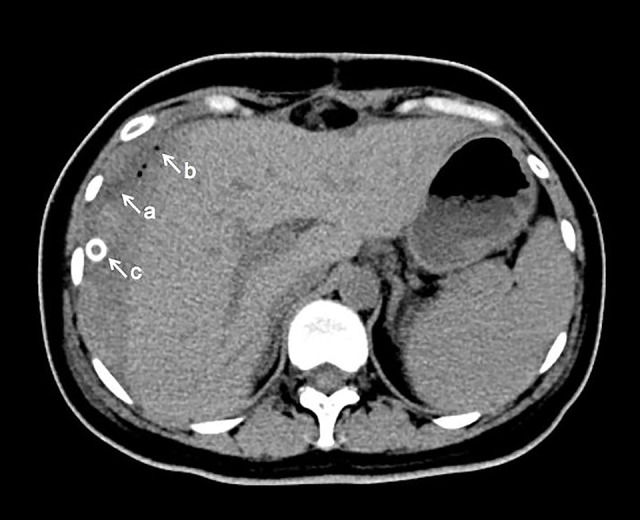
Computed tomography (CT) scan of the parturient on postpartum day 7. CT scan of the upper abdomen was performed 7 days after surgery, indicating the subcapsular hematoma on the surface of the right lobe of the liver. a: Subcapsular hematoma of the liver; b: Abdominal gas accumulation; c: Abdominal drainage tube.
Figure 3.
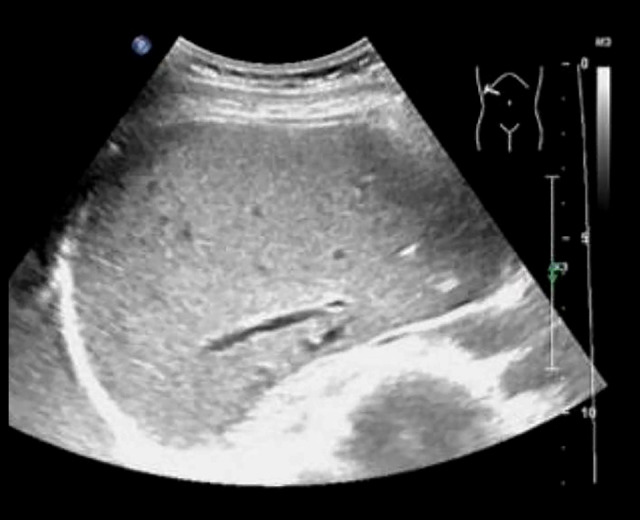
Liver ultrasound of the patient, performed 3 months after surgery. Three months after surgery, the patient came to our outpatient department for re-examination by abdominal ultrasound. The results indicated that the liver surface was smooth, the liver margin was sharp, and no obvious lesions were found where the hematoma had been detected by computed tomography scan during hospitalization, suggesting that the patient’s subcapsular liver hematoma and gas accumulation had been completely absorbed.
Discussion
HELLP syndrome is a rare but life-threatening pregnancy complication. Many investigators consider HELLP syndrome as a form of preeclampsia, and guidelines about HELLP syndrome are almost always included in the category of hypertensive disorders of pregnancy (HDP); however, the relationship between the 2 diseases is still controversial. In fact, 30% of cases defined as HELLP syndrome have no HDP, and some of the women with HELLP syndrome do not exhibit typical preeclampsia [3]. Multipara, older age (over 35 years old), obesity, HELLP syndrome history, and severe preeclampsia are high risk factors for HELLP syndrome. The pathogenesis of HELLP syndrome is still unclear, but immunological dysfunction, vasospasm, endothelial injury, or vascular fibrin deposition might be involved in the disease progression [4]. Symptoms of HELLP syndrome can be atypical, which make it difficult to be discovered and diagnosed. Most common symptoms are right upper quadrant or epigastric pain and headache, followed by nausea and vomiting, visual changes, malaise, and swollen feet and ankles [5,6].
As reported in our case, hepatic hematoma and hepatic capsular rupture are rare but severe complications of HELLP syndrome. Moreover, symptoms might be uncharacteristic, including abdominal pain, epigastric pain, anemia, right shoulder pain, and shock in severe cases. Considering this, a HELLP syndrome parturient with one or more symptoms should always be suspected to confirm potential liver hematoma [1], especially in women with markedly reduced platelet counts (≥20×109/L) [2], and imaging examination should be performed. In our case, the parturient’s abdominal ultrasound did not reveal any subcapsular hematoma upon admission to the emergency department. However, during the subsequent hospitalization and preoperative preparation process, we did not re-examine the abdominal ultrasound. When the parturient entered the operating room, her blood pressure had already dropped, but we overlooked this point. In fact, at this time, the parturient may have already developed a subcapsular hematoma and active bleeding. As was reported, any increase in abdominal pressure, such as vomiting, constipation, impact, compression, etc., may lead to the formation and rupture of subcapsular hematoma [7]. Therefore, based on the experience of this case, we believe that for such parturients, bedside ultrasound examination equipment should be present in the emergency room. When there is suspicion that the parturient may have a subcapsular liver hematoma, rapid ultrasound examination should be performed immediately to confirm the diagnosis. In our opinion, liver ultrasound examination is relatively easy to perform, and professional clinical doctors can master the preliminary screening methods for liver abnormalities after training. Indeed, we also recommend that doctors involved in the treatment of such parturients, whether they are obstetricians, emergency physicians, anesthesiologists, or critical care physicians, should receive relevant training and learn this skill. If necessary, computed tomography or magnetic resonance imaging, which have higher sensitivity for detecting liver rupture and evaluating the severity of hematoma, should be used [8].
For stable patients, conservative non-surgical methods can be considered, including supportive care and adequate infusion of fluids and blood products [9]. Surgical treatment is necessary when there is progressive aggravation of the hematoma, hemodynamics are unstable, or liver rupture has occurred. The surgical methods include packing hemostasis, compression hemostasis, and suturing of the liver capsule and parenchyma. Blocking the blood supply to the bleeding area is also a feasible method, such as surgical ligation of the blood supply artery [10]. Embolization of the bleeding hepatic arteries could be an alternative therapy, and has the advantages of being more effective and less invasive, but attention should be paid to postoperative ischemic complications such as liver injury or abnormal liver function [11]. In severe cases, when hepatic necrosis and irreversible deterioration of liver function happens, liver transplant should be considered, although its benefits are controversial [12].
Conclusions
Although rare, hepatic hematoma and hepatic capsular rupture in women with HELLP syndrome require sufficient attention. Imaging examinations can assist in diagnosis, especially for women with symptoms or signs. When hepatic capsular rupture or hemodynamic instability occurs, decisive surgical treatment is necessary. In selected cases, liver transplantation might also benefit patients.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Department of emergency, obstetrics and intensive care medicine, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, Shandong, PR China.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Ditisheim A, Sibai BM. Diagnosis and management of HELLP syndrome complicated by liver hematoma. Clin Obstet Gynecol. 2017;60(1):190–97. doi: 10.1097/GRF.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Clinical practice guidelines on the management of liver diseases in pregnancy. J Hepatol. 2023;79(3):768–828. doi: 10.1016/j.jhep.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Dusse LM, Alpoim PN, Silva JT, et al. Revisiting HELLP syndrome. Clin Chim Acta. 2015;451(Pt B):117–20. doi: 10.1016/j.cca.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Stojanovska V, Zenclussen AC. Innate and adaptive immune responses in HELLP syndrome. Front Immunol. 2020;11:667. doi: 10.3389/fimmu.2020.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64(4):933–45. doi: 10.1016/j.jhep.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Kappler S, Ronan-Bentle S, Graham A. Thrombotic microangiopathies (TTP, HUS, HELLP) Hematol Oncol Clin North Am. 2017;31(6):1081–103. doi: 10.1016/j.hoc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Chan AD, Gerscovich EO. Imaging of subcapsular hepatic and renal hematomas in pregnancy complicated by preeclampsia and the HELLP syndrome. J Clin Ultrasound. 1999;27(1):35–40. doi: 10.1002/(sici)1097-0096(199901)27:1<35::aid-jcu6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Pavlis T, Aloizos S, Aravosita P, et al. Diagnosis and surgical management of spontaneous hepatic rupture associated with HELLP syndrome. J Surg Educ. 2009;66(3):163–67. doi: 10.1016/j.jsurg.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Chou PY, Yu CH, Chen CC, Chen WT. Spontaneously ruptured subcapsular liver hematoma associated with hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome. Taiwan J Obstet Gynecol. 2010;49(2):214–17. doi: 10.1016/S1028-4559(10)60046-0. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekaran S, Simon R. Hepatic complications in preeclampsia. Clin Obstet Gynecol. 2020;63(1):165–74. doi: 10.1097/GRF.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 11.Augustin G, Hadzic M, Juras J, Oreskovic S. Hypertensive disorders in pregnancy complicated by liver rupture or hematoma: A systematic review of 391 reported cases. World J Emerg Surg. 2022;17(1):40. doi: 10.1186/s13017-022-00444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian Y, Dong Y, Mei S, et al. Liver transplantation for hemolysis, elevated liver enzymes, and low platelet count syndrome: A propensity-score matched long-term survival of the scientific registry of transplant recipients dataset. Transplant Proc. 2019;51(3):805–12. doi: 10.1016/j.transproceed.2018.10.032. [DOI] [PubMed] [Google Scholar]


