Abstract
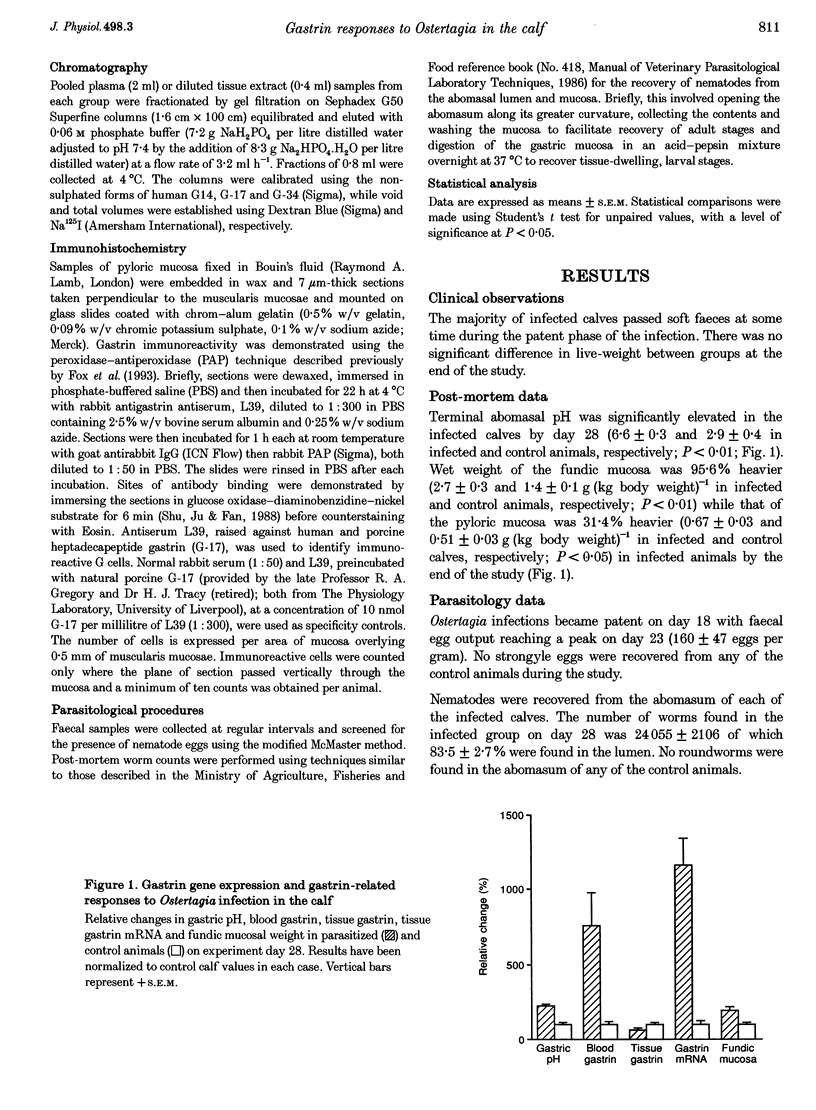
1. Infection with the bovine abomasal nematode Ostertagia ostertagi results in a loss of acid-secreting parietal cells and an increase in gastric pH. The effects of an experimental infection on gastrin mRNA expression, blood and tissue gastrin concentrations, the different molecular forms of gastrin in each, and pyloric mucosal chromogranin A-derived peptides were investigated in the calf. 2. An increase in blood gastrin concentrations in the infected group reached a peak by day 28 postinfection (635 pg ml-1; P < 0.01). Gel chromatography analysis of blood samples revealed that the hypergastrinaemia comprised largely gastrin-34 (G-34) in parasitized calves while gastrin-17 (G-17) predominated in control animals. 3. An 11-fold increase in gastrin mRNA expression was recorded in the parasitized animals which was accompanied by a 23.8% reduction in pyloric mucosal gastrin content and an apparent drop of 24.7% in the number of gastrin-producing G cells detected. There was no major change in the relative abundance of G-17 and G-34 in the pyloric mucosa of infected calves. No significant differences in the concentration of pyloric mucosal chromogranin A-derived peptides were recorded between infected and control groups. 4. These data suggest that the hypergastrinaemia seen in parasitized calves results largely from an increase in gastrin synthesis and that depletion of previously stored peptide makes virtually no contribution to elevated blood gastrin concentrations.
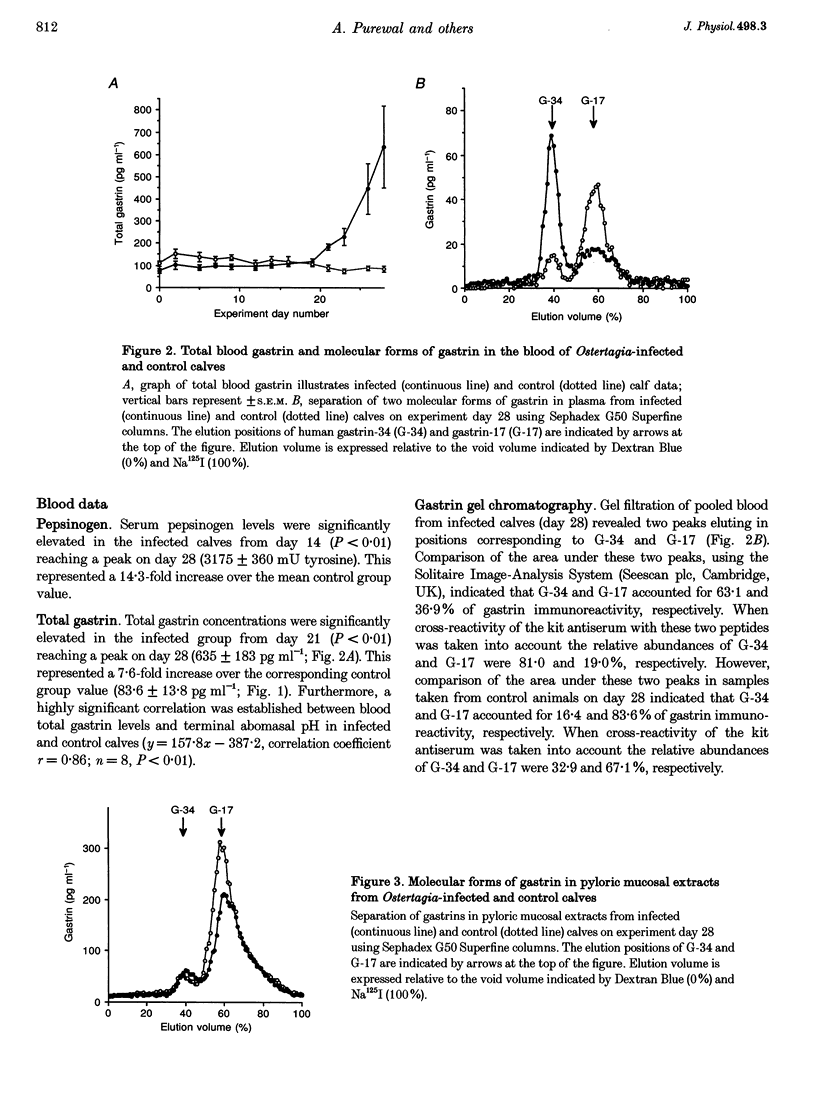
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N., Reynolds G. W., Titchen D. A. Changes in gastrointestinal mucosal mass and mucosal and serum gastrin in sheep experimentally infected with Ostertagia circumcincta. Int J Parasitol. 1988 Apr;18(3):325–331. doi: 10.1016/0020-7519(88)90141-5. [DOI] [PubMed] [Google Scholar]
- Cetin Y., Bargsten G., Grube D. Mutual relationships between chromogranins A and B and gastrin in individual gastrin cells. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2912–2916. doi: 10.1073/pnas.89.7.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dimaline R. Post-translational modification of peptide messengers in the gut. Q J Exp Physiol. 1988 Nov;73(6):873–902. doi: 10.1113/expphysiol.1988.sp003224. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Hamer C., Evans D., Varro A., Dimaline R. The secretory kinetics of the G cell in omeprazole-treated rats. Gastroenterology. 1991 May;100(5 Pt 1):1187–1194. [PubMed] [Google Scholar]
- Fox M. T., Gerrelli D., Pitt S. R., Jacobs D. E., Hart I. C., Simmonds A. D. Endocrine effects of a single infection with Ostertagia ostertagi in the calf. Int J Parasitol. 1987 Aug;17(6):1181–1185. doi: 10.1016/0020-7519(87)90170-6. [DOI] [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Halliday G. J., Mulligan W. Parasitic hypoalbuminaemia: studies on type II ostertagiasis of cattle. Res Vet Sci. 1968 May;9(3):224–230. [PubMed] [Google Scholar]
- Lewis J. J., Zdon M. J., Adrian T. E., Modlin I. M. Pancreastatin: a novel peptide inhibitor of parietal cell secretion. Surgery. 1988 Dec;104(6):1031–1036. [PubMed] [Google Scholar]
- Lund T., Olsen J., Rehfeld J. F. Cloning and sequencing of the bovine gastrin gene. Mol Endocrinol. 1989 Oct;3(10):1585–1588. doi: 10.1210/mend-3-10-1585. [DOI] [PubMed] [Google Scholar]
- Murray M., Jennings F. W., Armour J. Bovine ostertagiasis: structure, function and mode of differentiation of the bovine gastric mucosa and kinetics of the worm loss. Res Vet Sci. 1970 Sep;11(5):417–427. [PubMed] [Google Scholar]
- Mylrea P. J., Hotson I. K. Serum pepsinogen activity and the diagnosis of bovine ostertagiasis. Br Vet J. 1969 Aug;125(8):379–388. doi: 10.1016/s0007-1935(17)48808-7. [DOI] [PubMed] [Google Scholar]
- Pauwels S., Dockray G. J., Walker R. Comparison of the metabolism of sulfated and unsulfated heptadecapeptide gastrin in humans. Gastroenterology. 1987 May;92(5 Pt 1):1220–1225. doi: 10.1016/s0016-5085(87)91081-x. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Watkinson A., Jönsson A. C., Davison M., Young J., Lee C. M., Moore S., Dockray G. J. Heterogeneity of chromogranin A-derived peptides in bovine gut, pancreas and adrenal medulla. Biochem J. 1991 Jun 1;276(Pt 2):471–479. doi: 10.1042/bj2760471. [DOI] [PMC free article] [PubMed] [Google Scholar]


