Abstract
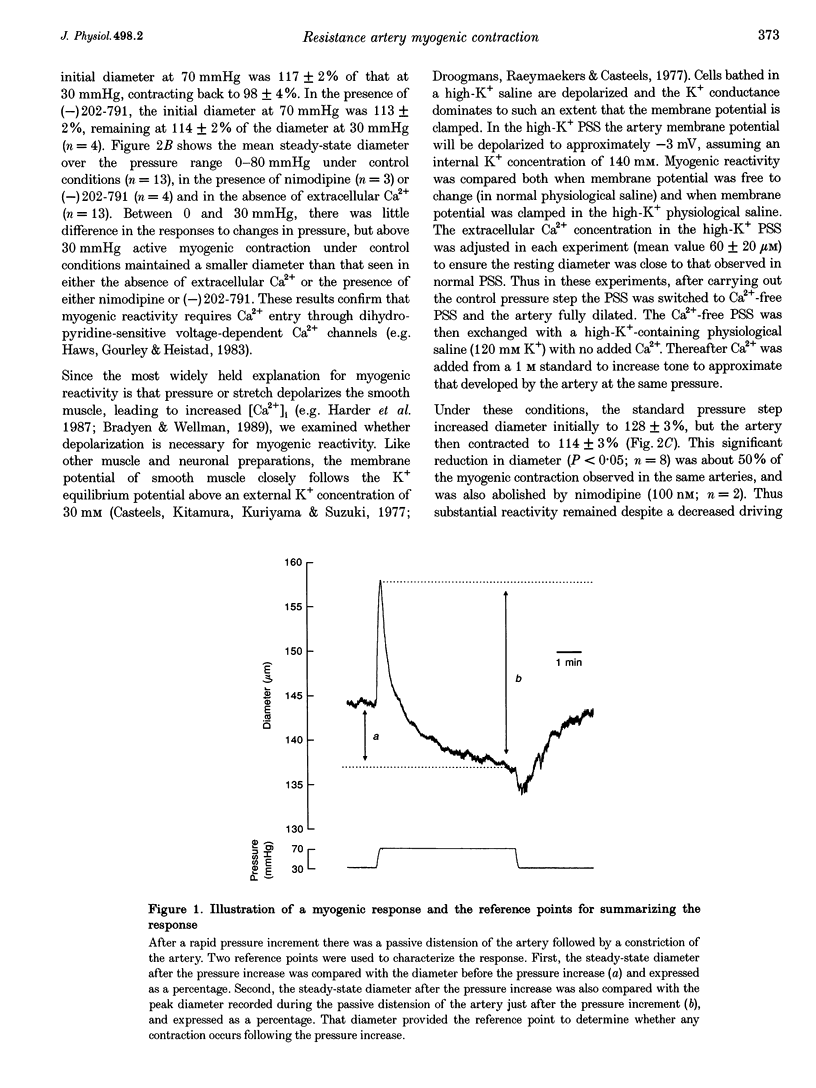
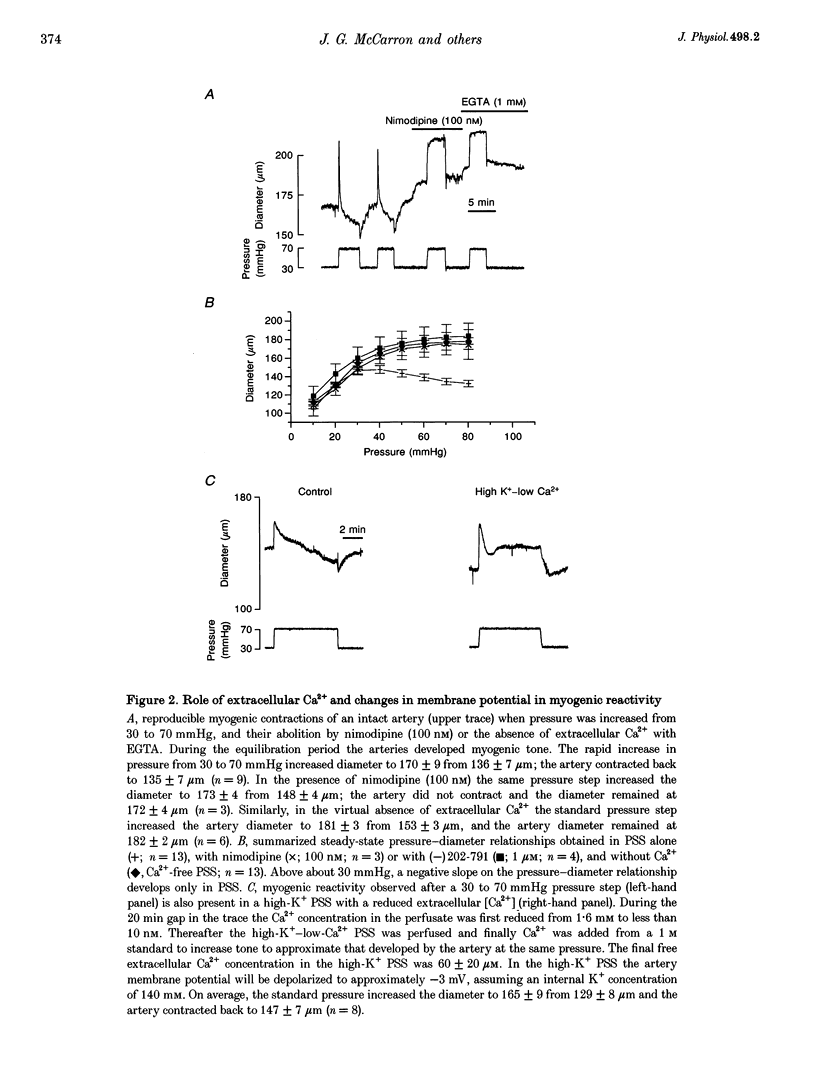
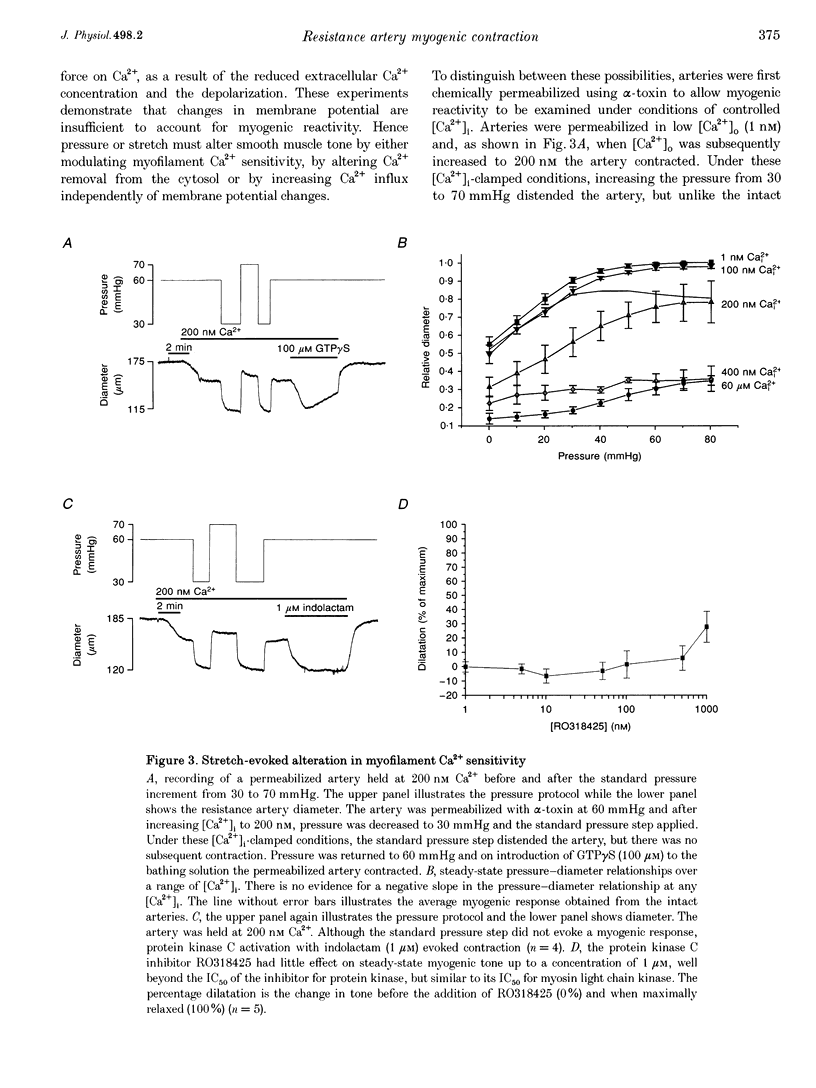
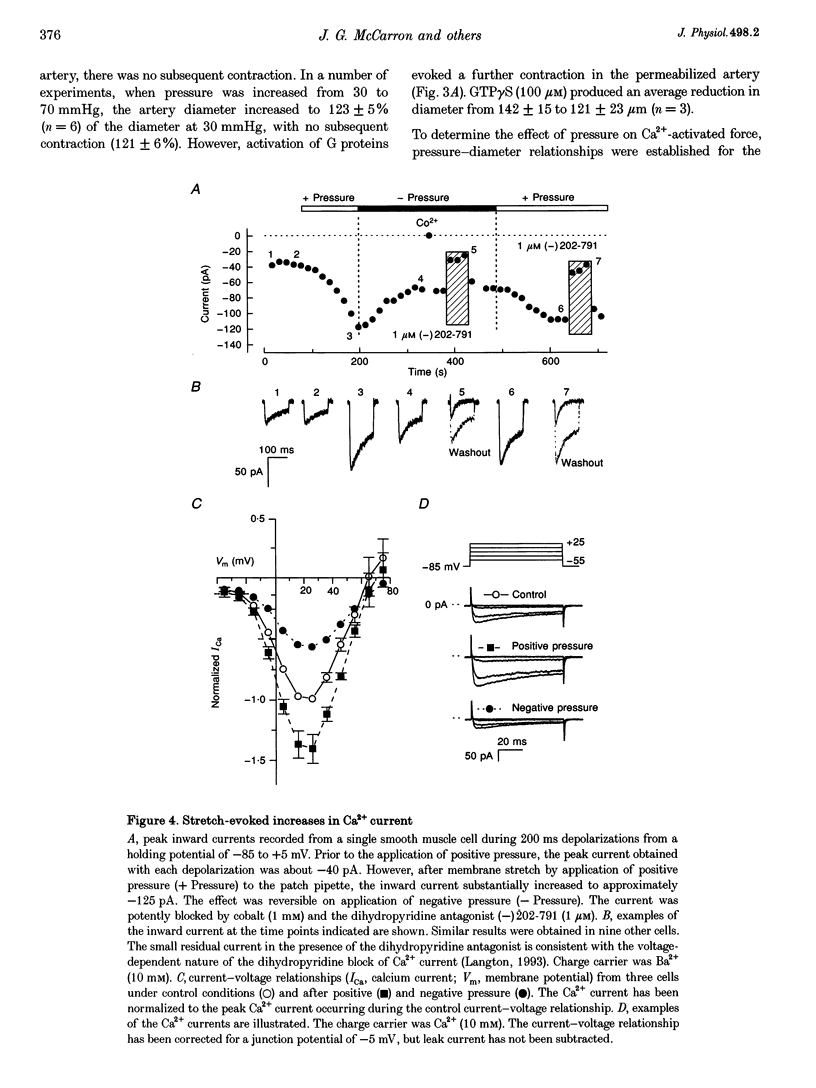
1. Tissue blood flow and blood pressure are regulated by the spontaneous, myogenic, contraction developed by resistance arteries. However, the cellular mechanisms underlying myogenic contraction are not understood. In this study, the mechanisms of myogenic contraction in cerebral resistance arteries were investigated. 2. The vasoconstriction observed in response to increased pressure in cerebral resistance arteries (myogenic reactivity) was dependent on Ca2+ entry through voltage-dependent Ca2+ channels, since it was abolished by Ca2+ removal and by dihydropyridine antagonists of voltage-dependent Ca2+ channels. 3. Myogenic reactivity persisted in a high-K+ saline, with reduced Ca2+, where membrane potential is presumed to be clamped. Therefore, membrane depolarization alone does not fully account for the increased voltage-dependent Ca2+ channel opening. 4. Voltage-dependent Ca2+ currents in single smooth muscle cells isolated from the resistance artery were substantially increased by applying positive pressure to the patch electrode evoking membrane stretch. 5. Myogenic reactivity remained unaffected by ryanodine and therefore was independent of internal ryanodine-sensitive Ca2+ stores. 6. The myofilament Ca2+ sensitivity was not increased by elevated pressure in alpha-toxin-permeabilized arteries. However, pharmacological activation of protein kinase C or G proteins did increase the myofilament Ca2+ sensitivity. 7. Myogenic contraction over the pressure range 30-70 mmHg could be accounted for by an increase in [Ca2+]i from 100 to 200 nM. 8. It is concluded that modest increases in [Ca2+]i within the range 100-200 nM can account for that myogenic contraction, and that stretch-evoked modulation of Ca2+ currents may contribute to the myogenic response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw D., Hill C. H., Nixon J. S., Wilkinson S. E. Therapeutic potential of protein kinase C inhibitors. Agents Actions. 1993 Jan;38(1-2):135–147. doi: 10.1007/BF02027225. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Wellman G. C. Endothelium-dependent dilation of feline cerebral arteries: role of membrane potential and cyclic nucleotides. J Cereb Blood Flow Metab. 1989 Jun;9(3):256–263. doi: 10.1038/jcbfm.1989.42. [DOI] [PubMed] [Google Scholar]
- Bárány K., Rokolya A., Bárány M. Stretch activates myosin light chain kinase in arterial smooth muscle. Biochem Biophys Res Commun. 1990 Nov 30;173(1):164–171. doi: 10.1016/s0006-291x(05)81036-8. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton C. A., Smith G. L. GTP and noradrenaline-induced force in isolated toxin-permeabilized rat anococcygeus and guinea-pig portal vein. J Physiol. 1991 Jun;437:543–561. doi: 10.1113/jphysiol.1991.sp018610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. J., Donovitz J. A., Hood J. D. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C1083–C1088. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai C. M. Length-dependent myosin phosphorylation and contraction of arterial smooth muscle. Pflugers Arch. 1991 Jul;418(6):564–571. doi: 10.1007/BF00370572. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Gilbert R., Lombard J. H. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am J Physiol. 1987 Oct;253(4 Pt 2):F778–F781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- Haws C. W., Gourley J. K., Heistad D. D. Effects of nimodipine on cerebral blood flow. J Pharmacol Exp Ther. 1983 Apr;225(1):24–28. [PubMed] [Google Scholar]
- Henrion D., Laher I. Effects of staurosporine and calphostin C, two structurally unrelated inhibitors of protein kinase C, on vascular tone. Can J Physiol Pharmacol. 1993 Jul;71(7):521–524. doi: 10.1139/y93-076. [DOI] [PubMed] [Google Scholar]
- Hill M. A., Falcone J. C., Meininger G. A. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol. 1990 Nov;259(5 Pt 2):H1586–H1594. doi: 10.1152/ajpheart.1990.259.5.H1586. [DOI] [PubMed] [Google Scholar]
- Homma T., Akai Y., Burns K. D., Harris R. C. Activation of S6 kinase by repeated cycles of stretching and relaxation in rat glomerular mesangial cells. Evidence for involvement of protein kinase C. J Biol Chem. 1992 Nov 15;267(32):23129–23135. [PubMed] [Google Scholar]
- Langton P. D. Calcium channel currents recorded from isolated myocytes of rat basilar artery are stretch sensitive. J Physiol. 1993 Nov;471:1–11. doi: 10.1113/jphysiol.1993.sp019887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte R., Haeberle J. R., Laher I. Phorbol ester-induced potentiation of myogenic tone is not associated with increases in Ca2+ influx, myoplasmic free Ca2+ concentration, or 20-kDa myosin light chain phosphorylation. J Mol Cell Cardiol. 1994 Mar;26(3):297–302. doi: 10.1006/jmcc.1994.1038. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Sage G. P., 2nd, Cipkus-Dubray L. A. Factors affecting rabbit mesenteric artery smooth muscle sensitivity to calcium antagonists. J Pharmacol Exp Ther. 1990 Mar;252(3):1167–1174. [PubMed] [Google Scholar]
- Moore E. D., Etter E. F., Philipson K. D., Carrington W. A., Fogarty K. E., Lifshitz L. M., Fay F. S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993 Oct 14;365(6447):657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Cheng H., Rubart M., Santana L. F., Bonev A. D., Knot H. J., Lederer W. J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995 Oct 27;270(5236):633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Osol G., Laher I., Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991 Feb;68(2):359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993 Apr;12(4):1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Hata S., Ishiro H., Ishii K., Nakayama K. Stretching releases Ca2+ from intracellular storage sites in canine cerebral arteries. Can J Physiol Pharmacol. 1994 Jan;72(1):19–24. doi: 10.1139/y94-004. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Tobe K., Hoh E., Maemura K., Kaida T., Komuro I., Tamemoto H., Kadowaki T., Nagai R., Yazaki Y. Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. J Biol Chem. 1993 Jun 5;268(16):12069–12076. [PubMed] [Google Scholar]
- Yazaki Y., Komuro I., Yamazaki T., Tobe K., Maemura K., Kadowaki T., Nagai R. Role of protein kinase system in the signal transduction of stretch-mediated protooncogene expression and hypertrophy of cardiac myocytes. Mol Cell Biochem. 1993 Feb 17;119(1-2):11–16. doi: 10.1007/BF00926847. [DOI] [PubMed] [Google Scholar]


