Abstract
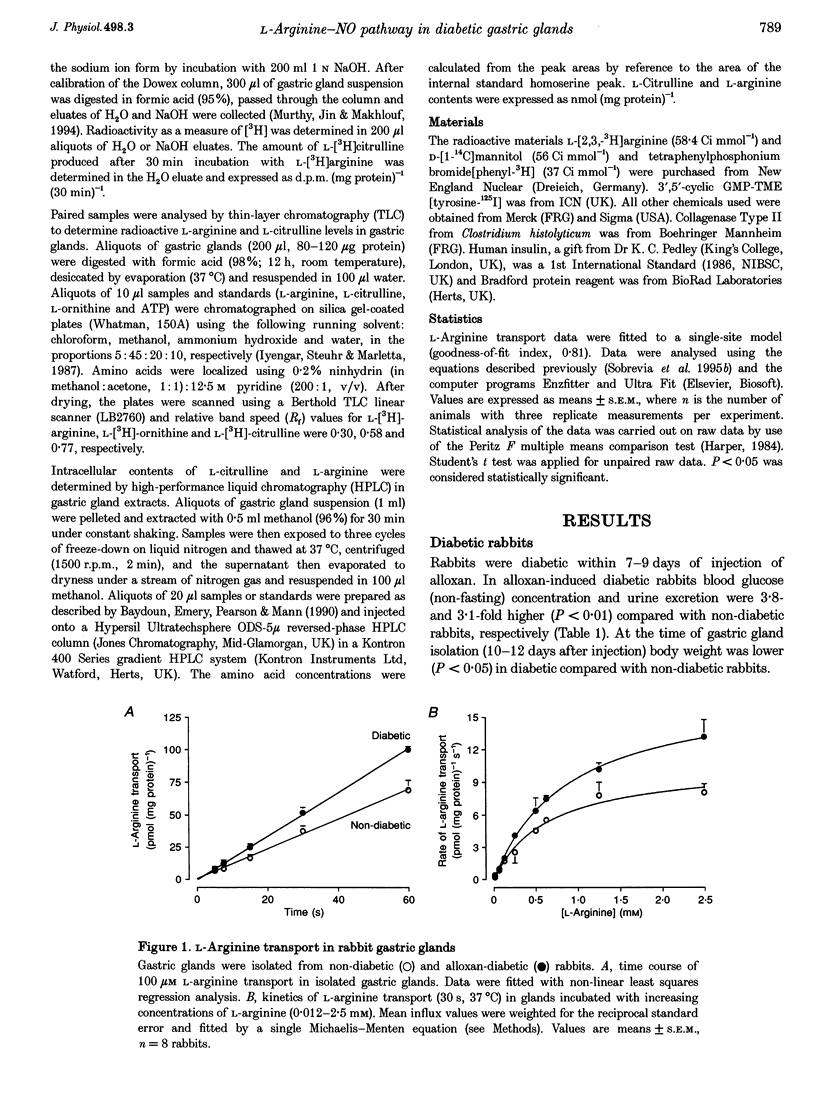
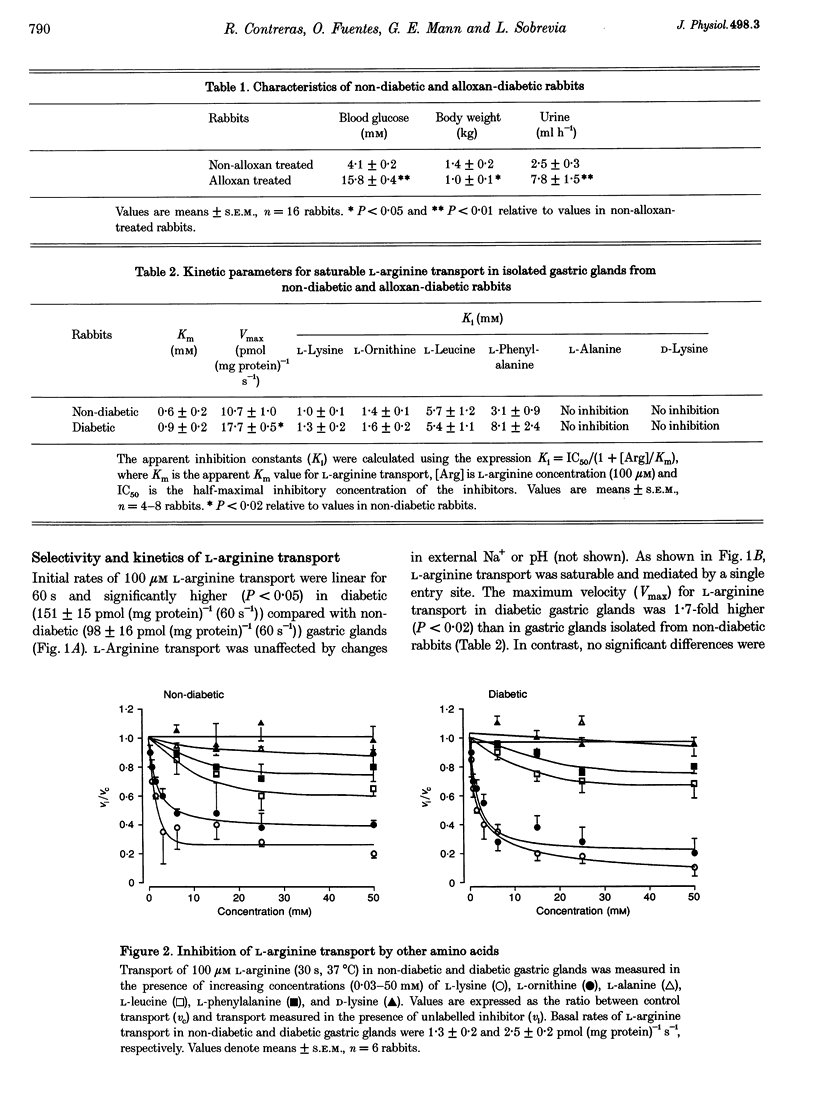
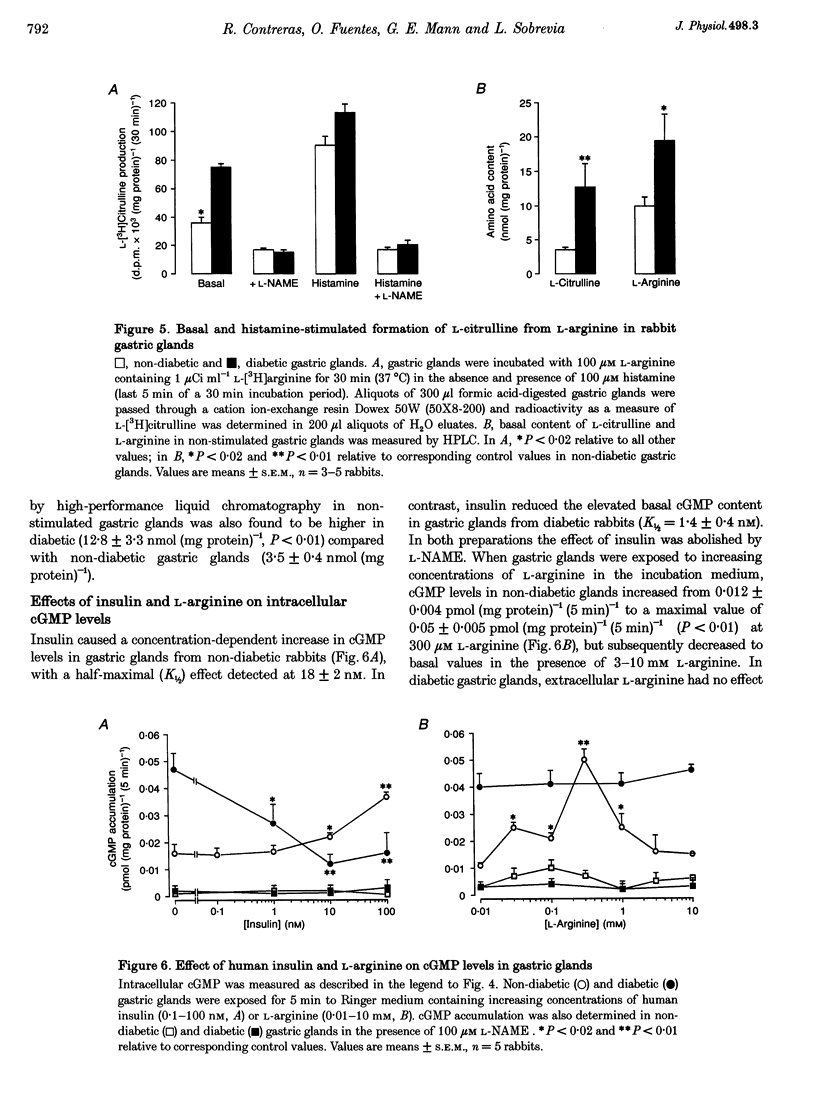
1. The properties of L-arginine transport have been characterized and correlated with cGMP production (index of nitric oxide (NO)) in whole gastric glands isolated from non-diabetic and alloxan-diabetic rabbits. 2. In non-diabetic and diabetic glands, transport of L-arginine was stereoselective, Na+ and pH independent and inhibited by other cationic amino acids. L-Arginine transport was slightly inhibited by L-leucine and L-phenylalanine, but unaffected by other neutral amino acids. 3. Diabetes enhanced the Vmax for saturable L-arginine transport from 10.7 +/- 1.0 to 17.7 +/- 0.5 pmol (mg protein)-1 s-1, with negligible changes in K(m). 4. Accumulation of the membrane potential-sensitive probe tetra[3H]phenylphosphonium (TPP+) was increased 2-fold in diabetic compared with non-diabetic gastric glands, suggesting a membrane hyperpolarization. 5. Basal intracellular cGMP levels were elevated 2-fold in diabetic gastric glands, and in non-diabetic glands histamine, vasoactive intestinal peptide, and bradykinin increased cGMP levels. The NO synthase inhibitor NG-nitro-L-arginine methyl ester (100 microM) abolished basal cGMP accumulation. 6. Addition of extracellular L-arginine induced a concentration-dependent increase in cGMP levels in gastric glands isolated from non-diabetic rabbits, but had no effect on elevated cGMP levels in diabetic glands. 7. Insulin induced a rapid (5 min) concentration-dependent increase in cGMP levels in non-diabetic gastric glands, but reduced elevated cGMP levels in diabetic gastric glands. 8. The present study has identified a specific transport system for L-arginine in gastric glands which resembles the classical system y+. Our findings also provide the first direct evidence that diabetes increases the basal activity of system y+ and NO synthase in gastric glands. The differential modulation of L-arginine transport by insulin and L-arginine identified in non-diabetic and diabetic glands, may be of importance in protecting the gastric mucosa from injuries associated with diabetes.
Full text
PDF

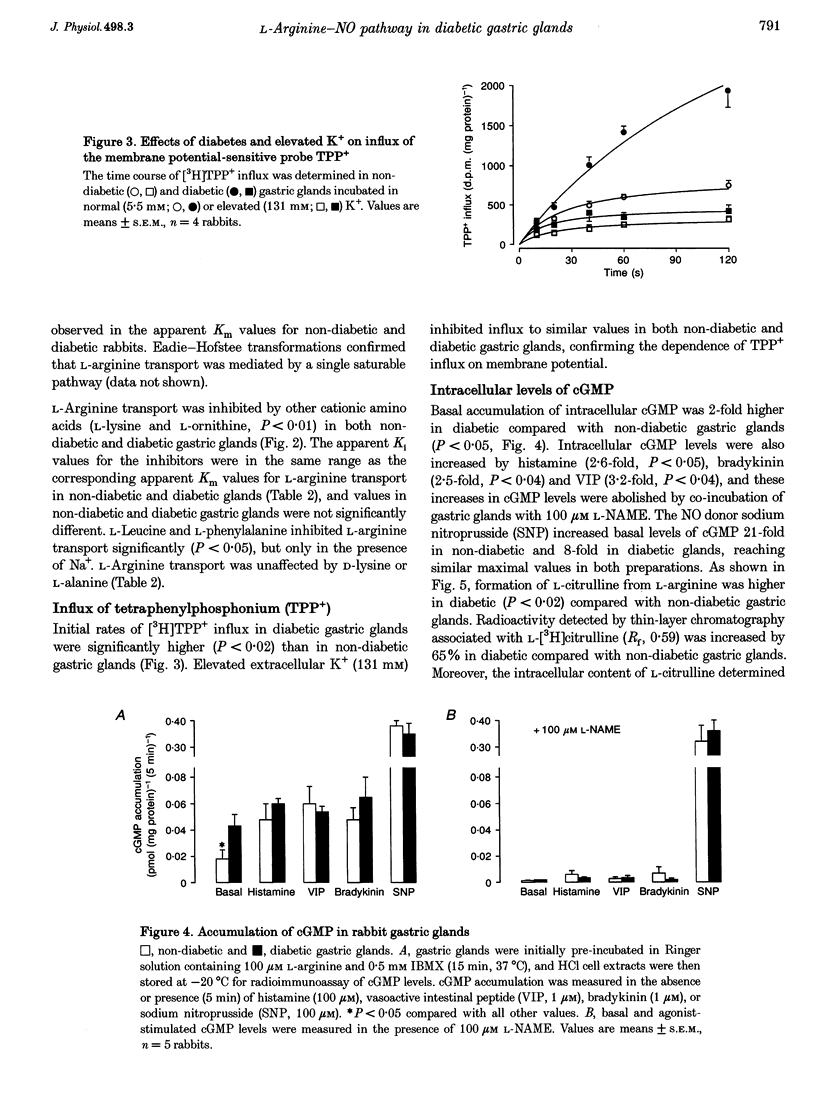




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assreuy J., Moncada S. A perfusion system for the long term study of macrophage activation. Br J Pharmacol. 1992 Oct;107(2):317–321. doi: 10.1111/j.1476-5381.1992.tb12744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona C., Bravo I. L-lysine transport through the basolateral surface of oxyntic glands and plasma membrane of parietal cells isolated from rabbit stomach. Cell Mol Biol (Noisy-le-grand) 1993 Sep;39(6):681–692. [PubMed] [Google Scholar]
- Baydoun A. R., Emery P. W., Pearson J. D., Mann G. E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990 Dec 31;173(3):940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Baydoun A. R., Pearson J. D., Moncada S., Mann G. E. L-arginine transport is increased in macrophages generating nitric oxide. Biochem J. 1992 May 15;284(Pt 1):15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bravo I., Sobrevía L. Kinetics and specificity of L-alanine transport across the basolateral cell surface in isolated oxyntic glands. Biochim Biophys Acta. 1990 Nov 2;1029(1):98–104. doi: 10.1016/0005-2736(90)90441-p. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Brosnan J. T., Man K. C., Hall D. E., Colbourne S. A., Brosnan M. E. Interorgan metabolism of amino acids in streptozotocin-diabetic ketoacidotic rat. Am J Physiol. 1983 Feb;244(2):E151–E158. doi: 10.1152/ajpendo.1983.244.2.E151. [DOI] [PubMed] [Google Scholar]
- Brown J. F., Tepperman B. L., Hanson P. J., Whittle B. J., Moncada S. Differential distribution of nitric oxide synthase between cell fractions isolated from the rat gastric mucosa. Biochem Biophys Res Commun. 1992 Apr 30;184(2):680–685. doi: 10.1016/0006-291x(92)90643-y. [DOI] [PubMed] [Google Scholar]
- Bussolati O., Laris P. C., Nucci F. A., Dall'Asta V., Franchi-Gazzola R., Guidotti G. G., Gazzola G. C. Influx of L-arginine is an indicator of membrane potential in human fibroblasts. Am J Physiol. 1989 Apr;256(4 Pt 1):C930–C935. doi: 10.1152/ajpcell.1989.256.4.C930. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Warner T. D., Bishop A. E., Polak J. M., Vane J. R. Nitric oxide, and not vasoactive intestinal peptide, as the main neurotransmitter of vagally induced relaxation of the guinea pig stomach. Br J Pharmacol. 1994 Dec;113(4):1197–1202. doi: 10.1111/j.1476-5381.1994.tb17124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R., Chavez P., Boyd C. A. Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol. 1992 Aug;454:491–501. doi: 10.1113/jphysiol.1992.sp019275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigel D. O., Metz D. C. Prevalence, etiology, and prognostic significance of upper gastrointestinal hemorrhage in diabetic ketoacidosis. Dig Dis Sci. 1996 Jan;41(1):1–8. doi: 10.1007/BF02208576. [DOI] [PubMed] [Google Scholar]
- Handlogten M. E., Kilberg M. S. Induction and decay of amino acid transport in the liver. Turnover of transport activity in isolated hepatocytes after stimulation by diabetes or glucagon. J Biol Chem. 1984 Mar 25;259(6):3519–3525. [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaap A. J., Tooke J. E. Pathophysiology of microvascular disease in non-insulin-dependent diabetes. Clin Sci (Lond) 1995 Jul;89(1):3–12. doi: 10.1042/cs0890003. [DOI] [PubMed] [Google Scholar]
- Jackson C. J., Garbett P. K., Nissen B., Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J Cell Sci. 1990 Jun;96(Pt 2):257–262. doi: 10.1242/jcs.96.2.257. [DOI] [PubMed] [Google Scholar]
- Jebbink H. J., Bravenboer B., Akkermans L. M., vanBerge-Henegouwen G. P., Smout A. J. Relationships between dyspeptic symptoms and gastrointestinal motility in patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993 Oct;36(10):948–954. doi: 10.1007/BF02374478. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P., Drenckhahn D. Intrinsic source of stomach NO. Nature. 1994 Jul 7;370(6484):25–26. doi: 10.1038/370025a0. [DOI] [PubMed] [Google Scholar]
- Malandro M. S., Kilberg M. S. Molecular biology of mammalian amino acid transporters. Annu Rev Biochem. 1996;65:305–336. doi: 10.1146/annurev.bi.65.070196.001513. [DOI] [PubMed] [Google Scholar]
- Middleton S. J., Reynolds P. D., Shorthouse M., Hunter J. O., Moss S. Nitric oxide synthase in gastric mucosa. Gut. 1995 Jun;36(6):942–942. doi: 10.1136/gut.36.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrevia L., Cesare P., Yudilevich D. L., Mann G. E. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: association with membrane hyperpolarization. J Physiol. 1995 Nov 15;489(Pt 1):183–192. doi: 10.1113/jphysiol.1995.sp021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrevia L., Nadal A., Yudilevich D. L., Mann G. E. Activation of L-arginine transport (system y+) and nitric oxide synthase by elevated glucose and insulin in human endothelial cells. J Physiol. 1996 Feb 1;490(Pt 3):775–781. doi: 10.1113/jphysiol.1996.sp021185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrevía L., Medina V., Reinicke K., Bravo I. Uptake of L-leucine and L-phenylalanine across the basolateral cell surface in isolated oxyntic glands. Biochim Biophys Acta. 1992 May 21;1106(2):257–263. doi: 10.1016/0005-2736(92)90004-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Kojima Y., Tsunoda Y., Beyer L. A., Kamijo M., Sima A. A., Owyang C. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol. 1996 Mar;270(3 Pt 1):G411–G417. doi: 10.1152/ajpgi.1996.270.3.G411. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Soper B. D. Interaction of nitric oxide and salivary gland epidermal growth factor in the modulation of rat gastric mucosal integrity. Br J Pharmacol. 1993 Sep;110(1):229–234. doi: 10.1111/j.1476-5381.1993.tb13797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttenthal L. O., Blázquez E. Characterization of high-affinity receptors for truncated glucagon-like peptide-1 in rat gastric glands. FEBS Lett. 1990 Mar 12;262(1):139–141. doi: 10.1016/0014-5793(90)80173-g. [DOI] [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990 Mar;99(3):607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993 Jul;49(3):642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- Wu G., Flynn N. E. The activation of the arginine-citrulline cycle in macrophages from the spontaneously diabetic BB rat. Biochem J. 1993 Aug 15;294(Pt 1):113–118. doi: 10.1042/bj2940113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Robinson D., Kung H. J., Hatzoglou M. Hormonal regulation of the gene for the type C ecotropic retrovirus receptor in rat liver cells. J Virol. 1994 Mar;68(3):1615–1623. doi: 10.1128/jvi.68.3.1615-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de'Angelis G. L., Apollionio G., Boselli E., De Fanti A., De Luca F., Fulgido S., Romanini E., Vanelli M. Anticorps antiestomac et gastrite au cours du diabète insulino-dépendant de l'enfant. Arch Fr Pediatr. 1993 Jun-Jul;50(6):475–478. [PubMed] [Google Scholar]


