Abstract
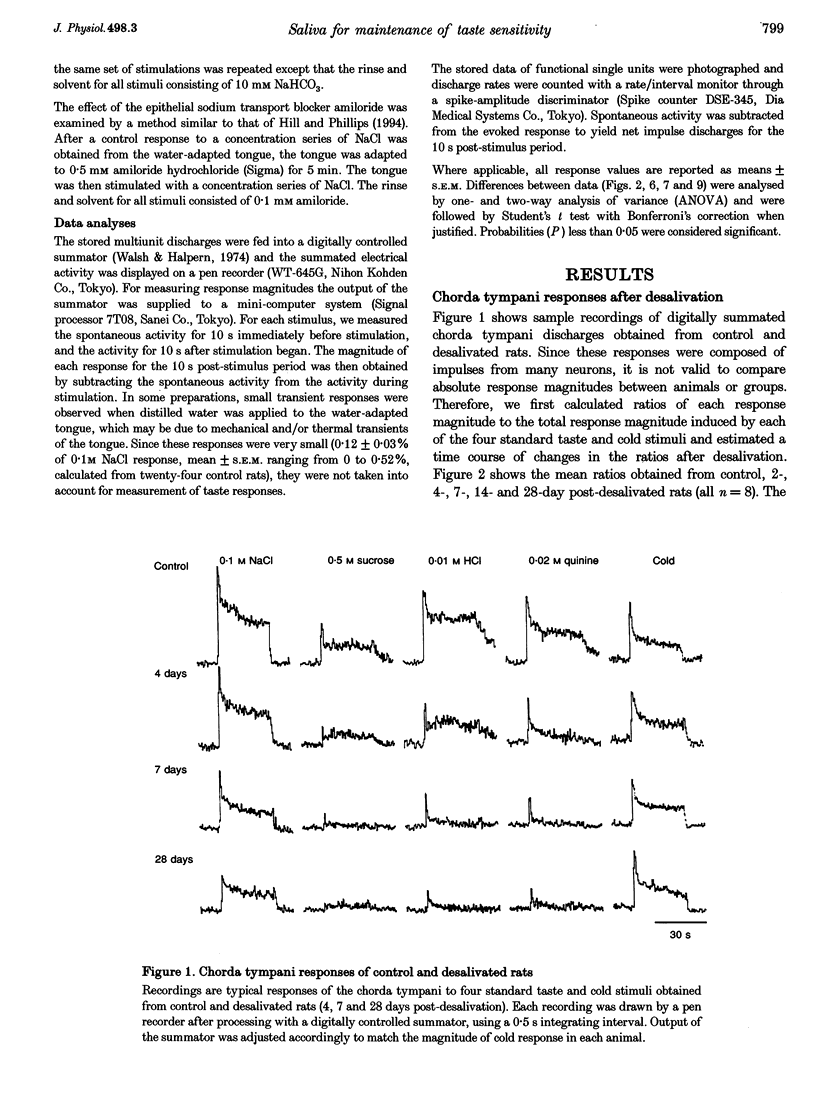
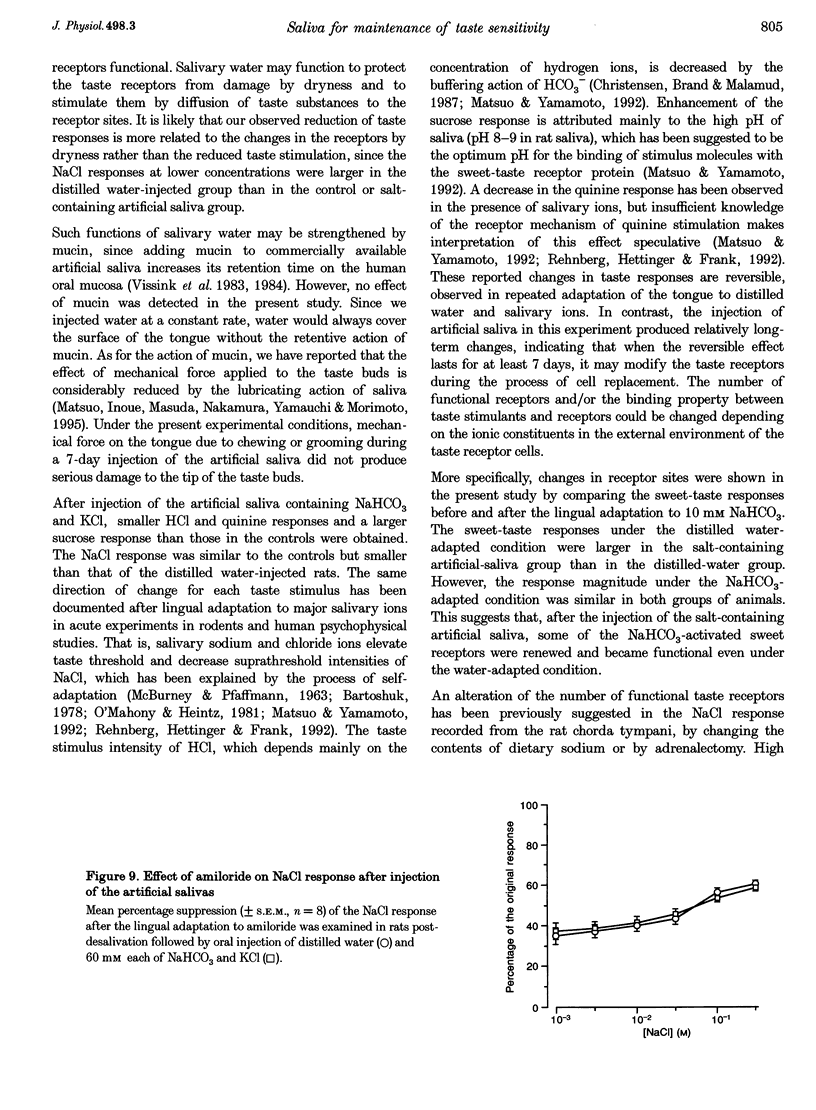
1. To evaluate the role of saliva in the maintenance of taste sensitivity, the activities in the rat chorda tympani innervating taste buds in the anterior part of the tongue were analysed. The effects of chronic extirpation of the submandibular and sublingual salivary glands were tested and compared with results after chronic oral administration of artificial saliva. 2. Removal of the salivary glands sharply decreased chorda tympani responses to four different taste stimuli by 7 days post-desalivation, while a stable response to cold water was observed by at least 28 days. 3. This selective decrease in taste responses was considerably recovered by 7-day-oral injection of artificial saliva (containing NaHCO3, KCl and/or mucin) or distilled water. However, the injection of the salt-containing artificial saliva induced significantly larger sucrose and smaller NaCl, HCl and quinine responses than did the injection of distilled water. 4. In our salivary manipulations, an alteration in the number of the functional sweet receptors was suggested by the cross-adaptation technique using NaHCO3, whereas sensitivity to the epithelial sodium transport blocker, amiloride, was stable in the NaCl response. 5. Salivary water and electrolytes which may participate in forming the external environment of the taste receptor cells modulated taste sensitivity in the chorda tympani.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Hidaka S., Ishibashi K., Yanabu M., Kamogashira K., Itoh T., Matsumoto M. Developmental changes in the volumes, protein, and some electrolyte concentrations of male and female rat submandibular saliva secreted in response to methoxamine and pilocarpine. J Dent Res. 1987 Mar;66(3):745–750. doi: 10.1177/00220345870660030801. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. M. The psychophysics of taste. Am J Clin Nutr. 1978 Jun;31(6):1068–1077. doi: 10.1093/ajcn/31.6.1068. [DOI] [PubMed] [Google Scholar]
- Beidler L. M., Smallman R. L. Renewal of cells within taste buds. J Cell Biol. 1965 Nov;27(2):263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosvic G. M., Hoey N. E. Dietary sodium deprivation does not alter taste sensitivity in the rat. Physiol Behav. 1990 May;47(5):881–888. doi: 10.1016/0031-9384(90)90013-t. [DOI] [PubMed] [Google Scholar]
- Brosvic G. M., Hoey N. E. Taste detection and discrimination performance of rats following selective desalivation. Physiol Behav. 1990 Nov;48(5):617–623. doi: 10.1016/0031-9384(90)90200-n. [DOI] [PubMed] [Google Scholar]
- Cano J., Rodríguez-Echandía E. L. Degenerating taste buds in sialectomized rats. Acta Anat (Basel) 1980;106(4):487–492. doi: 10.1159/000145218. [DOI] [PubMed] [Google Scholar]
- Catalanotto F. A., Sweeney E. A. Long-term effects of selective desalivation on taste acuity in rats. Arch Oral Biol. 1973 Aug;18(8):941–952. doi: 10.1016/0003-9969(73)90174-x. [DOI] [PubMed] [Google Scholar]
- Catalanotto F. A., Sweeney E. A. Salivary sodium and potassium concentrations in adrenalectomized rats. Behav Biol. 1978 Dec;24(4):467–473. doi: 10.1016/s0091-6773(78)90803-9. [DOI] [PubMed] [Google Scholar]
- Catalanotto F. A., Sweeney E. A. The effects of surgical desalivation of the rat upon taste acuity. Arch Oral Biol. 1972 Oct;17(10):1455–1465. doi: 10.1016/0003-9969(72)90105-7. [DOI] [PubMed] [Google Scholar]
- Christensen C. M., Brand J. G., Malamud D. Salivary changes in solution pH: a source of individual differences in sour taste perception. Physiol Behav. 1987;40(2):221–227. doi: 10.1016/0031-9384(87)90211-3. [DOI] [PubMed] [Google Scholar]
- Compton J., Martinez J. R., Martinez A. M., Young J. A. Fluid and electrolyte secretion from the isolated, perfused submandibular and sublingual glands of the rat. Arch Oral Biol. 1981;26(7):555–561. doi: 10.1016/0003-9969(81)90017-0. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Catalanotto F. A. Sodium deprivation in rats: salt thresholds are related to salivary sodium concentrations. Behav Neural Biol. 1980 Jul;29(3):303–314. doi: 10.1016/s0163-1047(80)90183-1. [DOI] [PubMed] [Google Scholar]
- Contreras R. J. Changes in gustatory nerve discharges with sodium deficiency: a single unit analysis. Brain Res. 1977 Feb;121(2):373–378. doi: 10.1016/0006-8993(77)90162-7. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979 May;73(5):569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili D., Maller O., Brightman V. J. The effects of desalivation by duct ligation or salivary gland extirpation on taste preference in rats. Arch Oral Biol. 1981;26(11):853–858. doi: 10.1016/0003-9969(81)90142-4. [DOI] [PubMed] [Google Scholar]
- Herness M. S. Aldosterone increases the amiloride-sensitivity of the rat gustatory neural response to NaCl. Comp Biochem Physiol Comp Physiol. 1992 Oct;103(2):269–273. doi: 10.1016/0300-9629(92)90578-e. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Phillips L. M. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994 May;14(5 Pt 1):2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. L., Przekop P. R., Jr Influences of dietary sodium on functional taste receptor development: a sensitive period. Science. 1988 Sep 30;241(4874):1826–1828. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- Hill D. L. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol. 1987 Dec;393:413–424. doi: 10.1113/jphysiol.1987.sp016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T., Contreras R. J. Adrenalectomy reduces peripheral neural responses to gustatory stimuli in the rat. Behav Neurosci. 1985 Aug;99(4):734–741. doi: 10.1037//0735-7044.99.4.734. [DOI] [PubMed] [Google Scholar]
- Martinez J. R., Quissell D. O., Wood D. L., Giles M. Abnormal secretory response to parasympathomimetic and sympathomimetic stimulations from the submaxillary gland of rats treated with reserpine. J Pharmacol Exp Ther. 1975 Aug;194(2):384–395. [PubMed] [Google Scholar]
- Matsuo R., Inoue T., Masuda Y., Nakamura O., Yamauchi Y., Morimoto T. Neural activity of chorda tympani mechanosensitive fibers during licking behavior in rats. Brain Res. 1995 Aug 21;689(2):289–298. doi: 10.1016/0006-8993(95)00582-b. [DOI] [PubMed] [Google Scholar]
- Matsuo R., Yamamoto T. Effects of inorganic constituents of saliva on taste responses of the rat chorda tympani nerve. Brain Res. 1992 Jun 26;583(1-2):71–80. doi: 10.1016/s0006-8993(10)80010-1. [DOI] [PubMed] [Google Scholar]
- Matsuo R., Yamamoto T., Ikehara A., Nakamura O. Effect of salivation on neural taste responses in freely moving rats: analyses of salivary secretion and taste responses of the chorda tympani nerve. Brain Res. 1994 Jun 27;649(1-2):136–146. doi: 10.1016/0006-8993(94)91057-x. [DOI] [PubMed] [Google Scholar]
- Matsuo R., Yamamoto T. Taste nerve responses during licking behavior in rats: importance of saliva in responses to sweeteners. Neurosci Lett. 1990 Jan 1;108(1-2):121–126. doi: 10.1016/0304-3940(90)90717-n. [DOI] [PubMed] [Google Scholar]
- Nanda R., Catalanotto F. A. Long-term effects of surgical desalivation upon taste acuity, fluid intake, and taste buds in the rat. J Dent Res. 1981 Jan;60(1):69–76. doi: 10.1177/00220345810600011401. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y., Katsukawa H., Funakoshi M. Alteration of salt taste sensitivity by the neonatal removal of sublingual glands in the rat. Comp Biochem Physiol A Comp Physiol. 1989;94(1):89–93. doi: 10.1016/0300-9629(89)90789-5. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Sato M., Yamashita S. Multiple sensitivity of chordat typani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol. 1968 Nov;199(1):223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissink A., Waterman H. A., s-Gravenmade E. J., Panders A. K., Vermey A. Rheological properties of saliva substitutes containing mucin, carboxymethylcellulose or polyethylenoxide. J Oral Pathol. 1984 Feb;13(1):22–28. doi: 10.1111/j.1600-0714.1984.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Vissink A., s-Gravenmade E. J., Panders A. K., Vermey A., Petersen J. K., Visch L. L., Schaub R. M. A clinical comparison between commercially available mucin- and CMC-containing saliva substitutes. Int J Oral Surg. 1983 Aug;12(4):232–238. doi: 10.1016/s0300-9785(83)80048-9. [DOI] [PubMed] [Google Scholar]
- Walsh L. F., Halpern B. P. Digitally controlled summator for quantizing multiunit responses. J Appl Physiol. 1974 Nov;37(5):748–751. doi: 10.1152/jappl.1974.37.5.748. [DOI] [PubMed] [Google Scholar]


