Simple Summary
Antibody Drug Conjugates (ADCs) are a newer class of therapeutic agents which present a promising therapeutic approach for non-small cell lung cancer (NSCLC). Several ADCs are in clinical trial, targeting various antigens including TROP2, HER2, MET and others. Despite the promise, ADCs present unique challenges including toxicity like interstitial lung disease (ILD). A more thorough analysis of target expression could lead to a better understanding of adverse events, such as ILD. This improved understanding may help reduce the occurrence of these adverse effects. Our study aims to analyze the ADC target expression in lung tissue to help us understand the relation between treatment with ADCs and onset of interstitial lung disease.
Keywords: antibody–drug conjugates, ADCs; interstitial lung disease; non-small-cell lung cancer, NSCLC; toxicity; ILD; TROP2; ERBB2
Abstract
Introduction: Non-small-cell lung cancer (NSCLC) remains a leading cause of cancer-related mortality worldwide, despite advances in immune checkpoint inhibitors and targeted therapies. Antibody–drug conjugates (ADCs) represent a promising therapeutic approach by delivering cytotoxic agents specifically to cancer cells, potentially reducing harm to healthy tissues. This study aims to explore the effectiveness and challenges associated with ADCs in NSCLC, with a focus on drug-induced interstitial lung disease (D-ILD). Methods: A comprehensive literature review was conducted across MEDLINE (Ovid), Embase (Elsevier), CENTRAL (Cochrane Library), and other sources up to March 2023, to identify ADCs used in NSCLC treatment and their associated risk of D-ILD. The incidence of ILD was analyzed from clinical trial data, while ADC target expression was examined through RNA and protein levels in normal and tumor lung tissues. Discussion: Our findings highlight the therapeutic potential of ADCs in NSCLC, as evidenced by significant clinical outcomes. However, the occurrence of D-ILD presents a notable challenge, as its incidence was not directly correlated with the expression levels of the target antigens. This suggests that D-ILD may result from factors beyond antigen expression, including the cytotoxic payload and linker characteristics of ADCs. Conclusion: ADCs offer a promising avenue for NSCLC treatment. Nonetheless, the risk of D-ILD necessitates a balanced approach in ADC development, focusing on optimizing linker and payload properties to mitigate this adverse effect. Further research is essential to better understand and manage D-ILD, ensuring the safe and effective use of ADCs in clinical practice.
1. Introduction
Non-small-cell lung cancer (NSCLC) is the most commonly encountered type of lung cancer worldwide [1]. Over the last decade, the advent of immune checkpoint inhibitors and genomically targeted therapy has dramatically improved the outcomes of patients with NSCLC [2,3]. However, mortality remains high in patients with NSCLC, and significant room for improvement remains; novel therapies with new mechanisms of action are still needed [4]. One promising approach for treatment of NSCLC is using antibody–drug conjugates (ADCs) to target specific tumor antigens. ADCs are monoclonal antibodies linked to cytotoxic drugs, which theoretically allow for the selective delivery of cytotoxic agents to cancer cells while avoiding harm to normal healthy cells and tissues [5].
The clinical development of ADCs has not been without challenge since the first clinically approved ADC, gemtuzumab ozogamicin, in hematological malignancies. More recently, improved ADC designs have led to several novel ADCs receiving FDA approval for gastric cancer, breast cancer, and, more recently, NSCLC [6,7]. The success of ADCs in NSCLC was demonstrated in the DESTINY-Lung 01 trial evaluating trastuzumab deruxtecan (TDX-d) in patients with metastatic, HER2-mutant NSCLC [8]. In 91 patients, TDX-d administration resulted in an objective response rate (ORR) of 55% with a median duration of response (mDOR) of 9.3 months. However, 26% of the patients in that study had any-grade drug-induced interstitial lung disease (D-ILD), using modestly higher doses of TDX-d as compared to breast cancer (6.4 vs. 5.4 mg/kg). Studies of anti-ERBB2 ADCs have also shown that D-ILD is a common dose-limiting toxicity, and the highest incidence of any-grade D-ILD was in patients with lung cancer [9]. The early and accurate diagnosis of D-ILD remains a significant challenge in the clinic because D-ILD can often mimic infectious pneumonia or cancer progression. Improving our understanding of this unique toxicity will help clinicians improve patient outcomes in those receiving ADCs for NSCLC.
Risk factors for D-ILD associated with ADCs may vary based upon cytotoxic payload, mechanism of action, underlying patient comorbidities, and the type of cancer. Patients with NSCLC may be at a higher risk for D-ILD given the association of NSCLC with smoking history and COPD, as well as lung surgery and thoracic radiation. With the ongoing development of multiple ADCs for use in NSCLC, developing a deeper understanding of the mechanism of ADC-induced ILD will enable the optimization of ADC development. A meta-analysis encompassed a comprehensive evaluation of 39 studies involving 7732 patients, to ascertain the incidence of market-approved ADC-related pneumonitis in solid tumors. The findings reveal that ADCs are associated with a significant incidence of all grade and grade ≥3 pneumonitis, with the highest incidence being observed in NSCLC 22.18% (95% CI, 2.14–52.61%) [10]. As such, interstitial lung disease (ILD) constitutes a significant challenge in the context of ADC use in lung cancer.
Beyond the mechanism of action of ADCs and the additional factors described above, ADC target expression could also impact ILD toxicity. In this review, we study the landscape of ADC target expression at the RNA, single-cell RNA, and protein level in the normal lung. We also review the incidence of ILD in clinical studies with ADCs to infer a potential relationship between ADC target antigen expression in the lungs and D-ILD.
2. Methods
A. Identification of targets: A systematic search of the MEDLINE (Ovid), Embase (Elsevier), and CENTRAL (Cochrane Library) databases, as well as peer-reviewed literature in the English language, conference abstracts, and trial registrations from major international oncology meetings up to March 2023 were conducted. The search was restricted to studies written in or translated into English and discussed keywords such as “Antibody Drug Conjugates”, “non-small cell lung cancer”, “NSCLC”, “ADC”, and “lung adenocarcinoma”. Clinical trials describing ADC-based interventions in NSCLC were identified through a review of each title and abstract. All ADCs regardless of their FDA approval status were studied. Targets of interest were then identified based on this information.
B. Incidence of ILD: The publicly available trial results of phase 1/2 clinical trials for each of the ADC corresponding to the target antigens of interest were included. Studies of trastuzumab deruxetecan (ERBB2) [8], patritumab deruxetecan (ERBB3) [11], datopotamab deruxetecan (TROP2) [12], tusamitamab ravtansine (CEACAM5) [13], and Telisotuzumab Vedotin (MET) [14] were reviewed to extract data on the incidence of ILD. We also reviewed the FDA label and package inserts for FDA-approved agents.
C. RNA and protein expression: We used publicly available datasets to study bulk RNA, single-cell RNA and protein expression via IHC of target antigens of interest for ADCs. The Genotype-Tissue Expression (GTEx) project is an ongoing effort to build a comprehensive public resource to study tissue-specific gene expression and regulation. These data were used to conduct the bulk RNA-seq analysis [15]. Data from The Cancer Genome Atlas (TCGA) were used to conduct the bulk RNA-seq analysis to understand RNA level expression of genes of interest [16]. The Human Protein Atlas (HPA) was used to obtain single-cell RNA sequencing (scRNA-seq) data and protein expression based on conventional immunohistochemistry profiling [17]. The HPA’s scRNA-seq data relies on the Smart-seq2 protocol, involving individual cell isolation from tissue samples, RNA reverse transcription, and amplification from each cell. Expression data are normalized using the transcripts per million (TPM) approach. Normalized mean transcript expression, represented as nTPM, serves as the expression-level measure for each target antigen. Detailed methods on how these data were obtained for the HPA database have been described elsewhere [18]. Protein expression data were available as antibody staining in the annotated cell types in the lung tissue reported as not detected, low, medium, or high, based on conventional immunohistochemistry profiling. This score was based on the combination of staining intensity and fraction of stained cells. Furthermore, staining intensity data were extracted from representative samples and scored as follows: 0—negative; 1—weak; 2—moderate; and 3—high. We used the highest antibody staining values for when multiple antibody staining patterns were described.
3. Results
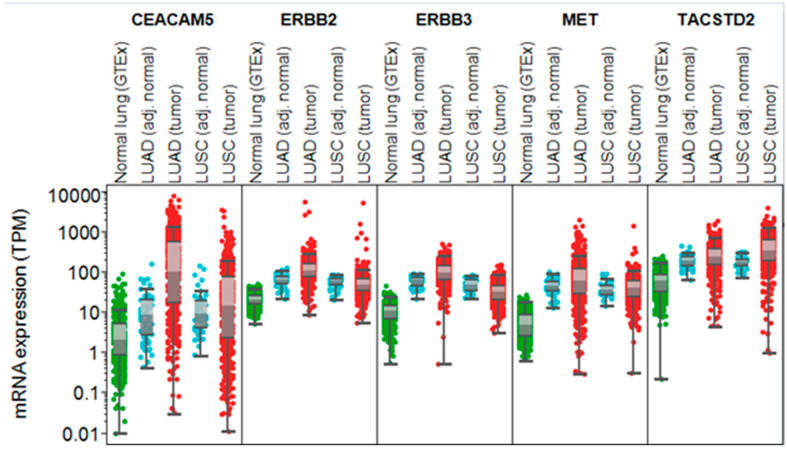
We found that, based on bulk RNA expression, these antigen targets of interest had higher expression in tumor tissues compared to normal lung tissue (obtained from GTEx and adjusted normal lung tissue from TCGA) (Figure 1) (Table 1).
Figure 1.
TCGA and GTEx bulk RNA expression of ADC-targetable antigens. Note: mRNA expression for the different targets in normal lung tissue was obtained from GTEx, while expression in lung adenocarcinoma (LUAD) and squamous carcinomas (LUSCs), as well as their adjacent non-cancerous tissue, was extracted from TCGA.
Table 1.
Characteristics of ADC, ILD incidence, and target antigen expression.
| ADC | Target | No. of Patients Included in Study | % All-Grade ILD | nMTP Expression | scRNA-seq Cell Type with the Highest Expression | Payload | Linker | DAR | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Trastuzumab Deruxetecan | HER2 | 91 | 26.4 | 73.7 | Type 1 | Topoisomerase 1 inhibitor (Dxd) | Cleavable | 8 | [8] |
| Patritumab Deruxetecan | HER3 | 57 | 7 | 88.5 | Type 2 | Topoisomerase 1 inhibitor (Dxd) | Cleavable | 8 | [11] |
| Datopotamab Deruxetecan | TROP2 | 34 | 3 | 1549.8 | Type 1 | Topoisomerase 1 inhibitor (Dxd) | Cleavable | 4 | [12] |
| Tusamitamab Ravtansine | CEACAM5 | 24 | 0 | 174.4 | Type 1 | Microtubule inhibitor (DM4) | Cleavable | 3.8 | [13] |
| Telisotuzumab Vedotin | MET | 136 | 6.6 | 155 | Type 2 | Microtubule inhibitor (MMAE) | Cleavable | 3 | [14] |
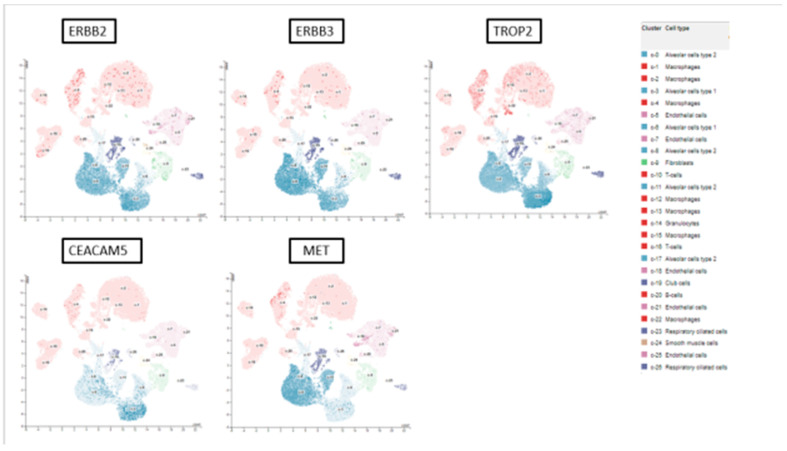
We evaluated single-cell RNA sequencing data from normal lungs and found that all target antigens were expressed in lung tissues, with the highest normalized transcript expression levels found in alveolar cells (type 1 or type 2). The nTPM expression for single-cell RNA across alveolar cells in lung tissue ranged from 73.7 to 1549.8 (Figure 2).
Figure 2.
scRNA-seq expression of ADC-targetable antigens in lung tissue (details: https://www.proteinatlas.org/ENSG00000141736-ERBB2/single+cell, accessed on 29 October 2024).
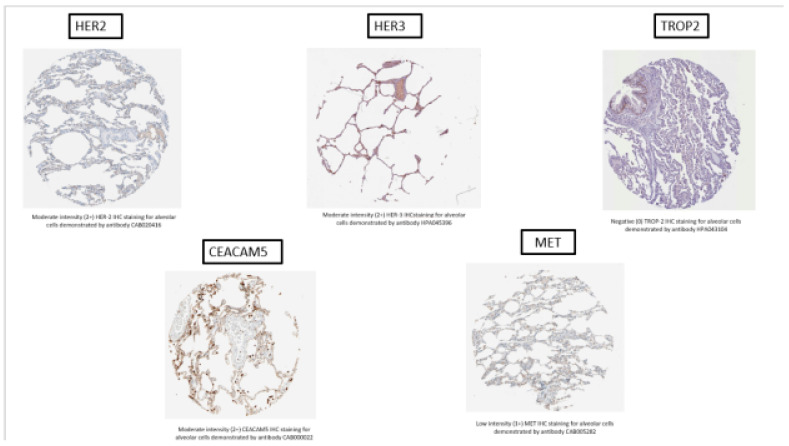
On analysis of protein expression from HPA via IHC, three of the five targets (ERBB2, ERBB3, and CEACAM5) demonstrated moderate-intensity staining (2+) on IHC, while MET demonstrated weak staining on alveolar cells. Similarly, IHC staining was moderate-to-high-intensity for macrophages in ERBB2, ERBB3, CEACAM5, and MET. Representative images for IHC antibody staining obtained directly from the HPA are shown in Figure 3.
Figure 3.
Representative IHC staining of normal lung tissue for HER2, HER3, TROP2, CEACAM5, and MET target antigens from the Human Protein Atlas (details: https://www.proteinatlas.org/ENSG00000141736-ERBB2/tissue/lung, accessed on 29 October 2024).
Our analysis revealed that the incidence of all-grade D-ILD across the ADCs of interest ranged from 0 to 26.4% (Table 1). Furthermore, there was no clear pattern of the incidence of ILD with IHC staining intensity across targets of interest, indicating that D-ILD may occur irrespective of the expression of proteins of interest and may in turn be related to linker and cytotoxic payload characteristics rather than target antigen expression.
4. Discussion
Beyond immune checkpoint inhibitors and genomically targeted therapy, ADCs are poised to enlarge the landscape of NSCLC therapy in the near future. It is important to understand the mechanisms of D-ILD. Our study identified that most of the ADC-targetable antigens were expressed in normal lung tissues, with the highest normalized transcript expression levels for these found within alveolar cells. We found that despite moderate-to-high-intensity IHC staining on alveolar cells, bulk or scRNA-seq expression of ADC-targetable antigens does not explain the incidence of any-grade ILD. Actually, there was no clear pattern of the incidence of ILD with IHC staining intensity across targets of interest, indicating that D-ILD may occur irrespective of the expression of proteins of interest. Although alveolar type 1 and type 2 cells most commonly expressed the targets of interest, single-cell RNA expression of these target genes were not proportionally associated with the occurrence of D-ILD. For example, the expression of TROP2 on alveolar type 1 cells exceeded ERBB2 expression (1549.8 vs. 73.7 nmTP); however, the incidence of ILD with datopotamab deruxtecan was found to be much lower that the results of DESTINY-lung 01 for trastuzumab deruxtecan (11 vs. 26%).
From a pathologic standpoint, D-ILD encompasses various lung conditions characterized by alveolitis, disarray in alveolar structures, and fibrosis in the alveolar interstitium. These processes culminate in a compromised gas exchange. The onset of this cascade is triggered by an acute or chronic lung injury caused by a drug, which subsequently advances to inflammation and manifests as overt D-ILD [19]. Many chemotherapeutic drugs, such as bleomycin, taxanes, irinotecan, tyrosine kinase inhibitors, and immune checkpoint inhibitors, have been associated with various forms of D-ILD [20]. Of interest, irinotecan, a topoisomerase I inhibitor, causes D-ILD, with higher incidence in patients with lung cancer specifically [21]. Many ADCs currently being developed in the clinic include a deruxtecan payload, which also acts via inhibition of topoisomerase I. Interestingly, in our review, we found that the incidence of ILD was higher with ADCs that have a DXd payload (Table 1). Although the specific mechanism of injury induced by ADCs is yet to be elucidated, it is possibly associated, at least in part, with the carried payload, with a low contribution of target antigen expression in lung tissue. For example, interstitial lung disease has been identified as a known adverse event for topotecan, a topoisomerase inhibitor [22,23].
Our results are indicative of D-ILD likely being an off-target, on-tissue toxicity of ADCs. It has been previously suggested that ADC-induced lung injury may be associated with bystander killing by free payload released from cancer cells, as well as circulating free payload resulting from deconjugation of the ADC. Furthermore, preclinical studies suggest that ADC-induced alveolar damage could be related to target-independent uptake of the conjugate by immune cells, rather than target-dependent uptake [24]. This further confirms our findings of a target-independent origin of ILD toxicity.
Finally, the type of linker and drug-to-antibody ratio used in the ADC construct may have important implications for the degree of bystander anti-tumor effect and off-target on-tissue toxicity. For example, the incidence of ILD was much higher in patients with HER2-positive breast cancer when treated with TDX-d compared with trastuzumab emtansine (28% vs. 15%), which may be related to the presence of a tumor-selective cleavable linker and a higher drug-to-antibody ratio in TDX-d [25,26]. Of note, TDX-d lung toxicity was shown to be strongly dose-dependent, arguing for the relevance of the level of exposure of normal lung tissue to the cytotoxic payload. Cleavable linkers were designed to improve ADC efficacy, as they can break down and release the cytotoxic payload in response to specific tumor-associated factors, such as acidic or reducing conditions, or proteolytic enzymes. On the other hand, non-cleavable linkers are more stable in the bloodstream but rely on the entire antibody–linker complex being degraded in lysosomes to release their payloads [27]. It is worth noting that while the extracellular release of the cytotoxic payload could be a crucial factor in the success of ADC therapy, this may have importance in the degree of on-target on-tissue toxicity observed [28]. Therefore, finding the right balance of linker stability is a complex challenge that depends on the specific target and payload selected, as well as the unique characteristics of the tumor microenvironment.
Optimization of ADCs should focus on improving activity in parallel to reducing bystander effects by selecting linker and payload classes that are least likely to cause ILD. This optimization should also take into account the dose and drug-to-antibody ratio. Our study supports this recommendation by identifying that ILD may be a bystander effect and highlighting the need to optimize ADCs to reduce such effects.
The limitations of our study include the use of scRNA-seq data from a single database and the limited sample size of clinical trial data for ILD incidence, given that many of these studies are currently in phase 1 and/or 2. Further studies using larger sample sizes and prospective IHC staining, bulk RNA sequencing, and scRNA-seq of tissue biopsies may provide a more comprehensive insight into the expression of these target antigens in normal and tumor lung tissue and the mechanism of ILD toxicity.
5. Conclusions
In conclusion, our study provides valuable insights into the incidence of all-grade ILD across different ADCs currently studied in NSCLC. Furthermore, there did not seem to be any correlation between the expression of target antigens and the incidence of ILD. This highlights the importance of well-designed translational and correlative studies to inform the mechanisms behind ADC-induced ILD. While it is important to promptly recognize and avoid inciting agents, there is a need to test effective therapies for ADC-induced ILD. Ultimately, better understanding of the pathogenesis of ADC-induced ILD will enable improved drug development of the ADCs currently in the pipeline while balancing bystander effects with target selectivity.
Author Contributions
Writing—original draft, A.D. and S.R.-C.; Writing—review & editing, V.S., A.S., S.D. and S.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be shared up on request.
Conflicts of Interest
A.D. reports advisory board participation for Foundation Medicine, Sanofi, and Amgen. S.R.-C. and A.S. report no COIs. V.S. reports advisory board participation for Bayer, Labcorp, Illumina, Jazz Pharmaceuticals, and Aadi biosciences. S.P. reports consultation/advisory board participation for AbbVie, AiCME, Amgen, Arcus, AstraZeneca, Bayer, Beigene, BerGenBio, Biocartis, BioInvent, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, ecancer, Eli Lilly, Elsevier, F-Star, Fishawack, Foundation Medicine, Genzyme, Gilead, GSK, Hutchmed, Illumina, Imedex, IQVIA, Incyte, Ipsen, iTeos, Janssen, Medscape, Medtoday, Merck Sharp and Dohme, Merck Serono, Merrimack, Mirati, Novartis, Novocure, OncologyEducation, Pharma Mar, Promontory Therapeutics, PER, Peerview, Pfizer, Regeneron, RMEI, Roche/Genentech, RTP, Sanofi, Seattle Genetics, Takeda, and Vaccibody; Board of Director position for Galenica; talk at company-organized public events for AiCME, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ecancer, Eli Lilly, Foundation Medicine, GSK, Illumina, Imedex, Ipsen, Medscape, Merck Sharp and Dohme, Mirati, Novartis, PER, Peerview, Pfizer, Roche/Genentech, RTP, Sanofi, and Takeda; and receipt of grants/research support for principal investigator position in trials (institutional financial support for clinical trials) sponsored by Amgen, Arcus, AstraZeneca, Beigene, Bristol-Myers Squibb, GSK, iTeos, Merck Sharp and Dohme, Mirati, Pharma Mar, Promontory Therapeutics, Roche/Genentech, and Seattle Genetics.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Forjaz G., Mooradian M.J., Meza R., Kong C.Y., Cronin K.A., Mariotto A.B., Lowy D.R., Feuer E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. New Engl. J. Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramagopalan S., Leahy T.P., Ray J., Wilkinson S., Sammon C., Subbiah V. The value of innovation: Association between improvements in survival of advanced and metastatic non-small cell lung cancer and targeted and immunotherapy. BMC Med. 2021;19:209. doi: 10.1186/s12916-021-02070-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Rous F., Singhi E.K., Sridhar A., Faisal M.S., Desai A. Lung Cancer Treatment Advances in 2022. Cancer Investig. 2022;41:12–24. doi: 10.1080/07357907.2022.2119479. [DOI] [PubMed] [Google Scholar]
- 5.Merle G., Friedlaender A., Desai A., Addeo A. Antibody Drug Conjugates in Lung Cancer. Cancer J. 2022;28:429–435. doi: 10.1097/PPO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 6.Drago J.Z., Modi S., Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021;18:327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai A., Abdayem P., Adjei A.A., Planchard D. Antibody-drug conjugates: A promising novel therapeutic approach in lung cancer. Lung Cancer. 2022;163:96–106. doi: 10.1016/j.lungcan.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Li B.T., Smit E.F., Goto Y., Nakagawa K., Udagawa H., Mazières J., Nagasaka M., Bazhenova L., Saltos A.N., Felip E. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. N. Engl. J. Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarantino P., Modi S., Tolaney S.M., Cortés J., Hamilton E.P., Kim S.-B., Toi M., Andrè F., Curigliano G. Interstitial Lung Disease Induced by Anti-ERBB2 Antibody-Drug Conjugates. JAMA Oncol. 2021;7:1873. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z., Shen G., Li J., Qiu T., Fang Q., Zheng Y., Xin Y., Liu Z., Zhao F., Ren D., et al. Incidence of antibody–drug conjugates-related pneumonitis in patients with solid tumors: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2023;184:103960. doi: 10.1016/j.critrevonc.2023.103960. [DOI] [PubMed] [Google Scholar]
- 11.Jänne P.A., Baik C., Su W.-C., Johnson M.L., Hayashi H., Nishio M., Kim D.-W., Koczywas M., Gold K.A., Steuer C.E. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor–resistant, EGFR-mutated non–small cell lung cancer. Cancer Discov. 2022;12:74–89. doi: 10.1158/2159-8290.CD-21-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garon E., Johnson M., Lisberg A., Spira A., Yamamoto N., Heist R., Sands J., Yoh K., Meric-Bernstam F., Kitazono S. LBA49 Efficacy of datopotamab deruxtecan (Dato-DXd) in patients (pts) with advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC) and actionable genomic alterations (AGAs): Preliminary results from the phase I TROPION-PanTumor01 study. Ann. Oncol. 2021;32:S1326–S1327. doi: 10.1016/j.annonc.2021.08.2128. [DOI] [Google Scholar]
- 13.Ricordel C., Barlesi F., Cousin S., Cho B.C., Calvo E., Kim T.M., Helissey C., Kim J.-S., Vieito M., Boni V. Safety and Efficacy of Tusamitamab Ravtansine (SAR408701) in Long-Term Treated Patients with Nonsquamous Non–Small Cell Lung Cancer (NSQ NSCLC) Expressing Carcinoembryonic Antigen-Related Cell Adhesion Molecule 5 (CEACAM5) American Society of Clinical Oncology; Alexandria, VA, USA: 2022. [Google Scholar]
- 14.Camidge D.R., Bar J., Horinouchi H., Goldman J.W., Moiseenko F.V., Filippova E., Cicin I., Bradbury P.A., Daaboul N., Tomasini P. Telisotuzumab Vedotin (Teliso-V) Monotherapy in Patients (pts) with Previously Treated c-Met–Overexpressing (OE) Advanced Non-Small Cell Lung Cancer (NSCLC) American Society of Clinical Oncology; Alexandria, VA, USA: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.C.G.A.R. Network Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pontén F., Jirström K., Uhlen M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008;216:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- 18.Uhlén M., Pontén F., Lindskog C. Charting the human proteome: Understanding disease using a tissue-based atlas. Science. 2015;347:1274. doi: 10.1126/science.347.6227.1274-c. [DOI] [Google Scholar]
- 19.Crystal R.G., Gadek J.E., Ferrans V.J., Fulmer J.D., Line B.R., Hunninghake G.W. Interstitial lung disease: Current concepts of pathogenesis, staging and therapy. Am. J. Med. 1981;70:542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- 20.Kuderer N.M., Desai A., Lustberg M.B., Lyman G.H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022;19:681–697. doi: 10.1038/s41571-022-00685-3. [DOI] [PubMed] [Google Scholar]
- 21.Ozawa Y., Koda K., Akahori D., Matsui T., Hasegawa H., Kakutani T., Amano T., Tanahashi M., Niwa H., Kunimoto Y., et al. Preexisting Interstitial Lung Disease and Lung Injury Associated with Irinotecan in Patients with Neoplasms. Anticancer Res. 2018;38:5937–5941. doi: 10.21873/anticanres.12939. [DOI] [PubMed] [Google Scholar]
- 22.FDA Topotecan: Prescribing Information. 1996. [(accessed on 1 September 2024)]. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=4638.
- 23.Enomoto Y., Inui N., Imokawa S., Karayama M., Hasegawa H., Ozawa Y., Matsui T., Yokomura K., Suda T. Safety of topotecan monotherapy for relapsed small cell lung cancer patients with pre-existing interstitial lung disease. Cancer Chemother. Pharmacol. 2015;76:499–505. doi: 10.1007/s00280-015-2816-6. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai K., Aida T., Tsuchiya Y., Kishino Y., Kai K., Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111:4636–4645. doi: 10.1111/cas.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., Tsurutani J., Ueno N.T., Prat A., Chae Y.S. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., Pegram M., Oh D.-Y., Diéras V., Guardino E. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombo R., Rich J.R. The therapeutic window of antibody drug conjugates: A dogma in need of revision. Cancer Cell. 2022;40:1255–1263. doi: 10.1016/j.ccell.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Tarantino P., Ricciuti B., Pradhan S.M., Tolaney S.M. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat. Rev. Clin. Oncol. 2023;20:558–576. doi: 10.1038/s41571-023-00783-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared up on request.