Abstract
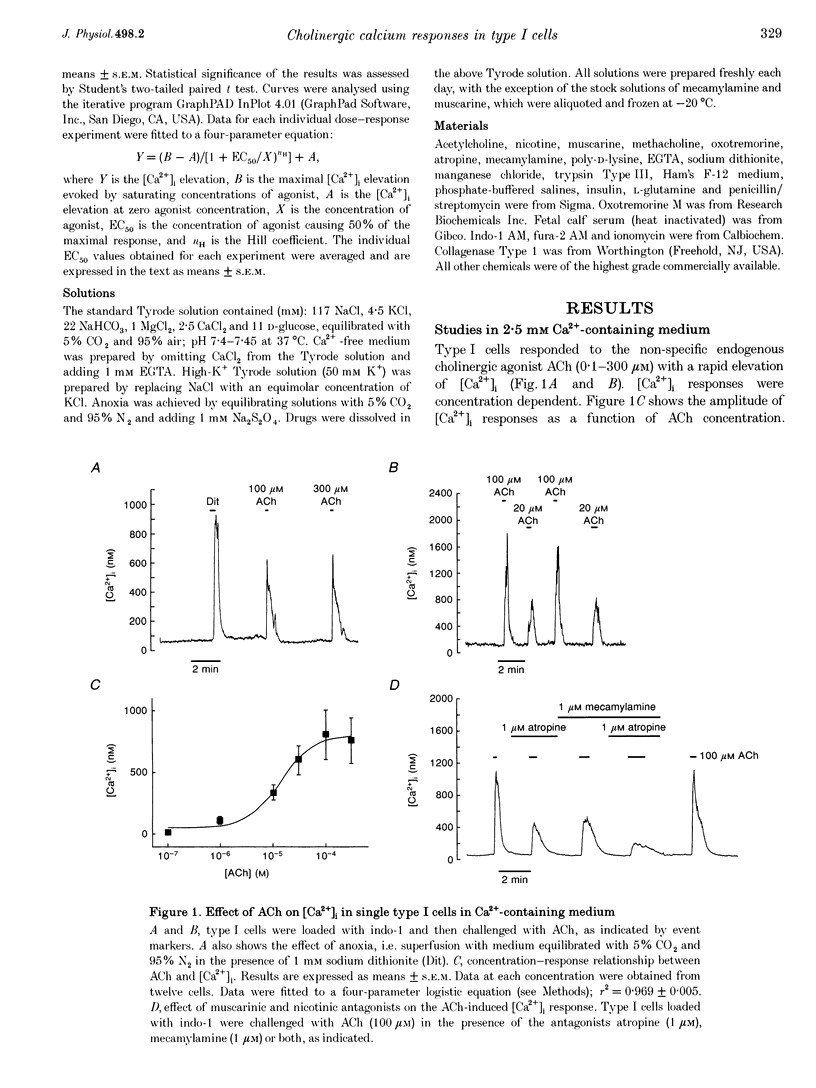
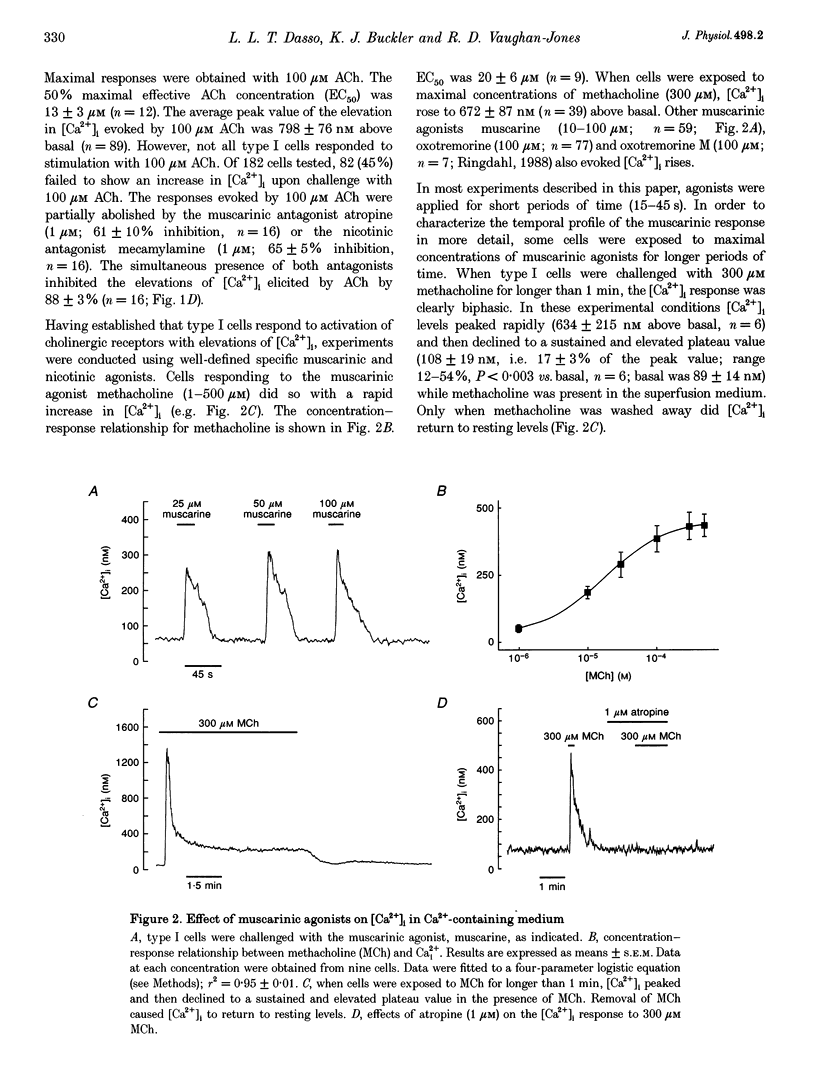
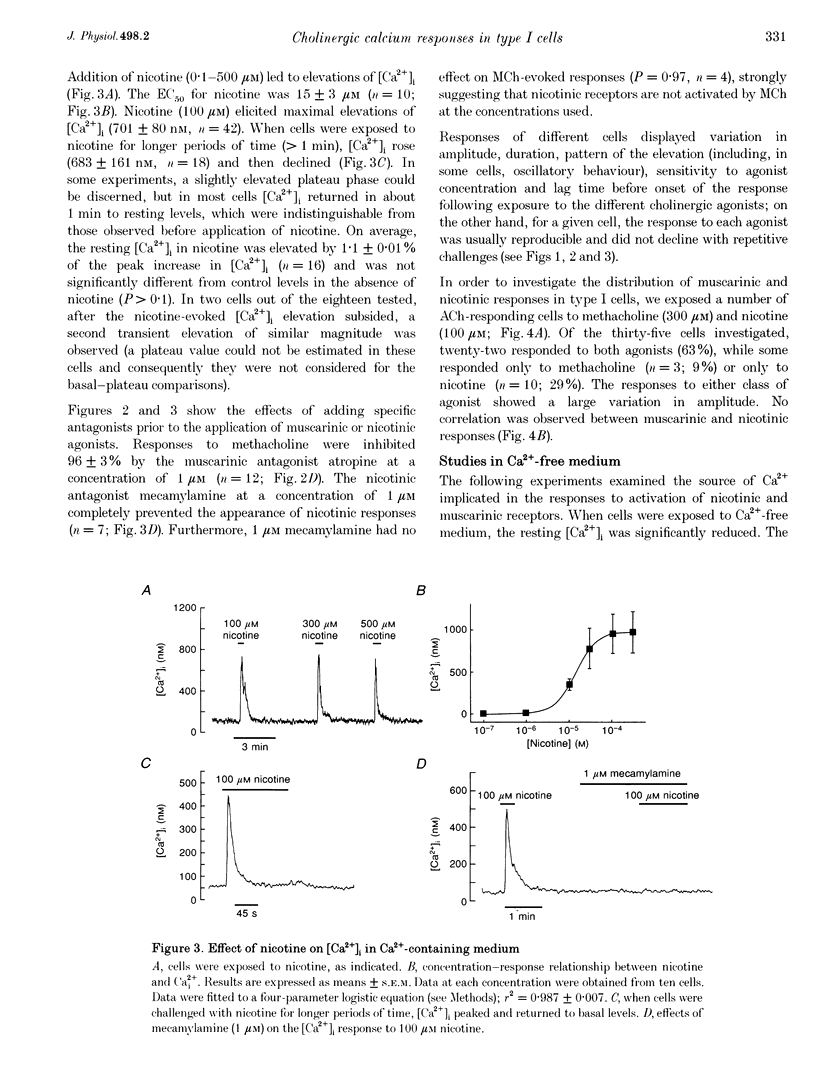
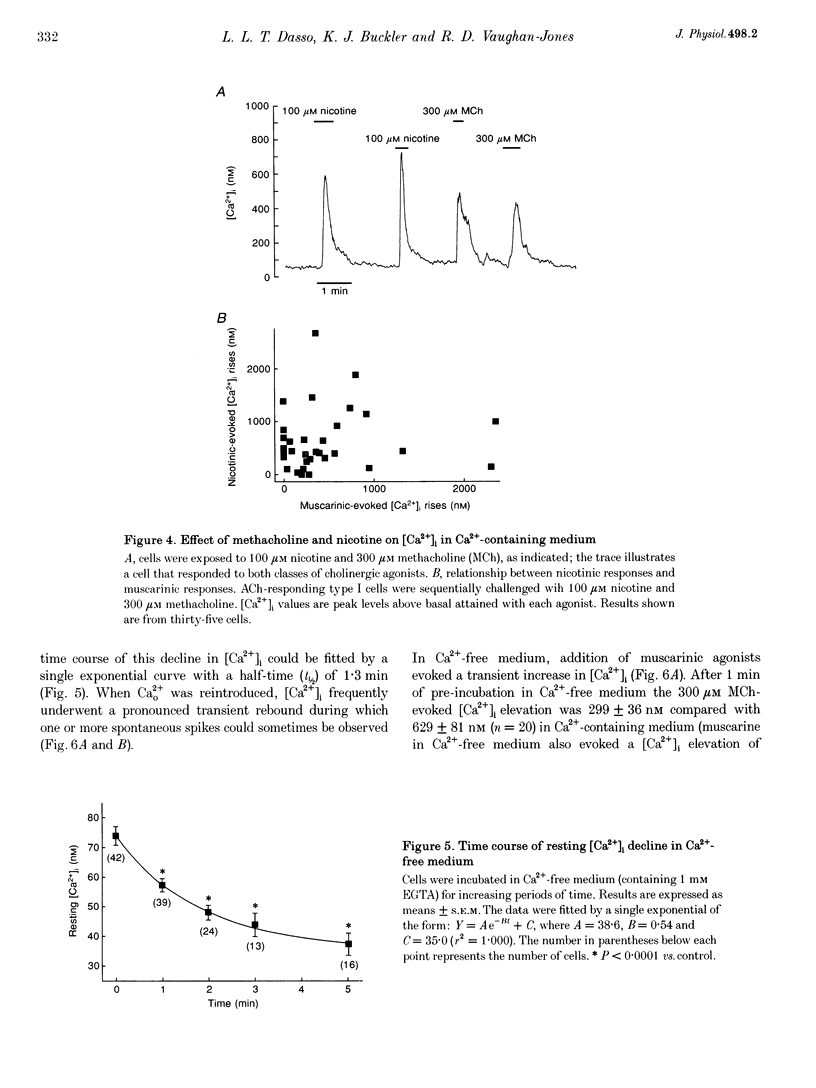
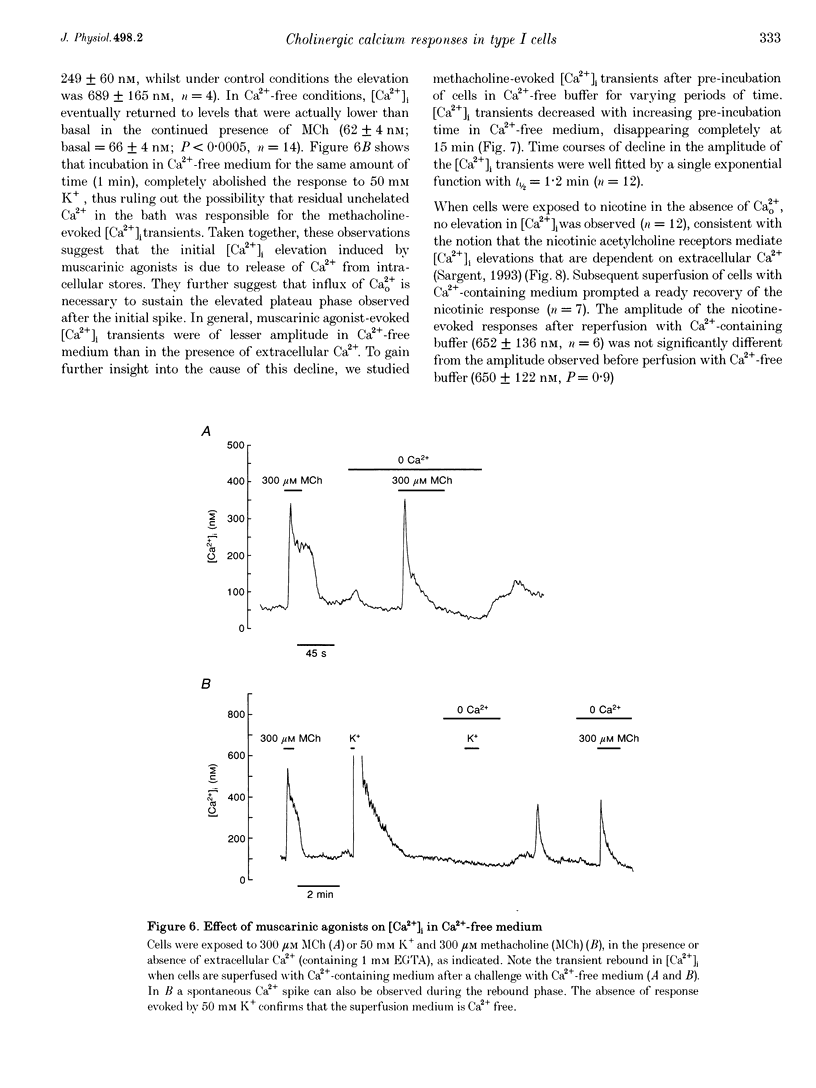
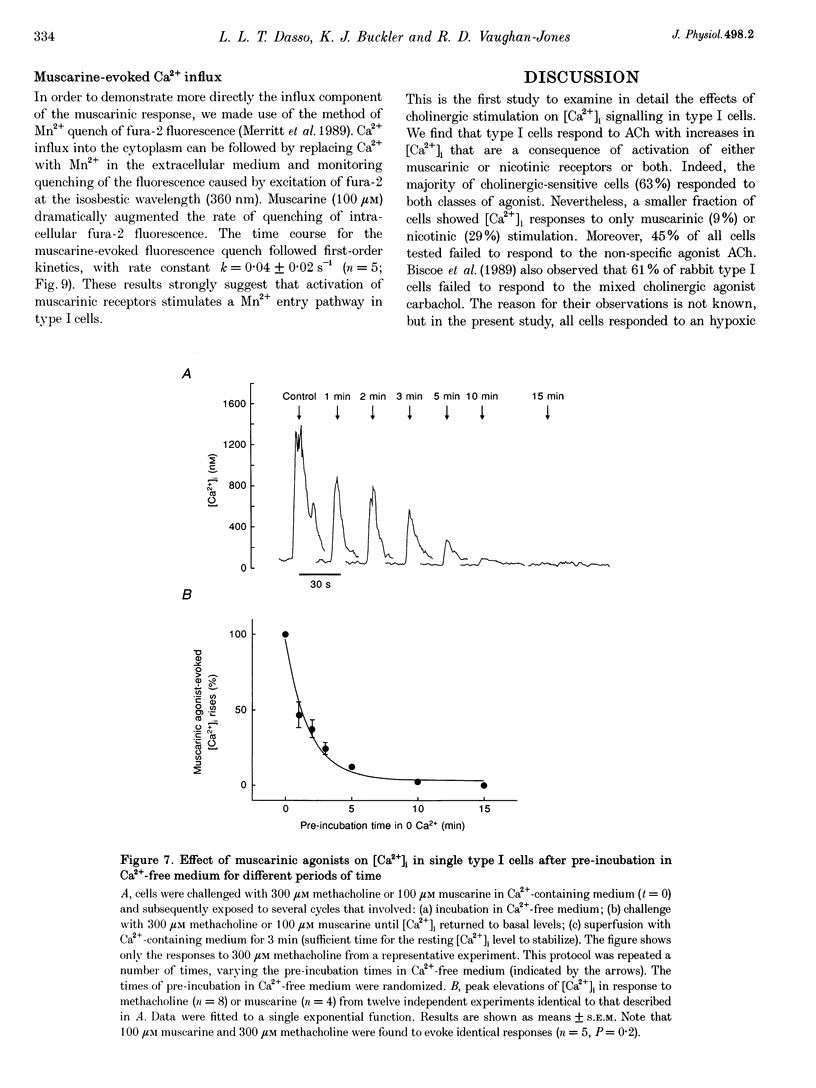
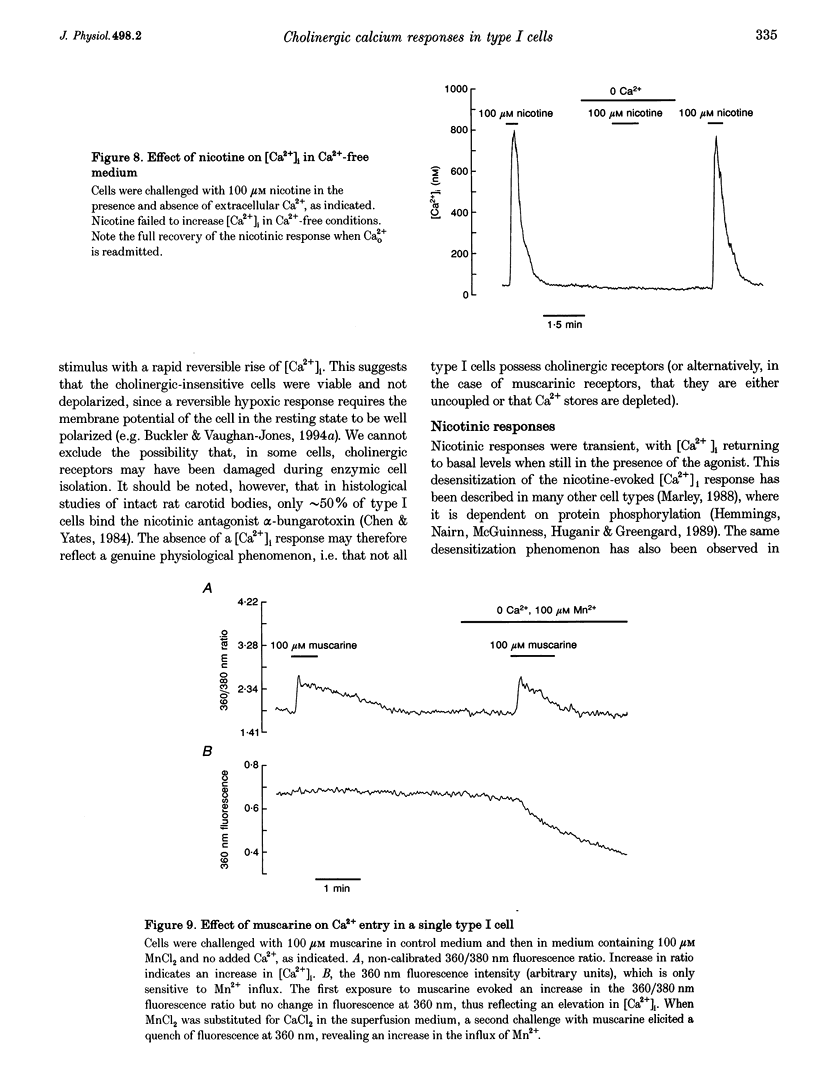
1. The effects of cholinergic agonists upon intracellular free Ca2+ levels ([Ca2+]i) have been studied in enzymically isolated rat carotid body single type I cells, using indo-1. 2. Acetylcholine (ACh) dose-dependently increased [Ca2+]i in 55% of cells studied (EC50 = 13 microM). These [Ca2+]i rises were partially inhibited by atropine or mecamylamine. 3. Specific nicotinic and muscarinic agonists also elevated [Ca2+]i in a dose-dependent manner (nicotine, EC50 = 15 microM; methacholine, EC50 = 20 microM). 4. While the majority of the ACh-sensitive cells responded to both classes of cholinergic agonist, 29% responded exclusively to nicotinic stimulation and 9% responded exclusively to muscarinic stimulation. 5. In the presence of nicotinic agonists, Ca2+i responses were transient. In the presence of muscarinic agonists, Ca2+i responses consisted of an initial rise, which then declined to a lower plateau level. 6. Nicotinic responses were rapidly abolished in Ca(2+)-free medium, suggesting that they are dependent on Ca2+ influx. 7. The plateau component of the muscarinic-activated response was also abolished in Ca(2+)-free conditions. The rapid initial [Ca2+]i rise, however, could still be evoked after several minutes in Ca(2+)-free medium. Muscarine also increased Mn2+ quenching of intracellular fura-2 fluorescence. These data suggest that the full muscarinic response depends on both Ca2+ release from intracellular stores and Ca2+o influx. 8. The results indicate that, in rat carotid body type I cells, both nicotinic and muscarinic acetylcholine receptors increase [Ca2+]i, but achieve this via different mechanisms. ACh may therefore play a role in carotid body function by modulating Ca2+i in the chemosensory type I cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R., Eisner D. A., O'Neill S. C., Valdeolmillos M. Measurements of intracellular Ca2+ in dissociated type I cells of the rabbit carotid body. J Physiol. 1989 Sep;416:421–434. doi: 10.1113/jphysiol.1989.sp017769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of acidic stimuli on intracellular calcium in isolated type I cells of the neonatal rat carotid body. Pflugers Arch. 1993 Oct;425(1-2):22–27. doi: 10.1007/BF00374499. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994 May 1;476(3):423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. P. Muscarinic receptors--characterization, coupling and function. Pharmacol Ther. 1993 Jun;58(3):319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Chen I. L., Yates R. D. Two types of glomus cell in the rat carotid body as revealed by alpha-bungarotoxin binding. J Neurocytol. 1984 Apr;13(2):281–302. doi: 10.1007/BF01148120. [DOI] [PubMed] [Google Scholar]
- Dinger B. G., Hirano T., Fidone S. J. Autoradiographic localization of muscarinic receptors in rabbit carotid body. Brain Res. 1986 Mar 5;367(1-2):328–331. doi: 10.1016/0006-8993(86)91612-4. [DOI] [PubMed] [Google Scholar]
- Dinger B., Gonzalez C., Yoshizaki K., Fidone S. Localization and function of cat carotid body nicotinic receptors. Brain Res. 1985 Jul 29;339(2):295–304. doi: 10.1016/0006-8993(85)90095-2. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. The effects of acetylcholine and dopamine on carotid chemosensory activity in the rabbit. J Physiol. 1979 Mar;288:411–423. [PMC free article] [PubMed] [Google Scholar]
- Eberhard D. A., Holz R. W. Cholinergic stimulation of inositol phosphate formation in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic mechanisms. J Neurochem. 1987 Nov;49(5):1634–1643. doi: 10.1111/j.1471-4159.1987.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Evans T., Martin M. W., Hughes A. R., Harden T. K. Guanine nucleotide-sensitive, high affinity binding of carbachol to muscarinic cholinergic receptors of 1321N1 astrocytoma cells is insensitive to pertussis toxin. Mol Pharmacol. 1985 Jan;27(1):32–37. [PubMed] [Google Scholar]
- Eyzaguirre C., Zapata P. Perspectives in carotid body research. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):931–957. doi: 10.1152/jappl.1984.57.4.931. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. S., Shirahata M. Acetylcholine and carotid body excitation during hypoxia in the cat. J Appl Physiol (1985) 1994 Apr;76(4):1566–1574. doi: 10.1152/jappl.1994.76.4.1566. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Marley P. D. Desensitization of the nicotinic secretory response of adrenal chromaffin cells. Trends Pharmacol Sci. 1988 Mar;9(3):102–107. doi: 10.1016/0165-6147(88)90177-0. [DOI] [PubMed] [Google Scholar]
- Mathes C., Thompson S. H. Calcium current activated by muscarinic receptors and thapsigargin in neuronal cells. J Gen Physiol. 1994 Jul;104(1):107–121. doi: 10.1085/jgp.104.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. S. A quantitative study of the effects of cholinergic drugs on carotid chemoreceptors in the cat. J Physiol. 1977 Dec;273(2):515–532. doi: 10.1113/jphysiol.1977.sp012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Monti-Bloch L., Eyzaguirre C. A comparative physiological and pharmacological study of cat and rabbit carotid body chemoreceptors. Brain Res. 1980 Jul 14;193(2):449–470. doi: 10.1016/0006-8993(80)90177-8. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Sasakawa N., Yamamoto S., Kato R. Functional shift from muscarinic to nicotinic cholinergic receptors involved in inositol trisphosphate and cyclic GMP accumulation during the primary culture of adrenal chromaffin cells. Biochem J. 1988 Apr 15;251(2):397–403. doi: 10.1042/bj2510397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Ca transport by plasma membrane and intracellular stores of gastric cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C843–C851. doi: 10.1152/ajpcell.1993.264.4.C843. [DOI] [PubMed] [Google Scholar]
- Nishi K., Eyzaguirre C. The action of some cholinergic blockers on carotid body chemoreceptors in vivo. Brain Res. 1971 Oct 8;33(1):37–56. doi: 10.1016/0006-8993(71)90304-0. [DOI] [PubMed] [Google Scholar]
- Peers C., Buckler K. J. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995 Mar;144(1):1–9. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- Peers C., Wyatt C. N., Buckler K. J. Actions of nicotinic agonists on isolated type I cells of the neonatal rat carotid body. Adv Exp Med Biol. 1994;360:155–157. doi: 10.1007/978-1-4615-2572-1_17. [DOI] [PubMed] [Google Scholar]
- Pietruschka F. Calcium influx in cultured carotid body cells is stimulated by acetylcholine and hypoxia. Brain Res. 1985 Nov 11;347(1):140–143. doi: 10.1016/0006-8993(85)90901-1. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Ringdahl B. Dimethylsulfonium and thiolanium analogues of the muscarinic agent oxotremorine. J Med Chem. 1988 Jan;31(1):164–168. doi: 10.1021/jm00396a025. [DOI] [PubMed] [Google Scholar]
- Sampson S. R. Effects of mecamylamine on responses of carotid body chemoreceptors in vivo to physiological and pharmacological stimuli. J Physiol. 1971 Feb;212(3):655–666. doi: 10.1113/jphysiol.1971.sp009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- Shaw K., Montague W., Pallot D. J. Biochemical studies on the release of catecholamines from the rat carotid body in vitro. Biochim Biophys Acta. 1989 Sep 4;1013(1):42–46. doi: 10.1016/0167-4889(89)90125-0. [DOI] [PubMed] [Google Scholar]
- Simpson P. B., Challiss R. A., Nahorski S. R. Divalent cation entry in cultured rat cerebellar granule cells measured using Mn2+ quench of fura 2 fluorescence. Eur J Neurosci. 1995 May 1;7(5):831–840. doi: 10.1111/j.1460-9568.1995.tb01070.x. [DOI] [PubMed] [Google Scholar]
- Ureña J., Fernández-Chacón R., Benot A. R., Alvarez de Toledo G. A., López-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Z., Stensaas L. J., Dinger B., Fidone S. J. Immunocytochemical localization of choline acetyltransferase in the carotid body of the cat and rabbit. Brain Res. 1989 Sep 25;498(1):131–134. doi: 10.1016/0006-8993(89)90407-1. [DOI] [PubMed] [Google Scholar]
- Wyatt C. N., Peers C. Nicotinic acetylcholine receptors in isolated type I cells of the neonatal rat carotid body. Neuroscience. 1993 May;54(1):275–281. doi: 10.1016/0306-4522(93)90399-z. [DOI] [PubMed] [Google Scholar]


