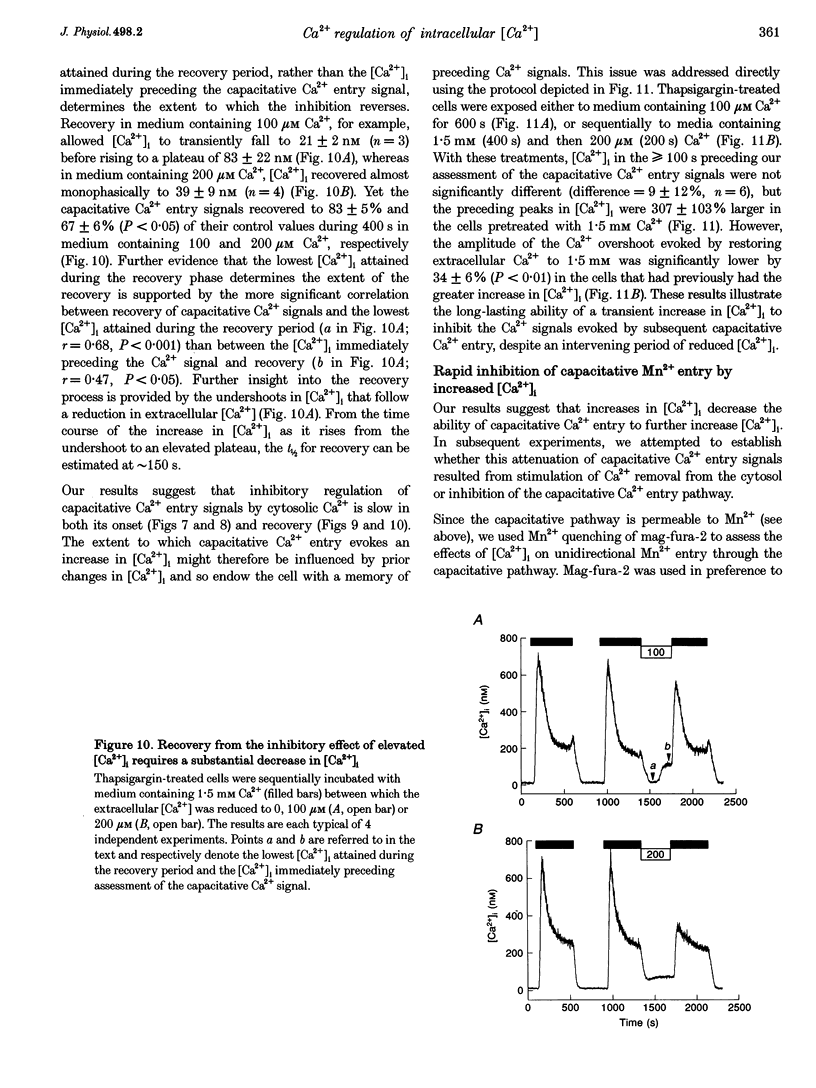
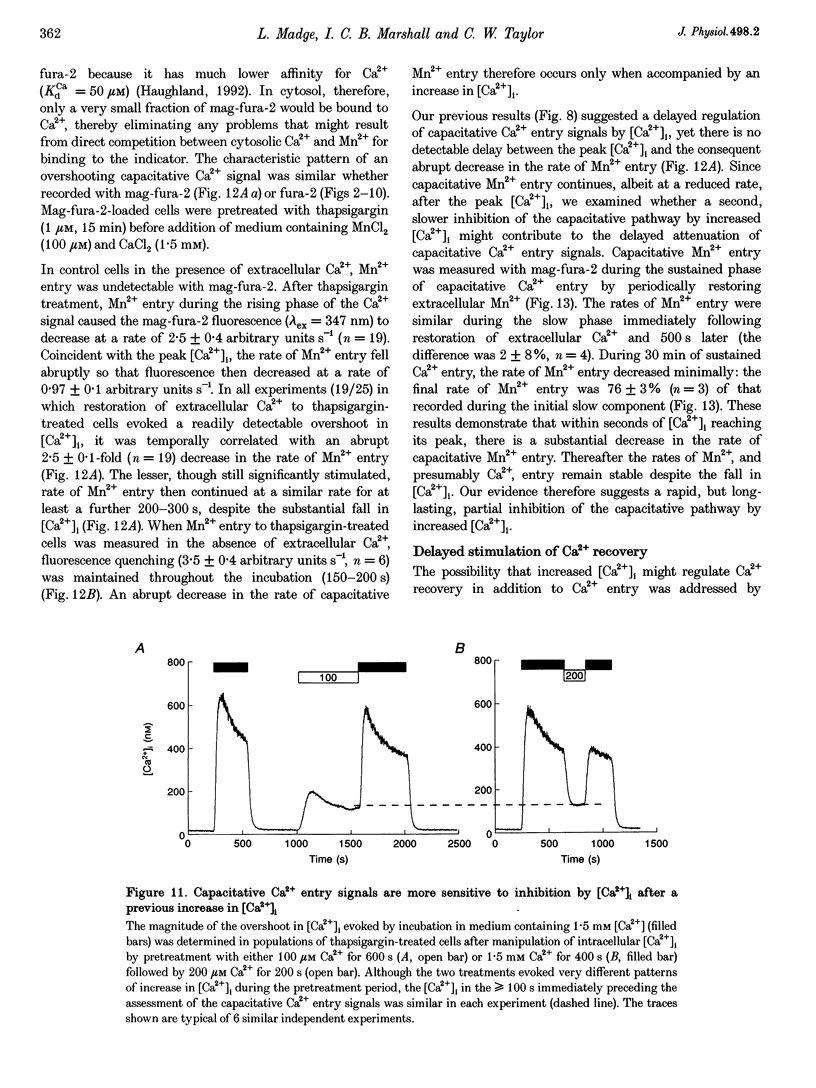
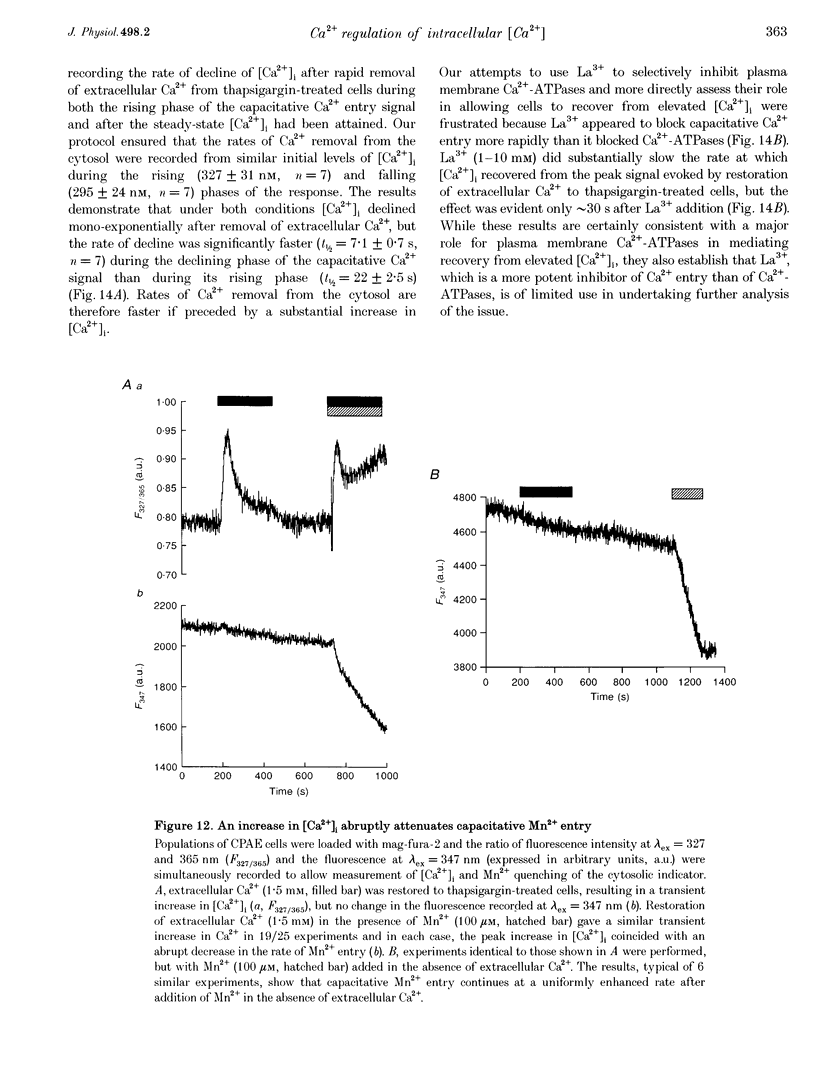
Abstract
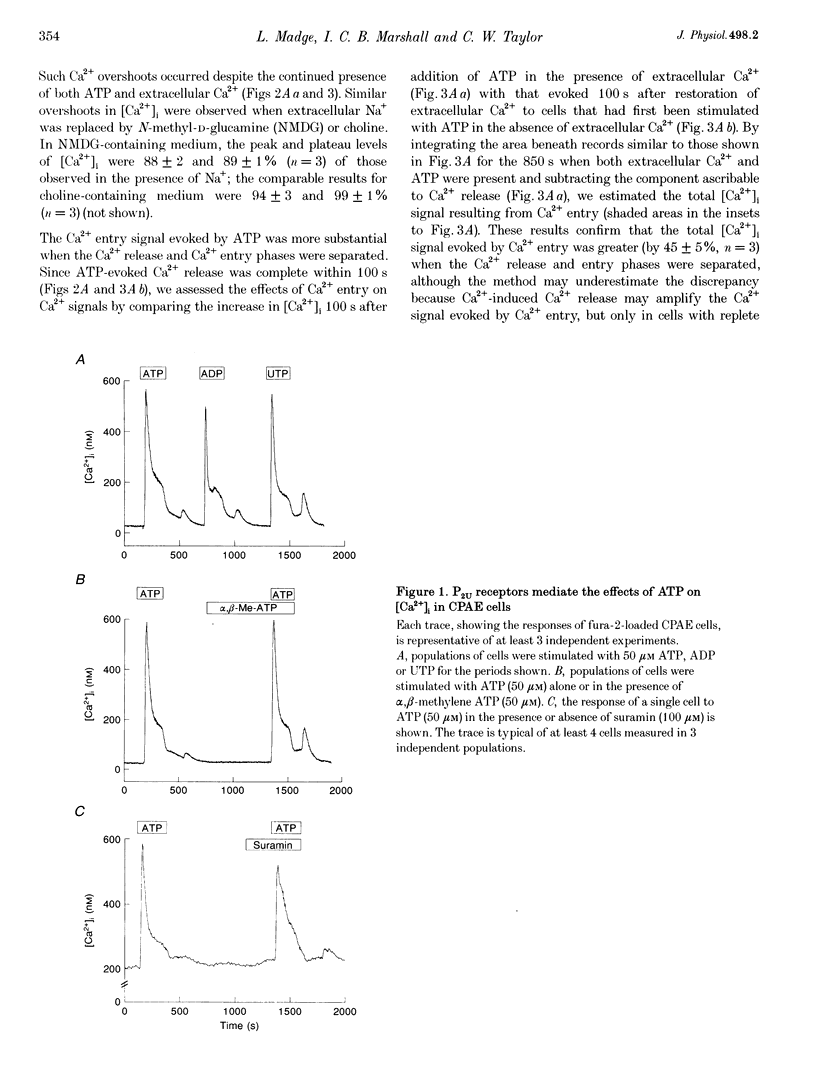
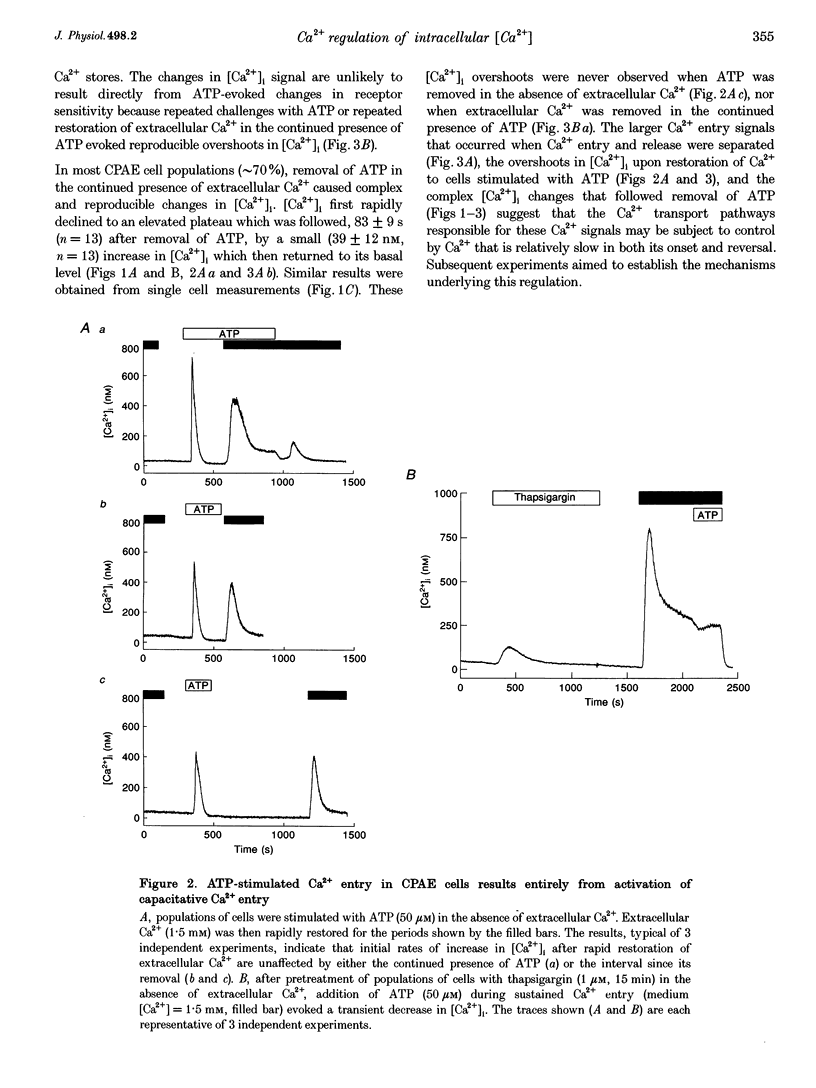
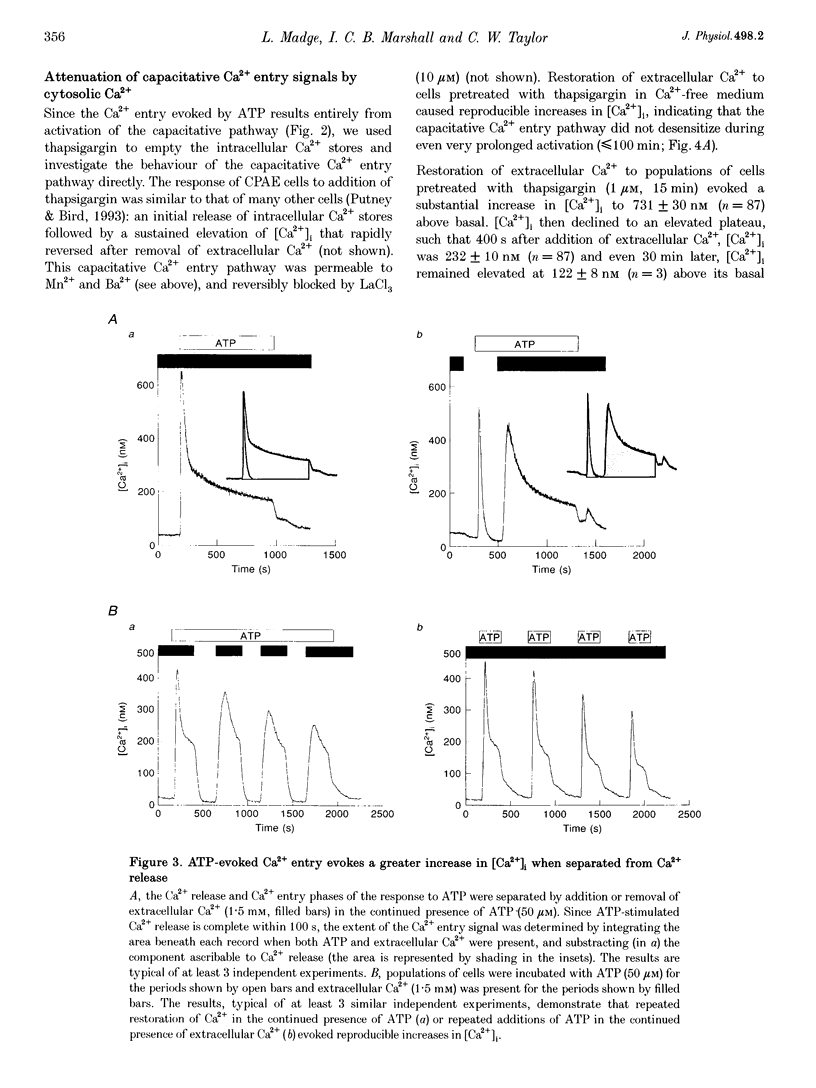
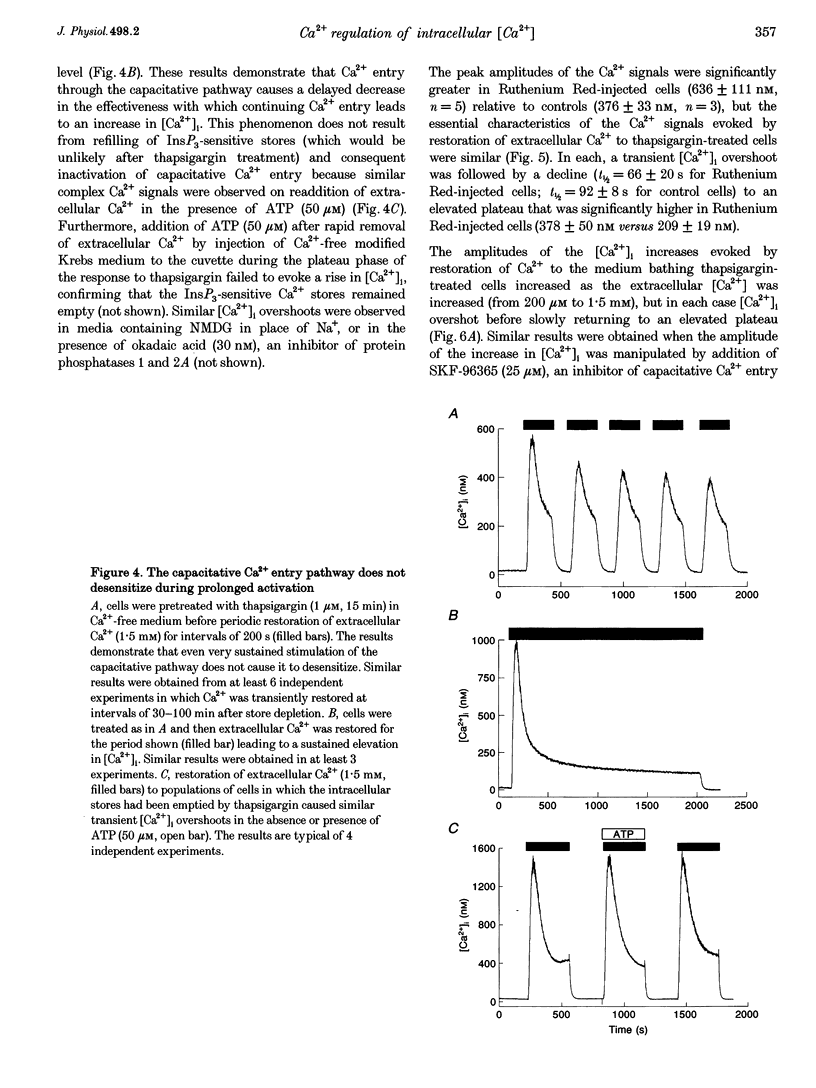
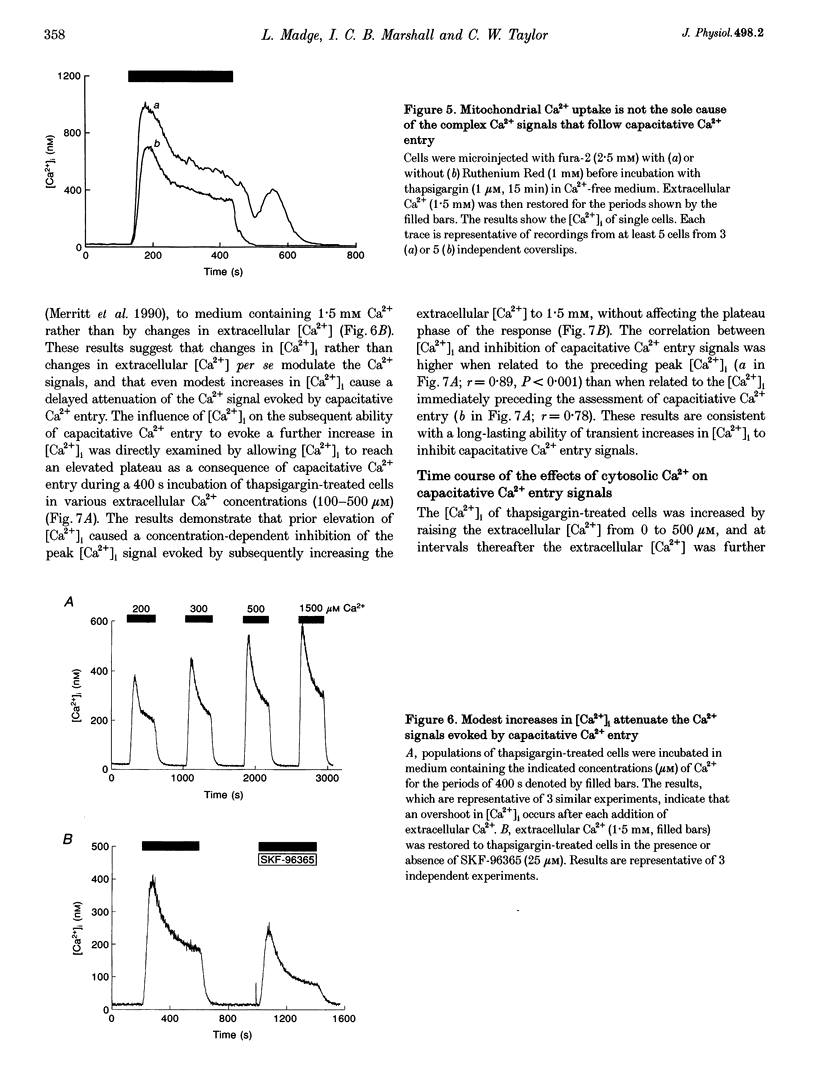
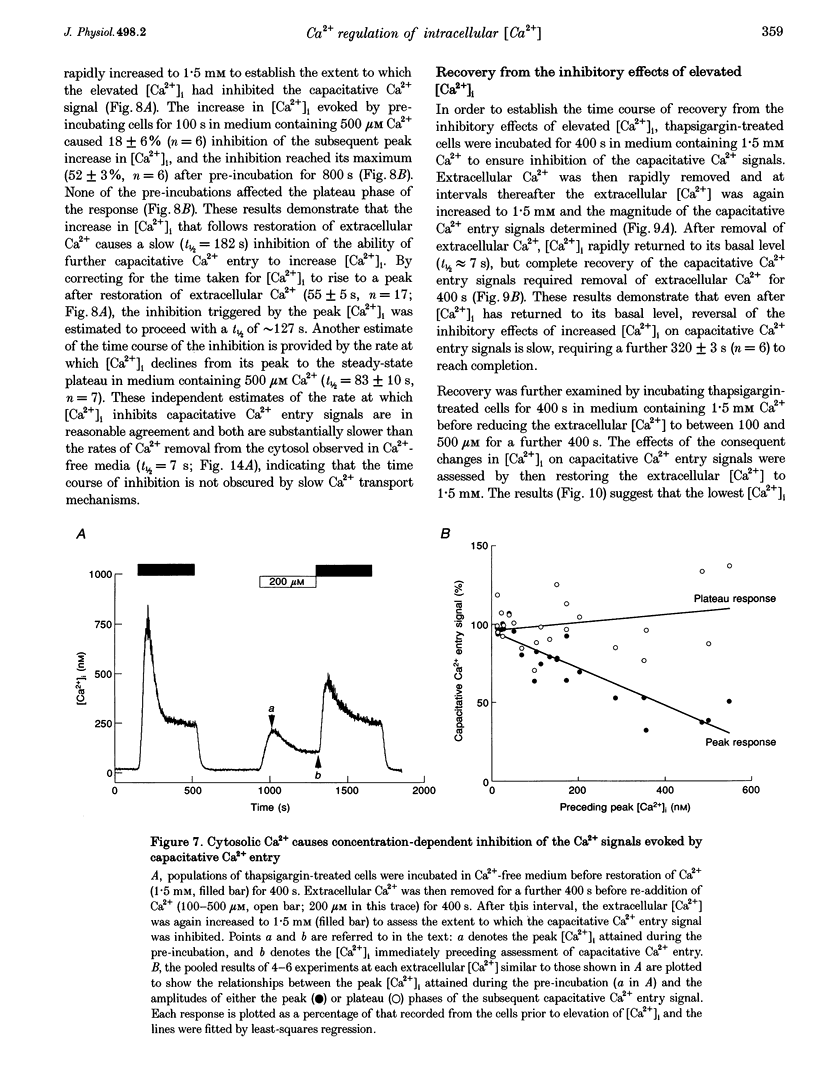
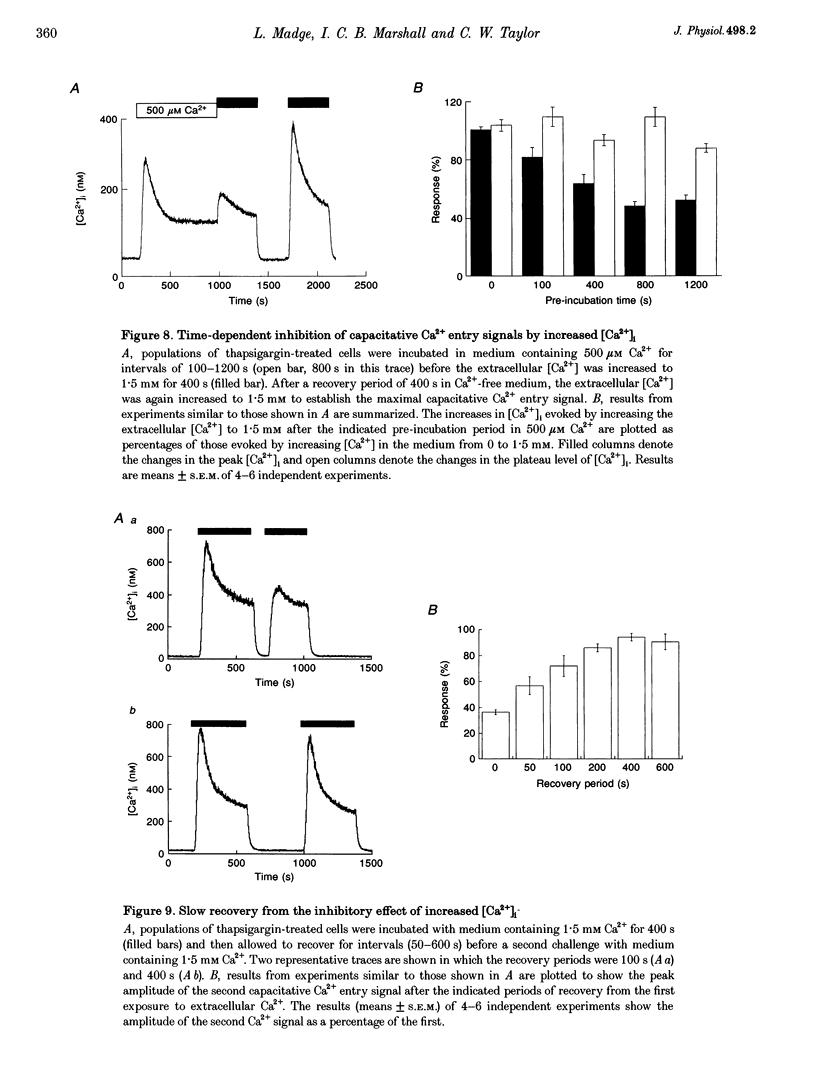
1. In calf pulmonary artery endothelial (CPAE) cells loaded with fura-2, the effects of ATP on Ca2+ entry were mediated entirely by the ability of P2U purinoceptors to stimulate InsP3 formation, empty intracellular Ca2+ stores and thereby activate capacitative Ca2+ entry. 2. Restoration of extracellular Ca2+ to cells with empty intracellular stores evoked transient increases in cytosolic [Ca2+] ([Ca2+]i) which then declined to an elevated plateau. These overshoots in [Ca2+]i were not a consequence of store refilling nor of desensitization of the capacitative pathway. Similar responses were recorded from cells in which Ca2+ uptake into mitochondria had been inhibited by microinjection of Ruthenium Red. The amplitudes of the capacitative Ca2+ signals decreased at lower extracellular [Ca2+], but [Ca2+]i invariably overshot before slowly declining to an elevated plateau. Even modest increases in [Ca2+]i therefore caused a delayed attenuation of the Ca2+ signal evoked by capacitative Ca2+ entry. 3. Modest pre-elevation of [Ca2+]i inhibited the ability of subsequent capacitative Ca2+ entry to further increase [Ca2+]i. The onset of the inhibition was slow (half-time (t1/2), approximately 100 s) and more tightly correlated with the preceding peak [Ca2+]i than with the [Ca2+]i immediately preceding Ca2+ entry. Recovery was also slow and complete only after [Ca2+]i had returned to its basal level for 320 +/- 3 s. 4. In thapsigargin-treated cells loaded with mag-fura-2, the peak [Ca2+]i that followed restoration of extracellular Ca2+ was accompanied by an abrupt approximately 2.5-fold decrease in the rate of Mn2+ entry, which then continued indefinitely at the reduced rate, demonstrating a rapid partial inactivation of the capacitative pathway. 5. The half-time for Ca2+ removal from the cytosol was significantly slower during the rising (t 1/2 = 22 +/- 2.5 s) than during the falling (t 1/2 = 7.1 +/- 0.7 s) phase of the Ca2+ overshoot evoked by addition of extracellular Ca2+ to thapsigargin-treated cells. 6. We conclude that an increase in [Ca2+]i rapidly inhibits the capacitative pathway and more slowly activates mechanisms that remove Ca2+ from the cytosol. Reversal of either or both of these regulatory mechanisms can occur only a considerable time after [Ca2+]i has been completely restored to its resting level. These mechanisms are likely to protect cells from excessive increases in [Ca2+]i and contribute to oscillatory changes in [Ca2+]i.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Capacitative calcium entry. Biochem J. 1995 Nov 15;312(Pt 1):1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Byron K. L., Taylor C. W. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem. 1993 Apr 5;268(10):6945–6952. [PubMed] [Google Scholar]
- Byron K., Taylor C. W. Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. J Physiol. 1995 Jun 1;485(Pt 2):455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991 Jan;71(1):129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Castro E., Mateo J., Tomé A. R., Barbosa R. M., Miras-Portugal M. T., Rosário L. M. Cell-specific purinergic receptors coupled to Ca2+ entry and Ca2+ release from internal stores in adrenal chromaffin cells. Differential sensitivity to UTP and suramin. J Biol Chem. 1995 Mar 10;270(10):5098–5106. doi: 10.1074/jbc.270.10.5098. [DOI] [PubMed] [Google Scholar]
- Drummond R. M., Fay F. S. Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflugers Arch. 1996 Feb;431(4):473–482. doi: 10.1007/BF02191893. [DOI] [PubMed] [Google Scholar]
- Foder B., Scharff O. Solitary calcium spike dependent on calmodulin and plasma membrane Ca2+ pump. Cell Calcium. 1992 Oct;13(9):581–591. doi: 10.1016/0143-4160(92)90038-t. [DOI] [PubMed] [Google Scholar]
- Foder B., Scharff O., Thastrup O. Ca2+ transients and Mn2+ entry in human neutrophils induced by thapsigargin. Cell Calcium. 1989 Oct;10(7):477–490. doi: 10.1016/0143-4160(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Wong D. C. [Ca2+]i inhibition of Ca2+ release-activated Ca2+ influx underlies agonist- and thapsigargin-induced [Ca2+]i oscillations in salivary acinar cells. J Biol Chem. 1994 Dec 16;269(50):31525–31532. [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. J Neurosci. 1994 Jul;14(7):4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G., Robb-Gaspers L. D., Seitz M. B., Thomas A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995 Aug 11;82(3):415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves A. C., Lummis S. C., Taylor C. W. Ca2+ permeability of cloned and native 5-hydroxytryptamine type 3 receptors. Mol Pharmacol. 1994 Dec;46(6):1120–1128. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Lawrie A. M., Rizzuto R., Pozzan T., Simpson A. W. A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J Biol Chem. 1996 May 3;271(18):10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- McDonald T. V., Premack B. A., Gardner P. Flash photolysis of caged inositol 1,4,5-trisphosphate activates plasma membrane calcium current in human T cells. J Biol Chem. 1993 Feb 25;268(6):3889–3896. [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Pary J. B., Oike M., Casteels R. Kinetics of empty store-activated Ca2+ influx in HeLa cells. J Biol Chem. 1994 Feb 25;269(8):5817–5823. [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Fasolato C., Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993 Jun;3(3):368–374. doi: 10.1016/0959-4388(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Petersen C. C., Berridge M. J. The regulation of capacitative calcium entry by calcium and protein kinase C in Xenopus oocytes. J Biol Chem. 1994 Dec 23;269(51):32246–32253. [PubMed] [Google Scholar]
- Putney J. W., Jr, Bird G. S. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993 Oct;14(5):610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Sage S. O., van Breemen C., Cannell M. B. Sodium-calcium exchange in cultured bovine pulmonary artery endothelial cells. J Physiol. 1991;440:569–580. doi: 10.1113/jphysiol.1991.sp018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O., Foder B. Delayed activation of plasma membrane Ca2+ pump in human neutrophils. Cell Calcium. 1994 Dec;16(6):455–466. doi: 10.1016/0143-4160(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Elliott S. J. Ca2+ signaling mechanisms of vascular endothelial cells and their role in oxidant-induced endothelial cell dysfunction. Am J Physiol. 1992 Jun;262(6 Pt 2):H1617–H1630. doi: 10.1152/ajpheart.1992.262.6.H1617. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989 Nov 5;264(31):18349–18355. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Taylor C. W., Traynor D. Calcium and inositol trisphosphate receptors. J Membr Biol. 1995 May;145(2):109–118. doi: 10.1007/BF00237369. [DOI] [PubMed] [Google Scholar]
- Toescu E. C., Petersen O. H. Region-specific activity of the plasma membrane Ca2+ pump and delayed activation of Ca2+ entry characterize the polarized, agonist-evoked Ca2+ signals in exocrine cells. J Biol Chem. 1995 Apr 14;270(15):8528–8535. doi: 10.1074/jbc.270.15.8528. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Du Y. S., Diglio C., Tsang W., Kuo T. H. Hormone-induced phosphorylation of the plasma membrane calcium pump in cultured aortic endothelial cells. Arch Biochem Biophys. 1991 Aug 15;289(1):103–108. doi: 10.1016/0003-9861(91)90448-r. [DOI] [PubMed] [Google Scholar]
- Zhu X., Jiang M., Peyton M., Boulay G., Hurst R., Stefani E., Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996 May 31;85(5):661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J Gen Physiol. 1995 Feb;105(2):209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]