Abstract
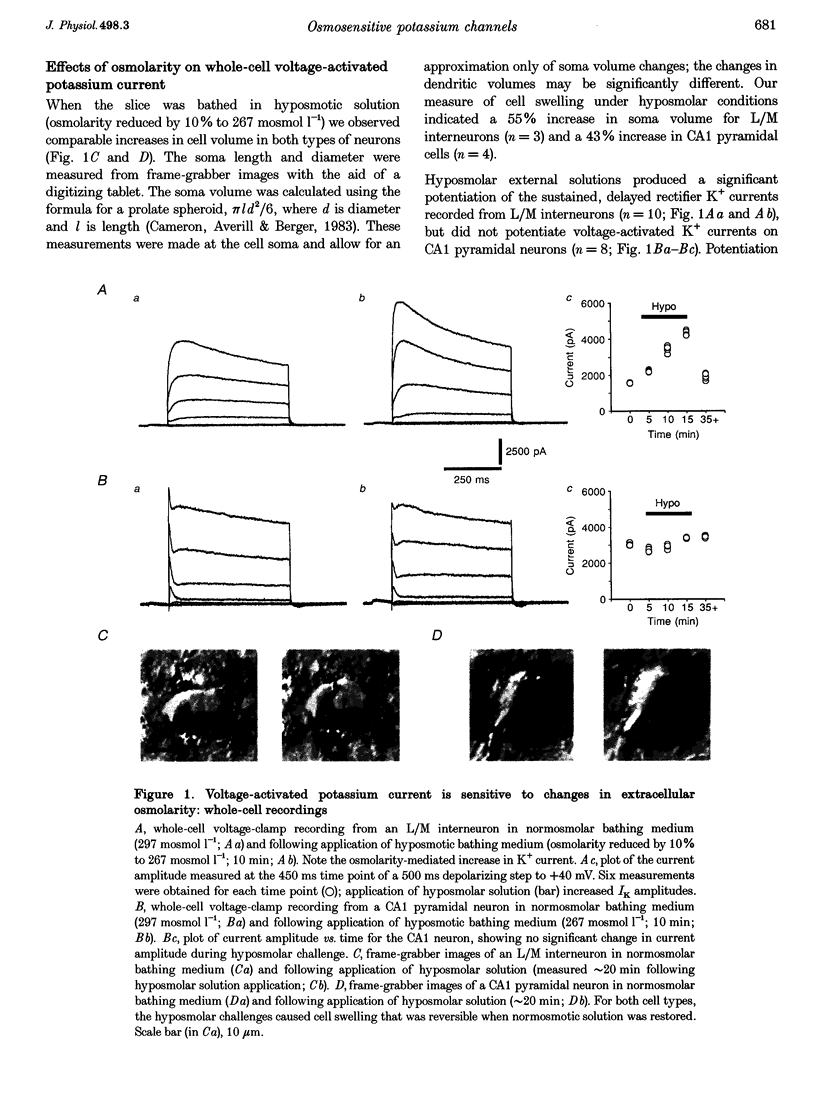
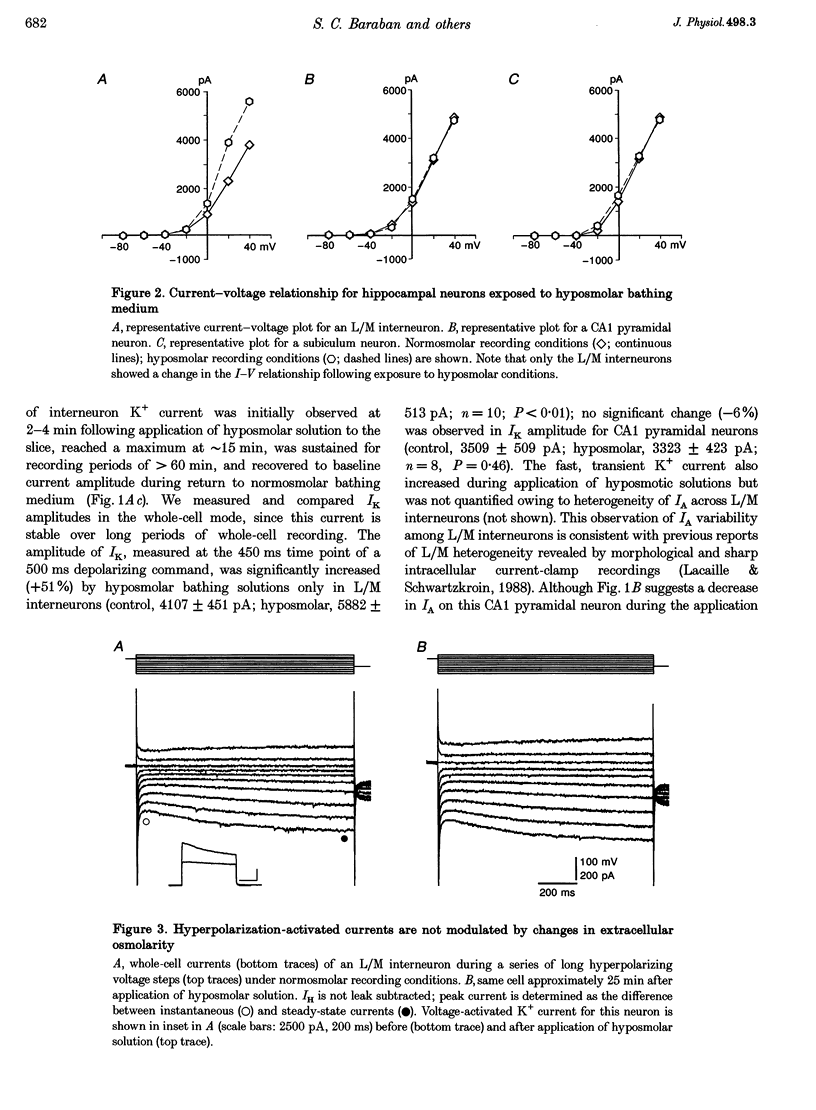
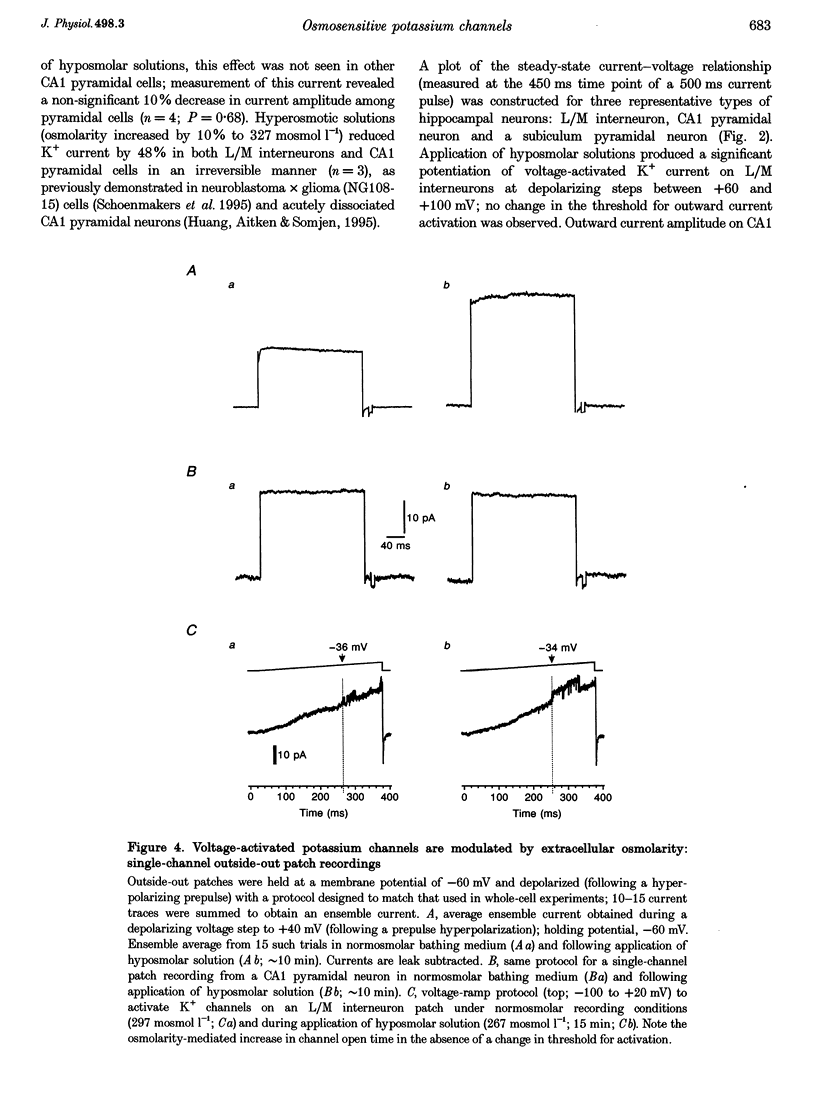
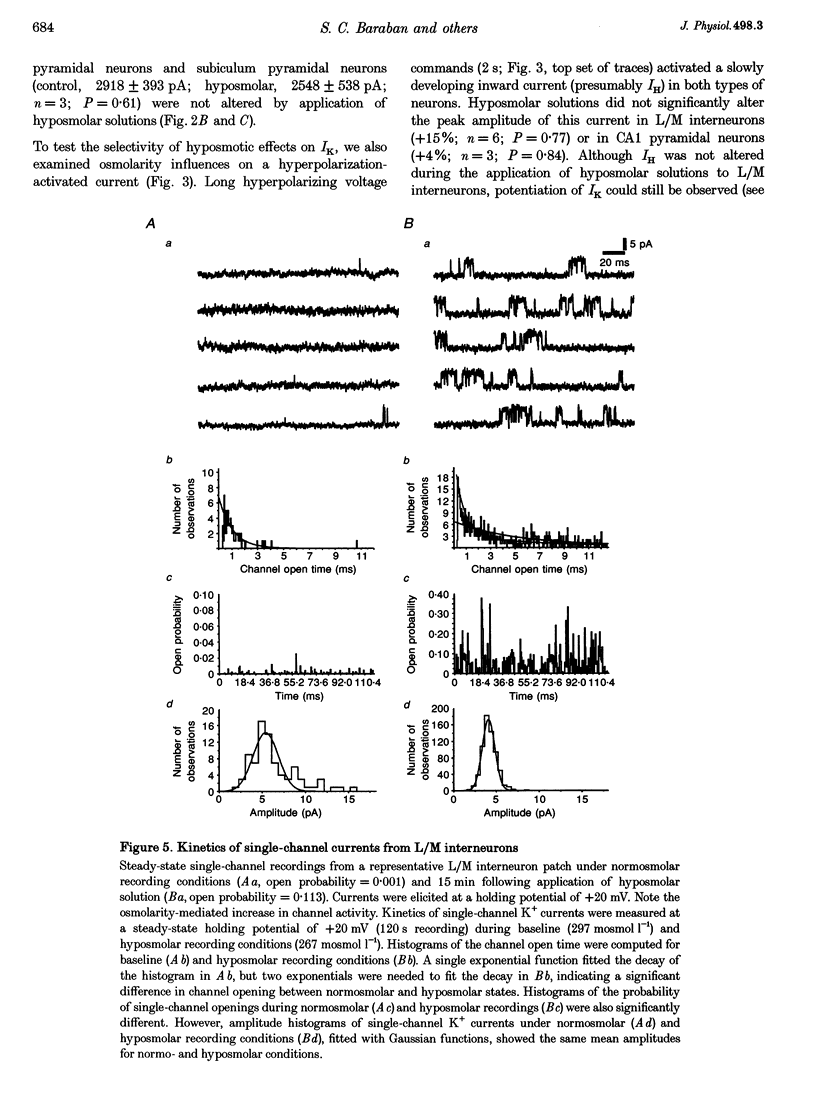
1. Whole-cell and single-channel recording methods were used in conjunction with infrared video microscopy techniques to examine the properties of voltage-activated potassium channels in hippocampal neurons during the application of hyposmolar solutions to hippocampal slices from rats. 2. Hyposmolar external solutions (osmolarity reduced by 10% to 267 mosmol l-1) produced a significant potentiation of voltage-activated K+ current on lacunosum/moleculare (L/M) hippocampal interneurons, but not on CA1 and subiculum pyramidal neurons. Hyperpolarization-activated (IH) and leak currents were not altered during the application of hyposmolar solutions in all cell types. 3. Mean channel open time and the probability of channel opening were dramatically increased under hyposmolar recording conditions for outside-out patches from L/M interneurons; no changes were observed for patches from CA1 pyramidal neurons. Mean current amplitude and the threshold for channel activation were not affected by hyposmotic challenge. 4. Hyposmolar external solutions produced a significant reduction in the firing frequency of L/M interneurons recorded in current-clamp mode. Hyposmolar solutions had no effect on resting membrane potential, action potential amplitude or duration, and spike after-hyperpolarization amplitude. 5. These results indicate that selective modulation of interneuron ion channel activity may be a critical mechanism by which osmolarity can regulate excitability in the central nervous system.
Full text
PDF


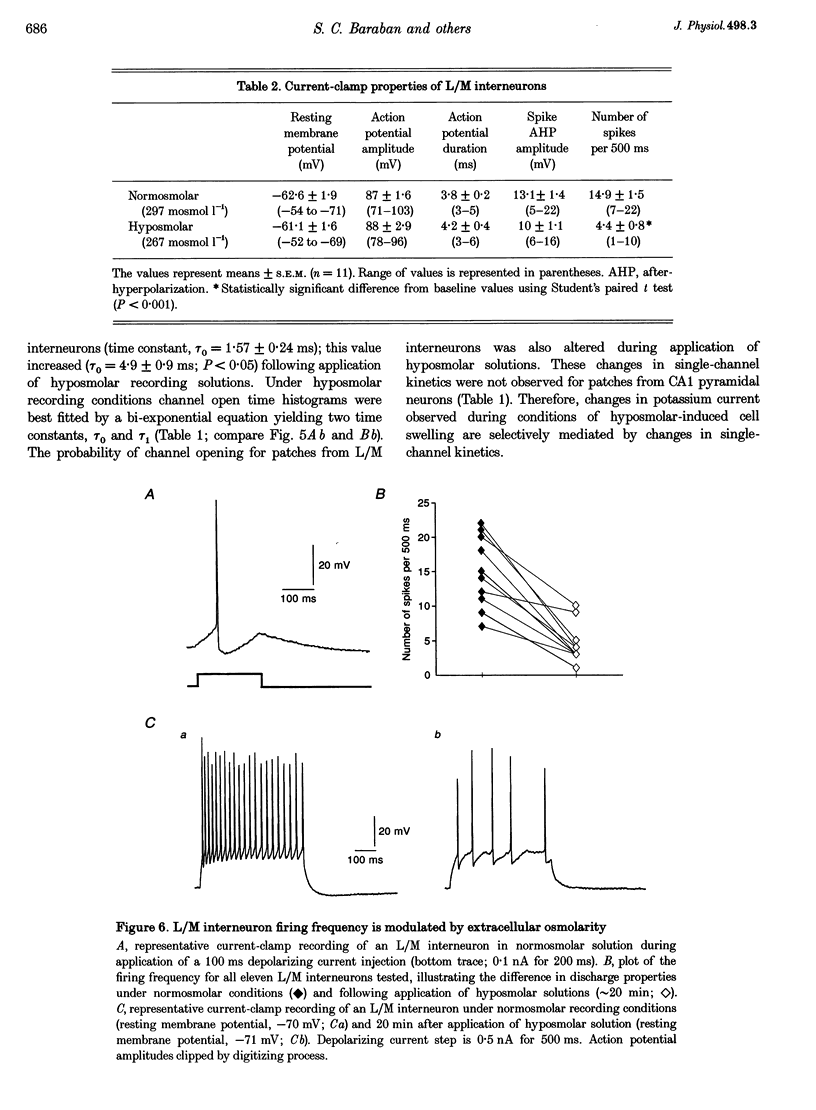



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K., Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. D. Seizure and acute osmotic change: clinical and neurophysiological aspects. J Neurol Sci. 1991 Jan;101(1):7–18. doi: 10.1016/0022-510x(91)90013-w. [DOI] [PubMed] [Google Scholar]
- Ballyk B. A., Quackenbush S. J., Andrew R. D. Osmotic effects on the CA1 neuronal population in hippocampal slices with special reference to glucose. J Neurophysiol. 1991 May;65(5):1055–1066. doi: 10.1152/jn.1991.65.5.1055. [DOI] [PubMed] [Google Scholar]
- Baraban S. C., Schwartzkroin P. A. Electrophysiology of CA1 pyramidal neurons in an animal model of neuronal migration disorders: prenatal methylazoxymethanol treatment. Epilepsy Res. 1995 Oct;22(2):145–156. doi: 10.1016/0920-1211(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Busch A. E., Hurst R. S., North R. A., Adelman J. P., Kavanaugh M. P. Current inactivation involves a histidine residue in the pore of the rat lymphocyte potassium channel RGK5. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1384–1390. doi: 10.1016/0006-291x(91)91726-s. [DOI] [PubMed] [Google Scholar]
- Busch A. E., Varnum M., Adelman J. P., North R. A. Hypotonic solution increases the slowly activating potassium current IsK expressed in xenopus oocytes. Biochem Biophys Res Commun. 1992 Apr 30;184(2):804–810. doi: 10.1016/0006-291x(92)90661-4. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Averill D. B., Berger A. J. Morphology of cat phrenic motoneurons as revealed by intracellular injection of horseradish peroxidase. J Comp Neurol. 1983 Sep 1;219(1):70–80. doi: 10.1002/cne.902190107. [DOI] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hochman D. W., Baraban S. C., Owens J. W., Schwartzkroin P. A. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995 Oct 6;270(5233):99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- Kim D., Fu C. Activation of a nonselective cation channel by swelling in atrial cells. J Membr Biol. 1993 Jul;135(1):27–37. doi: 10.1007/BF00234649. [DOI] [PubMed] [Google Scholar]
- Kunkel D. D., Lacaille J. C., Schwartzkroin P. A. Ultrastructure of stratum lacunosum-moleculare interneurons of hippocampal CA1 region. Synapse. 1988;2(4):382–394. doi: 10.1002/syn.890020405. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y., Suzuki S., Hashi K., Uede T., Matsumura S., Kawahara T., Yamaji I., Ujike Y., Kaneko M. [Elevation of intracranial pressure during hemodialysis--continuous measurement of cerebrospinal fluid pressure in a patient with acoustic neurinoma]. No To Shinkei. 1990 Jun;42(6):569–573. [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci. 1988 Apr;8(4):1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P. D. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol. 1993 Nov;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Lin L., Sigworth F. J. Substitution of a hydrophobic residue alters the conformational stability of Shaker K+ channels during gating and assembly. Biophys J. 1993 Oct;65(4):1740–1748. doi: 10.1016/S0006-3495(93)81202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Sigurdson W. J. Stretch-inactivated ion channels coexist with stretch-activated ion channels. Science. 1989 Feb 10;243(4892):807–809. doi: 10.1126/science.2536958. [DOI] [PubMed] [Google Scholar]
- Navaratnam D. S., Escobar L., Covarrubias M., Oberholtzer J. C. Permeation properties and differential expression across the auditory receptor epithelium of an inward rectifier K+ channel cloned from the chick inner ear. J Biol Chem. 1995 Aug 18;270(33):19238–19245. doi: 10.1074/jbc.270.33.19238. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Shimizu T., Imanaga H., Kubo O., Yoshida S. [Two cases of nonketotic hyperosmolar coma in neurosurgery (author's transl)]. No Shinkei Geka. 1977 Oct;5(11):1165–1170. [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron. 1996 Jan;16(1):175–181. doi: 10.1016/s0896-6273(00)80034-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P., Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994 Sep;13(3):645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Rees S. A., Vandenberg J. I., Wright A. R., Yoshida A., Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J Gen Physiol. 1995 Dec;106(6):1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson G. B., Messer C. Effect of composition of experimental solutions on neuronal survival during rat brain slicing. Exp Neurol. 1995 Jan;131(1):133–143. doi: 10.1016/0014-4886(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Roper S. N., Obenaus A., Dudek F. E. Osmolality and nonsynaptic epileptiform bursts in rat CA1 and dentate gyrus. Ann Neurol. 1992 Jan;31(1):81–85. doi: 10.1002/ana.410310115. [DOI] [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers T. J., Vaudry H., Cazin L. Osmo- and mechanosensitivity of the transient outward K+ current in a mammalian neuronal cell line. J Physiol. 1995 Dec 1;489(Pt 2):419–430. doi: 10.1113/jphysiol.1995.sp021062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R., Davies N. W., Shelton P. A., Sutcliffe M. J., Khan I. A., Brammar W. J., Conley E. C. A single aspartate residue is involved in both intrinsic gating and blockage by Mg2+ of the inward rectifier, IRK1. J Physiol. 1994 Jul 1;478(Pt 1):1–6. doi: 10.1113/jphysiol.1994.sp020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. F. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Dudek F. E., Snow R. W., Knowles W. D. Computer simulations indicate that electrical field effects contribute to the shape of the epileptiform field potential. Neuroscience. 1985 Aug;15(4):947–958. doi: 10.1016/0306-4522(85)90245-3. [DOI] [PubMed] [Google Scholar]
- Williams S., Samulack D. D., Beaulieu C., LaCaille J. C. Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994 Jun;71(6):2217–2235. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- Wong S. M., Chase H. S., Jr Role of intracellular calcium in cellular volume regulation. Am J Physiol. 1986 Jun;250(6 Pt 1):C841–C852. doi: 10.1152/ajpcell.1986.250.6.C841. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Bezanilla F., Parsegian V. A. Solute inaccessible aqueous volume changes during opening of the potassium channel of the squid giant axon. Biophys J. 1990 May;57(5):1049–1064. doi: 10.1016/S0006-3495(90)82623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg J., Parsegian V. A. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986 Sep 4;323(6083):36–39. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]



