Abstract
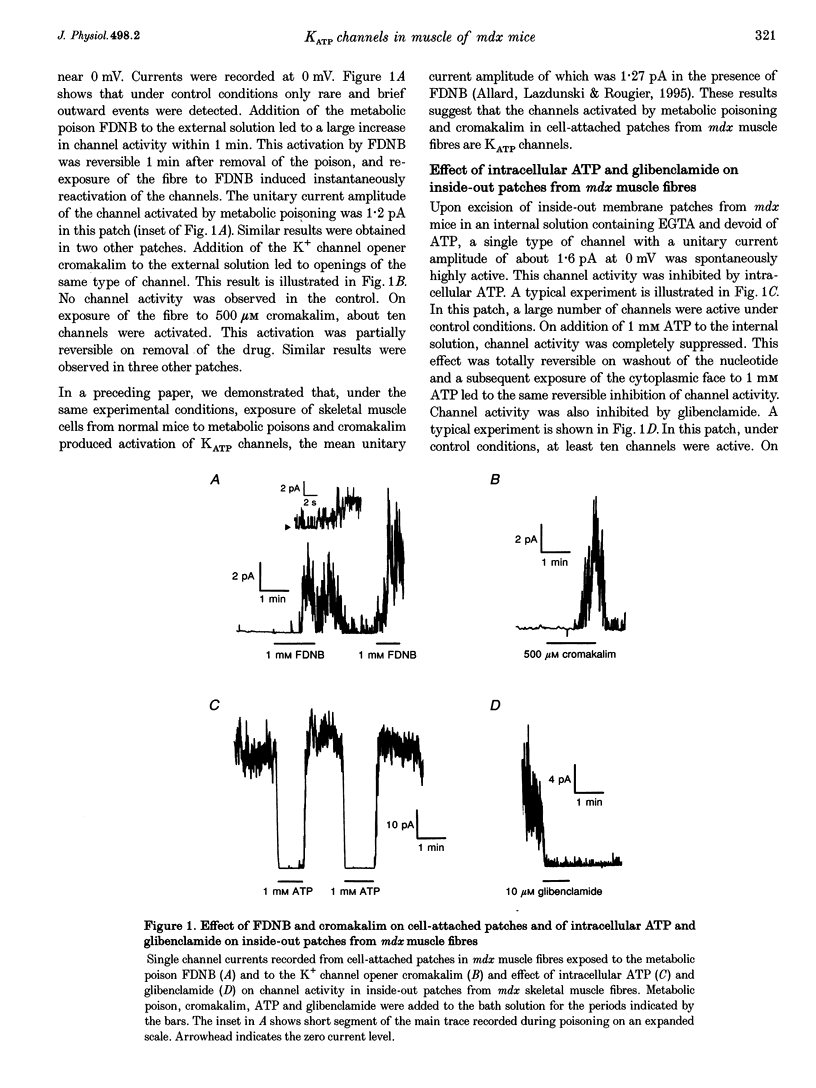
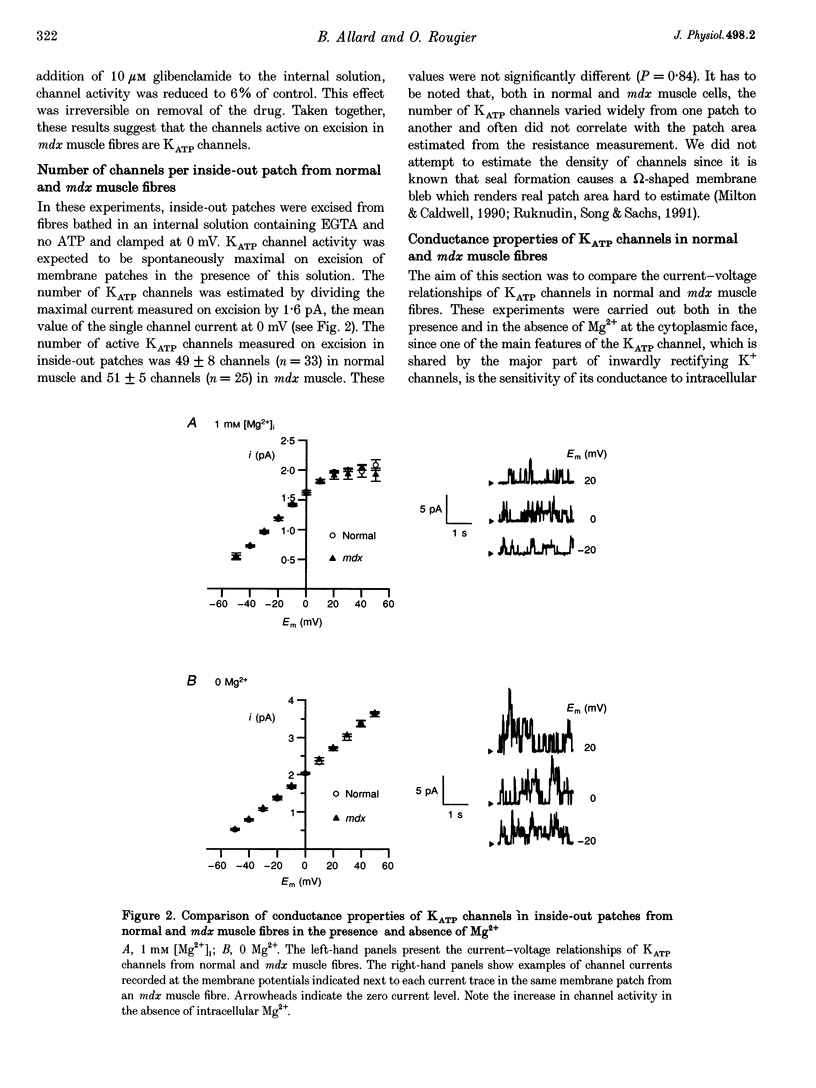
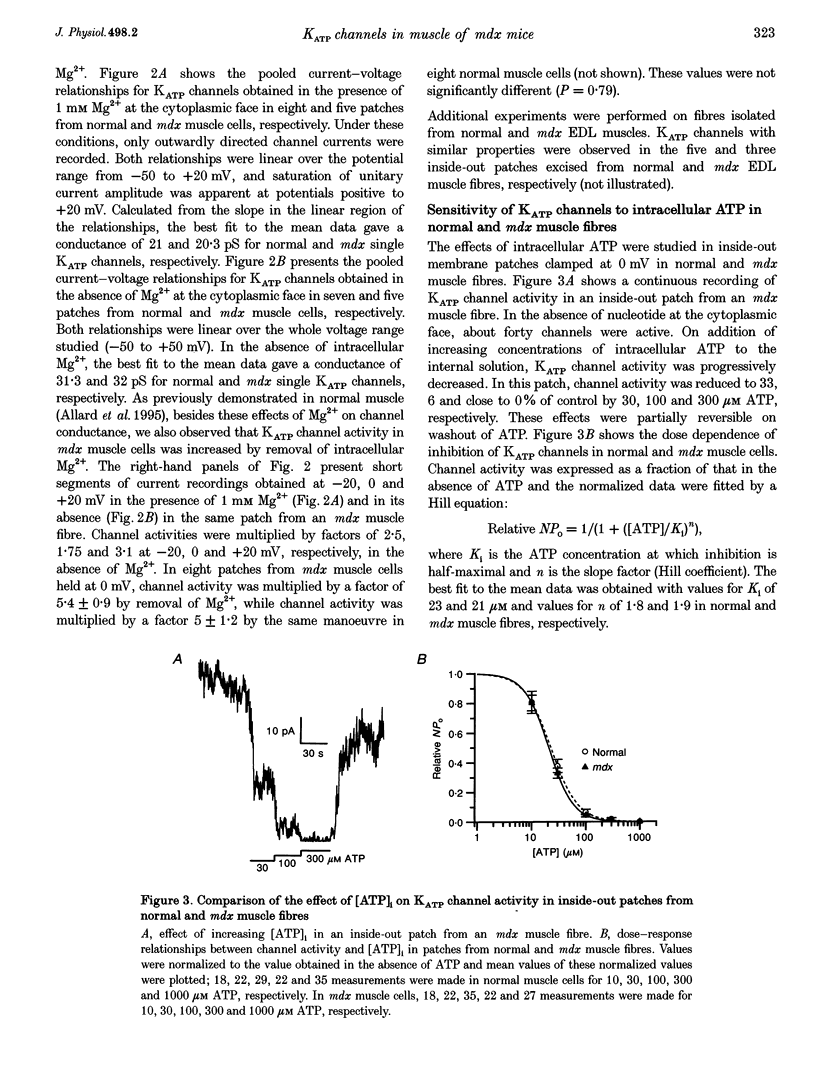
1. ATP-dependent K+ (KATP) channels were studied in fibres isolated from flexor digitorum brevis and interosseal skeletal muscles of normal and mutant mdx mice using the patch clamp technique in the presence of asymmetrical K+ concentrations (5 mM K+ in the pipette and in vivo intracellular [K+] or 145 mM K+ at the cytoplasmic face). 2. In cell-attached patches from mdx muscle fibres bathed in K(+)-rich solution, cell poisoning with fluorodinitrobenzene induced partially reversible opening of channels carrying an outward current of an amplitude of 1.2 pA at 0 mV. Exposure of fibres to the K+ channel opener cromakalim led to opening of the same type of channel. These channels were assumed to be KATP channels. 3. On excision of inside-out patches from mdx muscle fibres, in the absence of intracellular ATP, KATP channels were active: they carried a unitary outward current of 1.6 pA at 0 mV and were inhibited by intracellular ATP and glibenclamide. The number of KATP channels per patch was not significantly different in muscles from normal and mdx mice. 4. In inside-out patches, in the presence of 1 mM intracellular Mg2+, slope conductances of 21 and 20.3 pS were found for KATP channels in normal and mdx muscle, respectively. In the absence of Mg2+, slope conductances of KATP channels were 31.3 and 32 pS in normal and mdx muscle, respectively and KATP channel activity was augmented in mdx muscle in the same way as in normal muscle. Activity of the same KATP channel was observed in extensor digitorum longus muscle from normal and mdx mice. 5. In inside-out patches held at 0 mV, the relationship between KATP channel activity and intracellular ATP was described by a Hill equation: Ki values were 23 and 21 microM and Hill coefficients were 1.8 and 1.9 in normal and mdx muscle, respectively. 6. These results indicate that the distribution, the conductance properties and ATP sensitivity of KATP channels do not differ in normal and in mdx mouse skeletal muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard B., Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflugers Arch. 1992 Nov;422(2):185–192. doi: 10.1007/BF00370419. [DOI] [PubMed] [Google Scholar]
- Allard B., Lazdunski M., Rougier O. Activation of ATP-dependent K+ channels by metabolic poisoning in adult mouse skeletal muscle: role of intracellular Mg(2+) and pH. J Physiol. 1995 Jun 1;485(Pt 2):283–296. doi: 10.1113/jphysiol.1995.sp020730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Berthier C., Amsellem J., Blaineau S. Visualization of the subsarcolemmal cytoskeleton network of mouse skeletal muscle cells by en face views and application to immunoelectron localization of dystrophin. J Muscle Res Cell Motil. 1995 Oct;16(5):553–566. doi: 10.1007/BF00126439. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Standen N. B., Stanfield P. R. The effect of intracellular pH on ATP-dependent potassium channels of frog skeletal muscle. J Physiol. 1992 Jan;445:549–568. doi: 10.1113/jphysiol.1992.sp018939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. W., Standen N. B., Stanfield P. R. ATP-dependent potassium channels of muscle cells: their properties, regulation, and possible functions. J Bioenerg Biomembr. 1991 Aug;23(4):509–535. doi: 10.1007/BF00785809. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP-sensitive K+ channels in rat ventricular myocytes are blocked and inactivated by internal divalent cations. Pflugers Arch. 1987 Oct;410(3):313–320. doi: 10.1007/BF00580282. [DOI] [PubMed] [Google Scholar]
- Findlay I. ATP4- and ATP.Mg inhibit the ATP-sensitive K+ channel of rat ventricular myocytes. Pflugers Arch. 1988 Jul;412(1-2):37–41. doi: 10.1007/BF00583729. [DOI] [PubMed] [Google Scholar]
- Findlay I. The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J Physiol. 1987 Oct;391:611–629. doi: 10.1113/jphysiol.1987.sp016759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Yamane T., Terai T., Katayama Y., Hiraoka M. Functional linkage of the cardiac ATP-sensitive K+ channel to the actin cytoskeleton. Pflugers Arch. 1996 Feb;431(4):504–512. doi: 10.1007/BF02191896. [DOI] [PubMed] [Google Scholar]
- Galli A., DeFelice L. J. Inactivation of L-type Ca channels in embryonic chick ventricle cells: dependence on the cytoskeletal agents colchicine and taxol. Biophys J. 1994 Dec;67(6):2296–2304. doi: 10.1016/S0006-3495(94)80715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws C. M., Lansman J. B. Developmental regulation of mechanosensitive calcium channels in skeletal muscle from normal and mdx mice. Proc Biol Sci. 1991 Sep 23;245(1314):173–177. doi: 10.1098/rspb.1991.0105. [DOI] [PubMed] [Google Scholar]
- Head S. I., Williams D. A., Stephenson D. G. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci. 1992 May 22;248(1322):163–169. doi: 10.1098/rspb.1992.0058. [DOI] [PubMed] [Google Scholar]
- Hocherman S. D., Bezanilla F. A patch-clamp study of delayed rectifier currents in skeletal muscle of control and mdx mice. J Physiol. 1996 May 15;493(Pt 1):113–128. doi: 10.1113/jphysiol.1996.sp021368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Byerly L. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+. Neuron. 1993 May;10(5):797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994 Jan;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- McKillen H. C., Davies N. W., Stanfield P. R., Standen N. B. The effect of intracellular anions on ATP-dependent potassium channels of rat skeletal muscle. J Physiol. 1994 Sep 15;479(Pt 3):341–351. doi: 10.1113/jphysiol.1994.sp020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A., Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991 Jan 3;349(6304):69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Milton R. L., Caldwell J. H. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 1990 Aug;416(6):758–762. doi: 10.1007/BF00370626. [DOI] [PubMed] [Google Scholar]
- Petrof B. J., Shrager J. B., Stedman H. H., Kelly A. M., Sweeney H. L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A. G., Xiao Y. F., Ausiello D. A., Cantiello H. F. cAMP-independent regulation of CFTR by the actin cytoskeleton. Am J Physiol. 1995 Jun;268(6 Pt 1):C1552–C1561. doi: 10.1152/ajpcell.1995.268.6.C1552. [DOI] [PubMed] [Google Scholar]
- Ruknudin A., Song M. J., Sachs F. The ultrastructure of patch-clamped membranes: a study using high voltage electron microscopy. J Cell Biol. 1991 Jan;112(1):125–134. doi: 10.1083/jcb.112.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert E. M., Mills J. W., Stanton B. A. Actin-based cytoskeleton regulates a chloride channel and cell volume in a renal cortical collecting duct cell line. J Biol Chem. 1994 Mar 11;269(10):7081–7089. [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Miyazaki K., Ikeda M., Kawaguchi Y., Sakai O. F-actin network may regulate a Cl- channel in renal proximal tubule cells. J Membr Biol. 1993 May;134(1):31–39. doi: 10.1007/BF00233473. [DOI] [PubMed] [Google Scholar]
- Terzic A., Kurachi Y. Actin microfilament disrupters enhance K(ATP) channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996 Apr 15;492(Pt 2):395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. R., Fong P. Y., Denetclaw W. F., Steinhardt R. A. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991 Dec;115(6):1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. H., Cassola A., Giebisch G. Involvement of actin cytoskeleton in modulation of apical K channel activity in rat collecting duct. Am J Physiol. 1994 Oct;267(4 Pt 2):F592–F598. doi: 10.1152/ajprenal.1994.267.4.F592. [DOI] [PubMed] [Google Scholar]


