Abstract
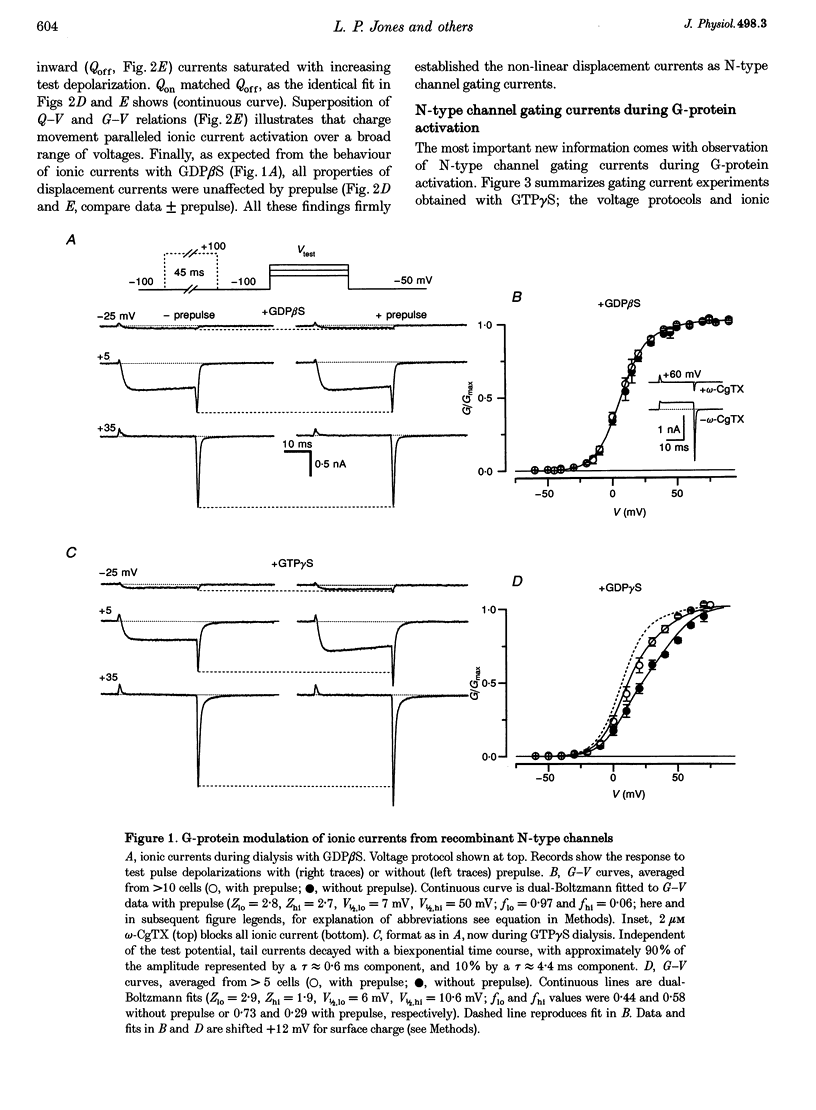
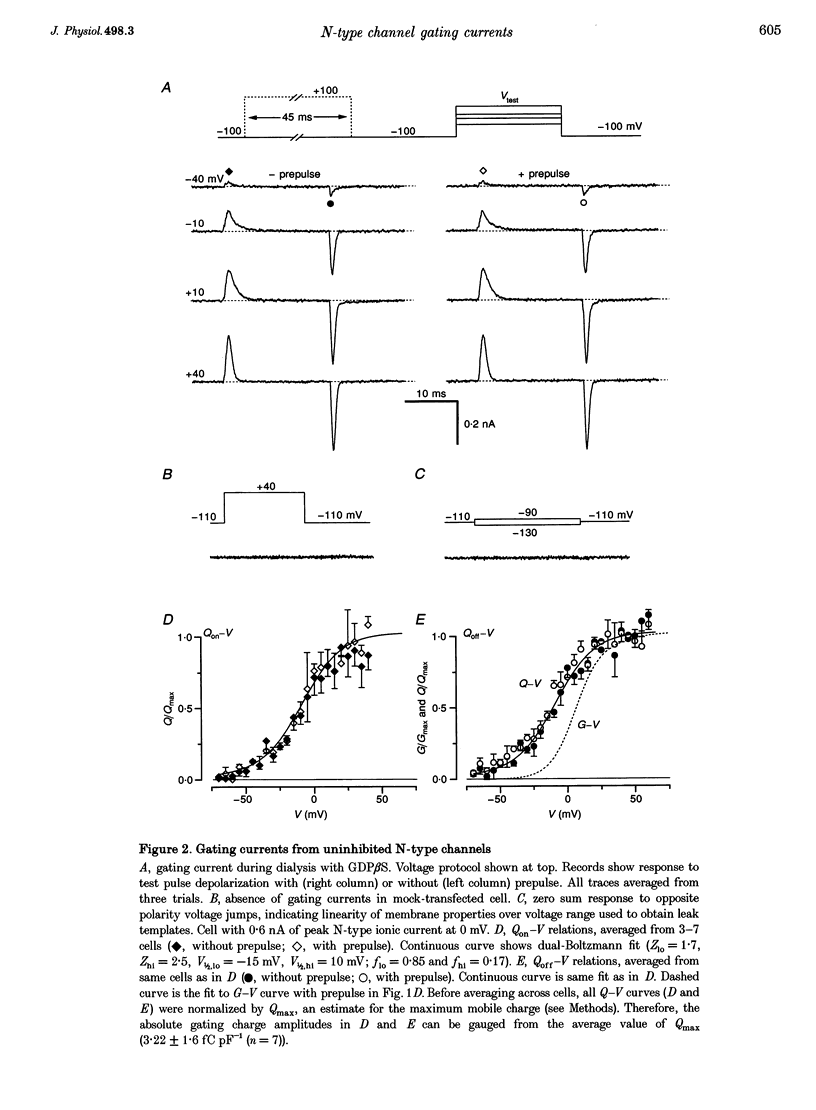
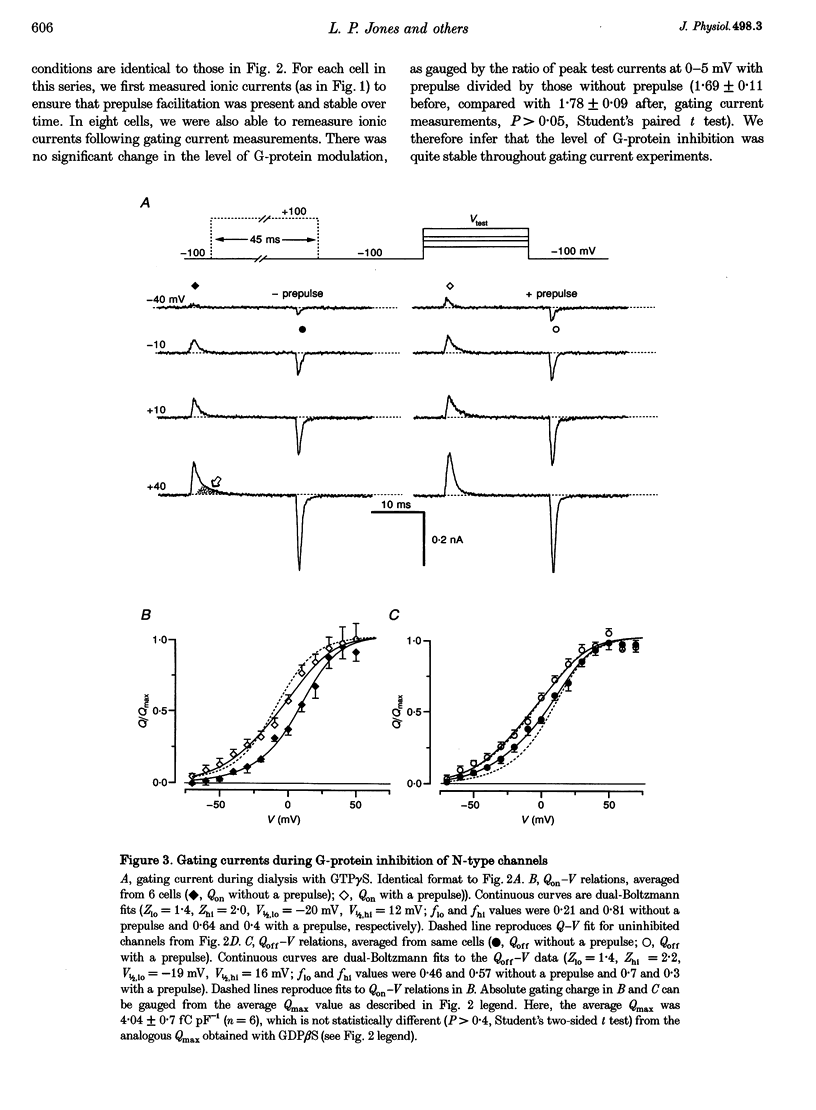
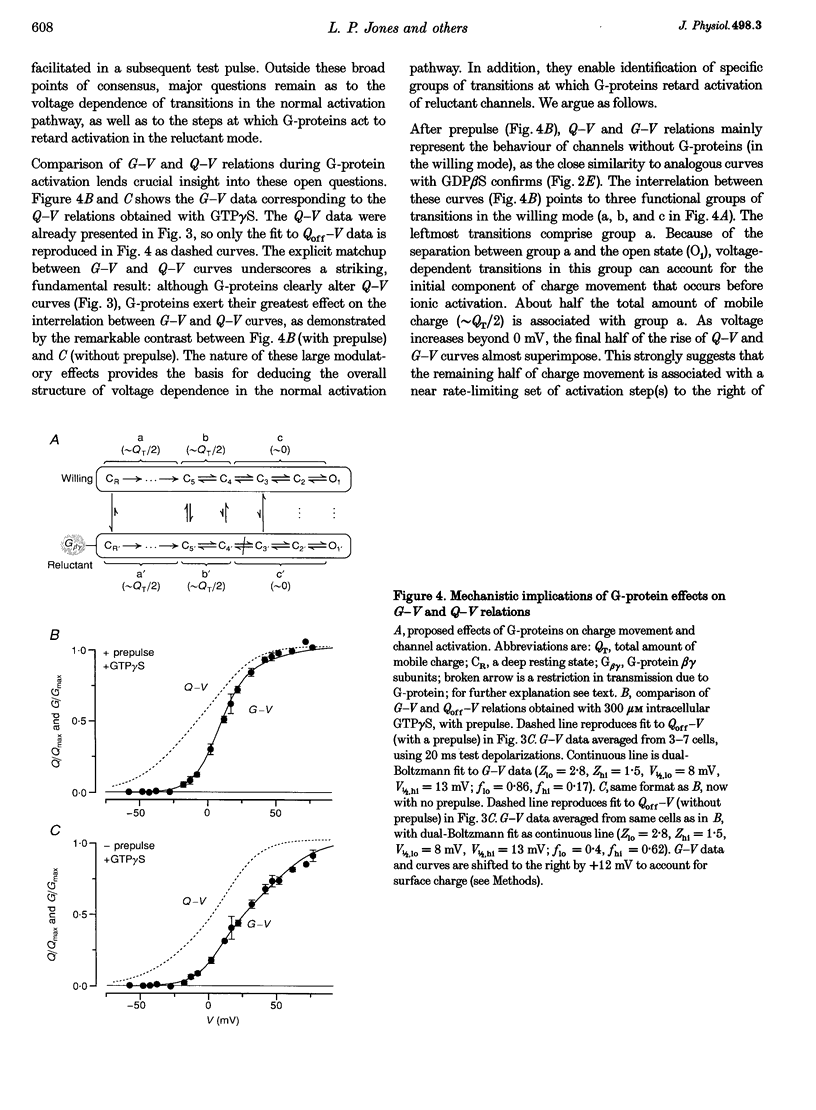
1. Voltage-dependent inhibition of N-type calcium currents by G-proteins contributes importantly to presynaptic inhibition. To examine the effect of G-proteins on key intermediary transitions leading to channel opening, we measured both gating and ionic currents arising from recombinant N-type channels (alpha 1B, beta 1b and alpha 2) expressed in transiently transfected human embryonic kidney cells (HEK 293). Recombinant expression of a homogeneous population of channels provided a favourable system for rigorous examination of the mechanisms underlying G-protein modulation. 2. During intracellular dialysis with GTP gamma S to activate G-proteins, ionic currents demonstrated classic features of voltage-dependent inhibition, i.e. strong depolarizing prepulses increased ionic currents and produced hyperpolarizing shifts in the voltage-dependent activation of ionic current. No such effects were observed with GDP beta S present to minimize G-protein activity. 3. Gating currents were clearly resolved after ionic current blockade with 0.1 mM free La3+, enabling this first report of gating charge translocation arising exclusively from N-type channels. G-proteins decreased the amplitude of gating currents and produced depolarizing shifts in the voltage-dependent activation of gating charge movement. However, the greatest effect was to induce a approximately 20 mV separation between the voltage-dependent activation of gating charge movement and ionic current. Strong depolarizing prepulses largely reversed these effects. These modulatory features provide telling clues about the kinetic steps affected by G-proteins because gating currents arise from the movement of voltage sensors that trigger channel activation. 4. The mechanistic implications of concomitant G-protein-mediated changes in gating and ionic currents are discussed. We argue that G-proteins act to inhibit both voltage-sensor movement and the transduction of voltage-sensor activation into channel opening.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjukow S., Döring F., Froschmayr M., Grabner M., Glossmann H., Hering S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol. 1996 Jun;118(3):748–754. doi: 10.1111/j.1476-5381.1996.tb15463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland L. M., Bean B. P. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: kinetics and voltage dependence. J Neurosci. 1993 Feb;13(2):516–533. doi: 10.1523/JNEUROSCI.13-02-00516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinet E., Soong T. W., Stea A., Snutch T. P. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust P. F., Simerson S., McCue A. F., Deal C. R., Schoonmaker S., Williams M. E., Veliçelebi G., Johnson E. C., Harpold M. M., Ellis S. B. Human neuronal voltage-dependent calcium channels: studies on subunit structure and role in channel assembly. Neuropharmacology. 1993 Nov;32(11):1089–1102. doi: 10.1016/0028-3908(93)90004-m. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Dhallan R. S., Yau K. W., Schrader K. A., Reed R. R. Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature. 1990 Sep 13;347(6289):184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- Dubel S. J., Starr T. V., Hell J., Ahlijanian M. K., Enyeart J. J., Catterall W. A., Snutch T. P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmslie K. S., Zhou W., Jones S. W. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990 Jul;5(1):75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Ca2+ and voltage inactivate Ca2+ channels in guinea-pig ventricular myocytes through independent mechanisms. J Physiol. 1991 Dec;444:257–268. doi: 10.1113/jphysiol.1991.sp018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. W., Lederer W. J. Nifedipine inhibits movement of cardiac calcium channels through late, but not early, gating transitions. Am J Physiol. 1995 Nov;269(5 Pt 2):H1784–H1790. doi: 10.1152/ajpheart.1995.269.5.H1784. [DOI] [PubMed] [Google Scholar]
- Herlitze S., Garcia D. E., Mackie K., Hille B., Scheuer T., Catterall W. A. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996 Mar 21;380(6571):258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994 Dec;17(12):531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S. R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996 Mar 21;380(6571):255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sperelakis N. Phosphorylation shifts the time-dependence of cardiac Ca++ channel gating currents. Biophys J. 1991 Aug;60(2):491–497. doi: 10.1016/S0006-3495(91)82075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Kongsamut S., Tsien R. W. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989 Aug 24;340(6235):639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- Marshall J., Molloy R., Moss G. W., Howe J. R., Hughes T. E. The jellyfish green fluorescent protein: a new tool for studying ion channel expression and function. Neuron. 1995 Feb;14(2):211–215. doi: 10.1016/0896-6273(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Patil P. G., de Leon M., Reed R. R., Dubel S., Snutch T. P., Yue D. T. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophys J. 1996 Nov;71(5):2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell M., Sakamoto J., Jay S. D., Campbell K. P. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 1991 Oct 21;291(2):253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. The agonist effect of Bay K 8644 on neuronal calcium channel currents is promoted by G-protein activation. Neurosci Lett. 1988 Jun 29;89(2):170–175. doi: 10.1016/0304-3940(88)90376-x. [DOI] [PubMed] [Google Scholar]
- Shirokov R., Levis R., Shirokova N., Ríos E. Ca(2+)-dependent inactivation of cardiac L-type Ca2+ channels does not affect their voltage sensor. J Gen Physiol. 1993 Dec;102(6):1005–1030. doi: 10.1085/jgp.102.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson W. J., Stea A., Bourinet E., Charnet P., Nargeot J., Snutch T. P. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993 Nov;32(11):1117–1126. doi: 10.1016/0028-3908(93)90006-o. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Bezanilla F. A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J. 1991 Dec;60(6):1511–1533. doi: 10.1016/S0006-3495(91)82186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with Shaker mRNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7243–7247. doi: 10.1073/pnas.86.18.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]