Abstract
Patient: Male, 78-year-old
Final Diagnosis: Elastofibroma dorsi
Symptoms: Asymptomatic • incidental finding
Clinical Procedure: —
Specialty: Surgery
Objective:
Rare disease
Background:
Elastofibroma dorsi is a rare, benign soft-tissue tumor, emerging in the subscapular area and exhibiting higher prevalence in elderly women. Despite its slow growth rate and asymptomatic nature in most patients, elastofibroma can cause swelling, pain, and discomfort during shoulder movements. Imaging and histopathologic data combined with a detailed history are essential to exclude malignancies and provide suitable treatment.
Case Report:
This report describes the case of a 78-year-old man with an incidental finding of elastofibroma dorsi, presenting as an asymptomatic left subscapular mass. Physical examination revealed the mass, the presence of which was later confirmed through an MRI scan. The tumor was surgically excised without any postoperative complications. Histopathologic findings from a biopsy supported the diagnosis of elastofibroma dorsi, showing an abundance of thick and irregular elastic fibers, giving a “rope-like” appearance in hematoxylin and eosin stain. Additionally, Verhoeff-Van Gieson stain highlighted the elastic fibers, making their characteristic arrangement and appearance evident. The patient was then discharged from our hospital and made a complete recovery.
Conclusions:
Despite its benign nature and rarity, elastofibroma dorsi should be included in the differential diagnosis of subscapular masses. Proper imaging and histopathological examination are crucial for a definitive diagnosis, to ensure that patients receive the appropriate and necessary treatment and guidance. Furthermore, additional research is needed to completely clarify the pathophysiologic mechanism responsible for the development of elastofibroma dorsi.
Key words: Back Pain, Case Reports, Rare Diseases, Scapula, Shoulder Pain, Soft Tissue Neoplasms
Introduction
Described for the first time in 1961 by Jarvi and Saxen, elastofibroma dorsi is an uncommon, ill-defined, and benign soft-tissue tumor with a slow growth rate [1–5]. It has an estimated prevalence of approximately 2%, predominately affecting elderly patients, especially women between the ages of 50 and 70 years [3–6]. The tumor typically develops in a very characteristic anatomical region, the subscapular area, located deep and adjacent to the serratus anterior, latissimus dorsi, and scapula elevator muscles [2,3,7]. While elastofibroma dorsi is usually asymptomatic, the most common symptoms include swelling, pain during movements of the shoulder, and snapping of the scapula [4,7]. Diagnosis is mainly made by imaging techniques such as CT scans and MRI, with the most important assessment being MRI [3,4,6,7].
Despite its non-malignant nature, treatment with surgical excision is often required to address compromised mobility and pain caused by the development of the mass [7–9].
In this paper, we document our experience in the diagnosis and treatment of elastofibroma dorsi, through a case of a 78-year-old male patient with an incidental finding of elastofibroma dorsi, presenting as an asymptomatic mass in the left subscapular area. Subsequently, we discuss the clinical, histopathologic, and radiologic characteristics of the tumor and review the corresponding scientific literature, to emphasize the importance of considering this benign tumor in the differential diagnosis of subscapular masses, especially in elderly patients.
Case Report
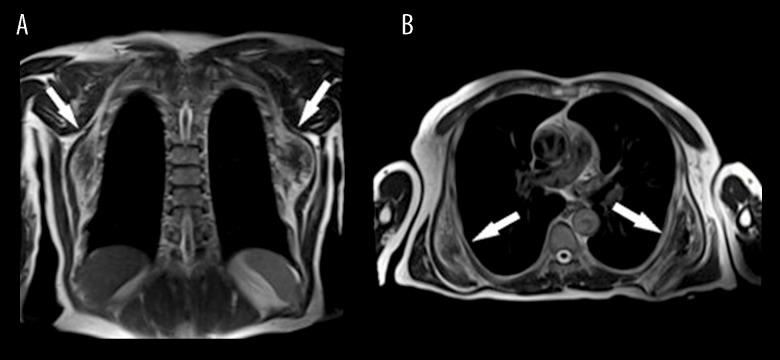
A 78-year-old male patient was admitted to the surgical department of our hospital, for the treatment of a mass in the left scapular area. The patient had no prior symptoms and discovered the mass coincidentally during his daily activities. Physical examination revealed the mass, the presence of which was later confirmed through an MRI scan. According to the radiologic findings, the tumor measured approximately 11×10×4.5 cm. The oval mass was indistinguishable from the surrounding muscles. Areas of higher signal intensity in T1 sequences, attributed to the presence of fatty tissue, were interspersed with fibrous tissue which emits low signal in T1, thus creating a zone of heterogenous signal. Radiologists also reported the presence of a right subscapular mass with similar imaging findings, which was not adjacent to the subcutaneous and therefore was not identifiable by clinical examination (Figure 1A, 1B). The excision of the left subscapular growth was scheduled after a surgical consultation.
Figure 1.
MRI image of elastofibroma dorsi; (A) coronal plane, (B) transverse plane. The images reveal bilateral soft-tissue lesions (white arrows).
Regarding his medical history, the patient had diabetes mellitus, arrythmias, and benign prostate hyperplasia, and was taking the following medications: metformin 850 mg/day, rabeprazole 200 mg/day, furosemide 40 mg/day, dutasteride/tamsulosin hydrochloride 0.5 mg/0.4 mg/day and bisoprolol 10 mg/day. Furthermore, he had undergone endoscopic removal of gastric polyps and surgical treatment of a gastric ulcer in recent years. Laboratory tests prior to the surgical procedure were within normal ranges.
Under general anesthesia, the patient underwent surgery, during which the mass was removed via an incision above the left subscapular area, without any intraoperative complications. The surgical specimens, 2 greyish-brown lesions with fibrous and elastic composition, were sent for histopathologic examination. The first, weighing 90 grams, measured 9.5×5.2×2 cm, while the second, weighing 110 grams, measured 10.5×6.5×5.5 cm. Macroscopic examination of the cut surface revealed multiple white-grey fibrous diaphragms, interspersed with fatty tissue.
Under microscopic evaluation and in multiple dissections, both lesions were comprised of fibroelastic tissue, with a vast number of randomly arranged collagenated elastic fibers. The fibers were thick, coarse, and irregular, giving a “rope-like” appearance in hematoxylin and eosin (H&E) stain (Figure 2). Among these fibers, atractoid, oval, or elongated cells, fatty tissue cells, and numerous small vessels could be observed. Myxoid degeneration of the extracellular matrix was detected in some areas; however, signs of cell atypia and malignant potential were absent. Fibroblasts were dispersed within the neoplasm, arranged in short fascicles or a haphazard pattern, and did not exhibit significant atypia or pleomorphism, while mitotic figures were rare. Verhoeff-Van Gieson stain was particularly useful in highlighting these elastic fibers, making their presence and arrangement more apparent (Figure 3). The collagen component was dense but less abundant than the elastic fibers. The tumor was non-encapsulated, and some degree of infiltration was observed into the adjacent tissue.
Figure 2.
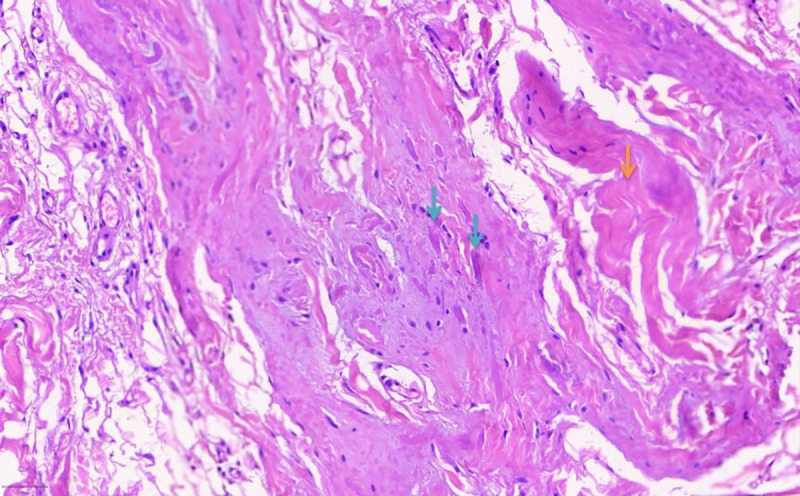
Abundant elastic fibers (indicated by blue arrows) are typically thick, coarse, and irregular, giving a “rope-like” appearance in hematoxylin and eosin (H&E) stain. The collagen fibers are indicated with the orange arrows. The fibroblasts dispersed within the neoplasm do not exhibit significant atypia or pleomorphism and are arranged in short fascicles or a haphazard pattern. Mitotic figures are rare.
Figure 3.
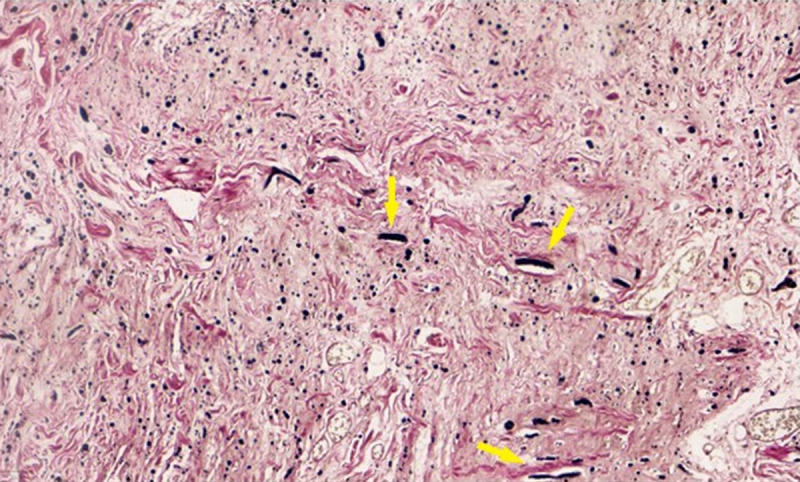
Elastic fibers highlighted with Verhoeff-Van Gieson stain, making their presence and arrangement more apparent (elastic fibers, indicated by yellow arrows, stain black with Verhoeff-Van Gieson). The collagen component tends to be dense but less abundant than the elastic fibers.
Histopathologic findings from the tumor were compatible with the diagnosis of elastofibroma dorsi. Surgical removal was successful, with negative macroscopic and microscopic margins (R0). The patient recuperated at a fast pace, as anticipated, and was discharged the next day, after receiving instructions for the care of his surgical wound and for returning for reassessment.
Discussion
The presented case report of a 78-year-old man with a subscapular mass that proved to be an elastofibroma dorsi shares numerous similarities with other case reports, indicating the necessity of considering this type of soft-tissue tumor in the differential diagnosis of subscapular masses, particularly in elderly patients. Neagoe et al (2021) highlighted the complicated process and diagnostic challenges, especially in asymptomatic patients, which correspond to our case [10]. Additionally, the case report emphasizes the importance of imaging, as noted by Goyal et al (2017), and the significance of histopathological examination [11]. Through characteristic patterns, clinicians can be guided towards a safe diagnosis and appropriate management plan, distinguishing elastofibroma dorsi from other soft-tissue tumors and masses.
Elastofibroma is a rare type of soft-tissue tumor, reported for the first time in 1961 by Jarvi and Saxen [1]. It typically appears in elderly patients as a painless dorsal mass, with a higher incidence in middle-aged to older women, especially between the ages of 50 and 70 years old [4,7,11,12]. Accounting for approximately 2% of thoracic wall tumors, elastofibromas in most cases develop in the subscapular anatomical region between the 6th and 8th rib, hence the name elastofibroma dorsi [3,5,13]. They are located deep and adjacent to the serratus anterior, latissimus dorsi, and scapula elevator muscles, with connections to ligament, muscle, and bone tissue [3,6]. Even though it is considered a rare tumor, studies using CT imaging with patients without mobility disorder, lateral or posterior thoracic wall pain, or the presence of a palpable mass, showed the presence of elastofibromas in 2% of adults over the age of 60 [13]. Autopsy series have indicated a higher prevalence, with rates of 24% in women and 11% in men [7,11,14].
Most incidents are asymptomatic. However, symptomatic patients typically present with pain in the region of the mass which affects the mobility of an upper limb, especially the abduction of the scapula [4,7]. Bilateral lesions can appear in approximately 10–66% of patients [3,6,15,16]. In symptomatic cases, patients complain of mild posterior and lateral thoracic pain with swelling, low-degree discomfort, or a clucking sound during motion and impairment of shoulder and upper-limb movement, especially during abduction [17]. No studies have ever reported malignant transformation or metastatic potential, reinforcing the benign profile of the tumor. Recognition and differential diagnosis of the tumor is essential to implement the correct and adequate treatment, avoiding costly errors for the patients. In the process of differential diagnosis, both benign and malignant tumors should be taken into consideration, such as fibroma, fibrolipoma, aggressive fibromatosis, sarcoma, and subcutaneous metastatic tumors [18,19].
The exact pathophysiologic mechanism responsible for the development of this tumor has not yet been clarified. Most researchers suggest mechanical trauma correlated with movements of the scapula, especially abduction of the upper limb, leading to reactive increased multiplication of fibroblastic tissue cells [4,5,11,20]. Other studies have suggested immunological and genetic factors. In a study in Okinawa in Japan by Nagamine et al, out of 170 cases presented, 55 arose from a single family line (32.3%), indicating a possible involvement of inheritable factors [21]. Various studies indicate that elastofibromas generally appear with higher prevalence in patients involved in manual labor that confers strain on the scapular anatomical region [6,11,22].
Regarding histopathological examination of these tumors, observation of the cut surface of elastofibromas reveals bands of white fibers interwoven with yellow adipose tissue [4,19,22]. Microscopically, eosinophilic, elongated, branched, and unbranched elastic fibers are scattered in a collagenous matrix mixed with fatty cells [19,22,23]. Verhoeff-Van Gieson stain can highlight the fibers, which are organized in globules [19,22]. Signs of cell atypia and increased mitotic activity are not detected [22]. According to the research by Di Vito et al, immunohistological analysis in recent years has revealed the involvement of CD34-positive cells in the perivascular transformation of endothelial and mesenchymal cells, instigating the proliferation of fibroblastic tissue [23]. CD34-positive spindle cells and tryptase-positive mast cells are scattered in a concentrated mix of fibers in an unstructured matrix consisting of proteoglycans across the lesion, more distinctively around peripheral vessels [23]. Moreover, it is highlighted that periostin and tenascin-c in conjunction with elastin and collagen have a detrimental role in the fibrotic procedure [24].
Cytogenetic studies and karyotype analysis have indicated the presence of chromosomal instability with numerous aberrations, the most recurrent affecting chromosome 1; particularly the short arm (1p) [25]. Nishio et al (2002) reported changes in the number of copies of specific chromosomal regions in 33% of patients, with the most common being an acquisition of Xq12-q22 [26]. In 2 cases of elastofibromas, a non-random inactivation of the X-linked androgen receptor gene was observed by Hisaoka and Hashimoto in 2006 [27]. A more contemporary paper by Hernandez et al in 2010 detected deletions in CASR(3q21), GSTP1(11q13), and BRCA2(13q12); and gains in APC(5q21) and PAH(12q23), by implementing multiplex ligation-dependent probe amplification of DNA samples from both patients in the study [4,28]. The presented evidence points to a neoplastic origin and reinforces the classification of elastofibroma dorsi as a tumor. Nevertheless, the common phenomenon of bilateral occurrence attests that there is not a malignant origin [20,26]. Further study is required to shed light on the exact genetic and chromosomal anomalies that lead to the tumor formation.
Precise diagnosis, especially in asymptomatic patients, requires familiarity with the fundamental and crucial imaging findings. X-ray imaging of the chest area can reveal a soft-tissue lesion, usually without calcification [4,20]. Utilizing ultrasounds, elastofibromas can be categorized into 4 groups based on the imaging patterns created [29]. The most common is type 1, which exhibits a nonhomogeneous clustered and fasciculated image [29]. In MRI scans, elastofibromas form an oval mass with indistinct and unclear margins in all planes; displayed by an area of heterogenous signal [4,20,29,30]. Fibrous tissue emits low-intensity signal in T1W and T2W sequences, while interwoven fatty tissue produces higher signal in T1W and intermediate signal in T2W, thus creating the characteristic banded pattern [4,20,29,30]. Elastofibromas appear in CT with an attenuation like skeletal muscle fiber, combined with areas of fatty tissue, thus creating an image of multiple layers [4,20,29]. In patients undergoing treatment for malignancies, the utilization of positron emission tomography-computed tomography (PET-CT) with fluorodeoxyglucose (18-FDG) contrast revealed an elastofibroma frequency of 1.66%, with a higher incidence in women (75.86%) than in men (24.14%) [31]. Mild and moderate absorption of 18-FDG is a characteristic discovery that can assist in diagnosis, particularly in the instance of incidentalomas [31,32].
Diagnosis is usually based on imaging and histopathologic findings due to the ambiguous clinical and macroscopic characteristics. Although the performance of a fine needle biopsy or a core biopsy was a necessary procedure in the past, the most recent approach does not consider these methods as standard and is instead based on imaging techniques [4,10,20]. Nishio et al (2021) stated that in symptomatic cases, radiologic findings alone can lead to a safe and confident diagnosis [4]. MRI scans offer a positive predictive value of 93.3% and a sensitivity of 100% [33]. However, if the typical MRI findings are absent or the tumor exhibits an accelerated growth rate in a time span of months, a biopsy can be useful and essential [4]. Some researchers suggest that ultrasound can also be utilized as a first-line imaging technique [29,34]. The decision for surgical removal should be made cooperatively by both surgeon and patient, and should be reserved for symptomatic cases, those in which malignancy cannot be ruled out, and masses with a diameter over 5 cm and with suspicious imaging findings [8,35]. Resection with negative macroscopic and microscopic margins (R0) is performed through a subscapular incision of the skin and splitting of the dorsal muscles covering the lesion [9]. Elastofibromas can adhere firmly to the muscle or thoracic wall, increasing the complexity of the operation [9]. Postoperative complications include seromas, hematomas, infections of the wound, and textiloma [3,8,9,34]. Difficulty in mobility in the surgical area, in conjunction with the capacious dead space from the removal, results in a high incidence of seromas, ranging between 35.9% to 87.5%, as shown in the research by Muramatsu et al [3]. The possibility of complications is correlated with the size of the tumor [35].
Conclusions
Despite its rarity and benign nature, elastofibroma dorsi should be considered in the differential diagnosis of subscapular masses, especially in elderly women. The lesion’s distinctive location, combined with findings from proper imaging and histopathologic examination, can lead to a safe and secure diagnosis, ensuring that patients receive appropriate treatment and guidance. However, further research is needed to completely understand the pathophysiologic mechanism behind the development of elastofibroma.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Department of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Ethics statement
The study was approved by the Institutional Review Board of AHEPA University Hospital, Thessaloniki (protocol code 16162, date of approval 16 April 2024).
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Jarvi O, Saxen E. Elastofibroma dorse. Acta Pathol Microbiol Scand Suppl. 1961;51(Suppl. 144):83–84. [PubMed] [Google Scholar]
- 2.Muratori F, Esposito M, Rosa F, et al. Elastofibroma dorsi: 8 case reports and a literature review. J Orthop Traumatol. 2008;9(1):33–37. doi: 10.1007/s10195-008-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muramatsu K, Ihara K, Hashimoto T, et al. Elastofibroma dorsi: Diagnosis and treatment. J Shoulder Elbow Surg. 2007;16(5):591–95. doi: 10.1016/j.jse.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Nishio J, Nakayama S, Nabeshima K, Yamamoto T. Current update on the diagnosis, management and pathogenesis of elastofibroma dorsi. Anticancer Res. 2021;41(5):2211–15. doi: 10.21873/anticanres.14997. [DOI] [PubMed] [Google Scholar]
- 5.De Weerdt G, Verhoeven V, Vrints I, et al. Elastofibroma dorsi: A case report of bilateral occurrence and review of literature. Acta Chirurgica Belgica. 2019;121(2):122–26. doi: 10.1080/00015458.2019.1642595. [DOI] [PubMed] [Google Scholar]
- 6.Deveci MA, Özbarlas HS, Erdoğan KE, et al. Elastofibroma dorsi: Clinical evaluation of 61 cases and review of the literature. Acta Orthop Traumatol Turc. 2017;51(1):7–11. doi: 10.1016/j.aott.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parratt MT, Donaldson JR, Flanagan AM, et al. Elastofibroma dorsi: Management, outcome and review of the literature. J Bone Joint Surg Br. 2010;92(2):262–66. doi: 10.1302/0301-620X.92B2.22927. [DOI] [PubMed] [Google Scholar]
- 8.Fabien J, Patel V, Timpone M. Management of symptomatic elastofibroma dorsi: A case report and literature review. Cureus. 2022;14(9):e29163. doi: 10.7759/cureus.29163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagano S, Yokouchi M, Setoyama T, et al. Elastofibroma dorsi: Surgical indications and complications of a rare soft tissue tumor. Mol Clin Oncol. 2014;2(3):421–24. doi: 10.3892/mco.2014.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neagoe O, Faur CI, Ionică M, et al. Elastofibroma dorsi, a rare condition, with challenging diagnosis. Case report and literature review. Medicina (Kaunas) 2021;57(4):370. doi: 10.3390/medicina57040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P, Gandhi D, Gupta S, et al. Elastofibroma dorsi. Proc (Bayl Univ Med Cent) 2017;30(3):340–42. doi: 10.1080/08998280.2017.11929641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haihua R, Xiaobing W, Jie P, Xinxin H. Retrospective analysis of 73 cases of elastofibroma. Ann R Coll Surg Engl. 2020;102(2):84–93. doi: 10.1308/rcsann.2019.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandser EA, Goree JC, El-Khoury GY. Elastofibroma dorsi: Prevalence in an elderly patient population as revealed by CT. Am J Roentgenol. 1998;171(4):977–80. doi: 10.2214/ajr.171.4.9762978. [DOI] [PubMed] [Google Scholar]
- 14.Järvi OH, Länsimies PH. Subclinical elastofibromas in the scapular region in an autopsy series. Acta Pathol Microbiol Scand A. 1975;83(1):87–108. doi: 10.1111/j.1699-0463.1975.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 15.Suliman MS, Higginbothom ZS, Amro A, et al. Elastofibroma dorsi: A rare connective tissue tumor. Cureus. 2020;12(2):e6874. doi: 10.7759/cureus.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guha AR, Raja RCS, Devadoss VG. Elastofibroma dorsi – a case report and review of literature. Int J Clin Pract. 2004;58(2):218–20. doi: 10.1111/j.1368-5031.2004.0051.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith HG, Hannay JA, Thway K, et al. Elastofibroma dorsi: The clunking tumour that need not cause alarm. Ann R Coll Surg Engl. 2016;98(3):208–11. doi: 10.1308/rcsann.2016.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patnayak R, Jena A, Settipalli S, Nagesh N. Elastofibroma: An uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9(1):34–37. doi: 10.4103/0974-2077.178543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daigeler A, Vogt PM, Busch K, et al. Elastofibroma dorsi – differential diagnosis in chest wall tumours. World J Surg Oncol. 2007;5:15. doi: 10.1186/1477-7819-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haykir R, Karakose S, Karabacakoglu A. Elastofibroma dorsi: Typical radiological features. Australas Radiol. 2007;51:B95–B97. doi: 10.1111/j.1440-1673.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa. A clinicopathologic study of 170 cases. Cancer. 1982;50(9):1794–805. doi: 10.1002/1097-0142(19821101)50:9<1794::aid-cncr2820500925>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Go PH, Meadows MC, Deleon EM, Chamberlain RS. Elastofibroma dorsi: A soft tissue masquerade. Int J Shoulder Surg. 2010;4(4):97–101. doi: 10.4103/0973-6042.79797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Vito A, Scali E, Ferraro G, et al. Elastofibroma dorsi: A histochemical and immunohistochemical study. Eur J Histochem. 2015;59(1):2459. doi: 10.4081/ejh.2015.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumaratilake JS, Krishnan R, Lomax-Smith J, Cleary EG. Elastofibroma: Disturbed elastic fibrillogenesis by periosteal-derived cells? An immunoelectron microscopic and in situ hybridization study. Hum Pathol. 1991;22(10):1017–29. doi: 10.1016/0046-8177(91)90010-m. [DOI] [PubMed] [Google Scholar]
- 25.McComb EN, Feely MG, Neff JR, et al. Cytogenetic instability, predominantly involving chromosome 1, is characteristic of elastofibroma. Cancer Genet Cytogenet. 2001;126(1):68–72. doi: 10.1016/s0165-4608(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 26.Nishio JN, Iwasaki H, Ohjimi Y, et al. Gain of Xq detected by comparative genomic hybridization in elastofibroma. Int J Mol Med. 2002;10(3):277–80. [PubMed] [Google Scholar]
- 27.Hisaoka M, Hashimoto H. Elastofibroma: Clonal fibrous proliferation with predominant CD34-positive cells. Virchows Arch. 2006;448(2):195–99. doi: 10.1007/s00428-005-0053-9. [DOI] [PubMed] [Google Scholar]
- 28.Hernández JL, Rodríguez-Parets JO, Valero JM, et al. High-resolution genome-wide analysis of chromosomal alterations in elastofibroma. Virchows Arch. 2010;456(6):681–87. doi: 10.1007/s00428-010-0911-y. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia M, Vanel D, Pollastri P, et al. Imaging patterns in elastofibroma dorsi. Eur J Radiol. 2009;72(1):16–21. doi: 10.1016/j.ejrad.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Tsubakimoto M, Yamashiro T, Tsuchiya N, et al. MRI findings and demographics of elastofibroma dorsi: Assessment of diffusion-weighted imaging and contrast enhancement patterns. Acta Radiol. 2018;59(6):709–15. doi: 10.1177/0284185117732099. [DOI] [PubMed] [Google Scholar]
- 31.Blumenkrantz Y, Bruno GL, González CJ, et al. Characterization of elastofibroma dorsi with (18)FDG PET/CT: A retrospective study. Rev Esp Med Nucl. 2011;30(6):342–45. doi: 10.1016/j.remn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Fang N, Wang YL, Zeng L, et al. Characteristics of elastofibroma dorsi on PET/CT imaging with (18)F-FDG. Clin Imaging. 2016;40(1):110–13. doi: 10.1016/j.clinimag.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Tamimi Mariño I, Sesma Solis P, Pérez Lara A, et al. Sensitivity and positive predictive value of magnetic resonance imaging in the diagnosis of elastofibroma dorsi: Review of fourteen cases. J Shoulder Elbow Surg. 2013;22(1):57–63. doi: 10.1016/j.jse.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 34.El Hammoumi M, Qtaibi A, Arsalane A, et al. Elastofibroma dorsi: Clinicopathological analysis of 76 cases. Korean J Thorac Cardiovasc Surg. 2014;47(2):111–16. doi: 10.5090/kjtcs.2014.47.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lococo F, Cesario A, Mattei F, et al. Elastofibroma dorsi: Clinicopathological analysis of 71 cases. Thorac Cardiovasc Surg. 2013;61(3):215–22. doi: 10.1055/s-0032-1328932. [DOI] [PubMed] [Google Scholar]