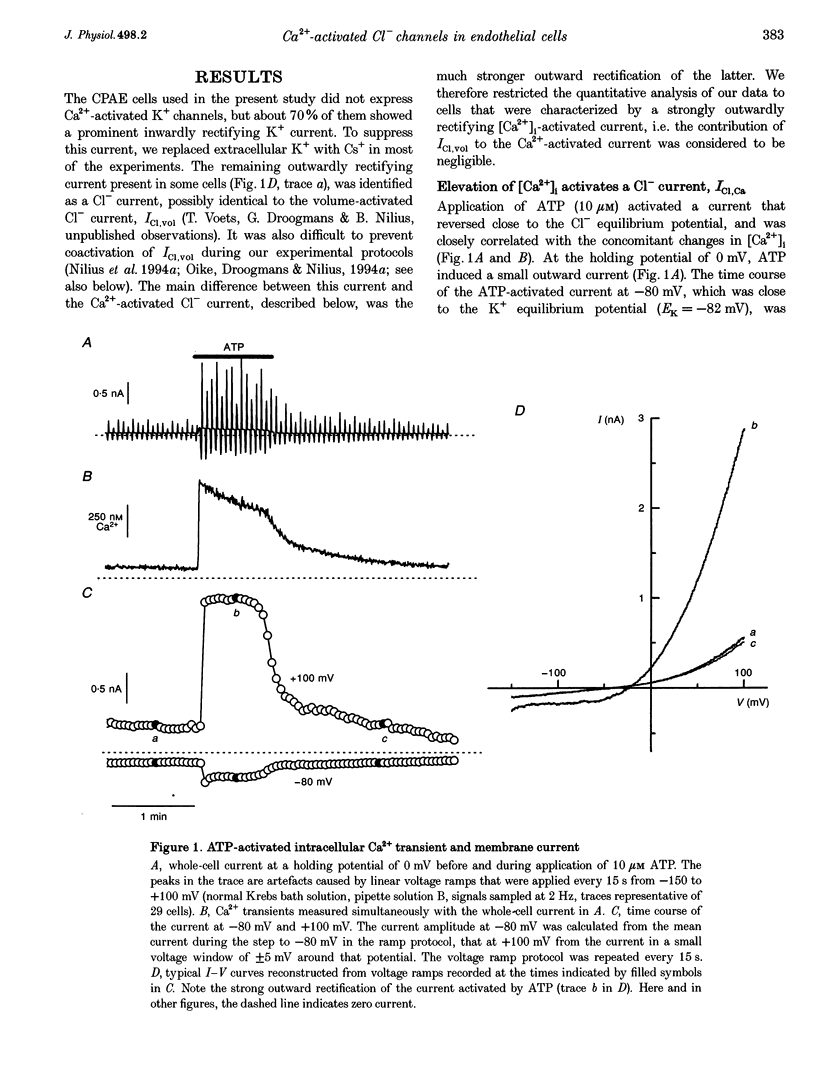
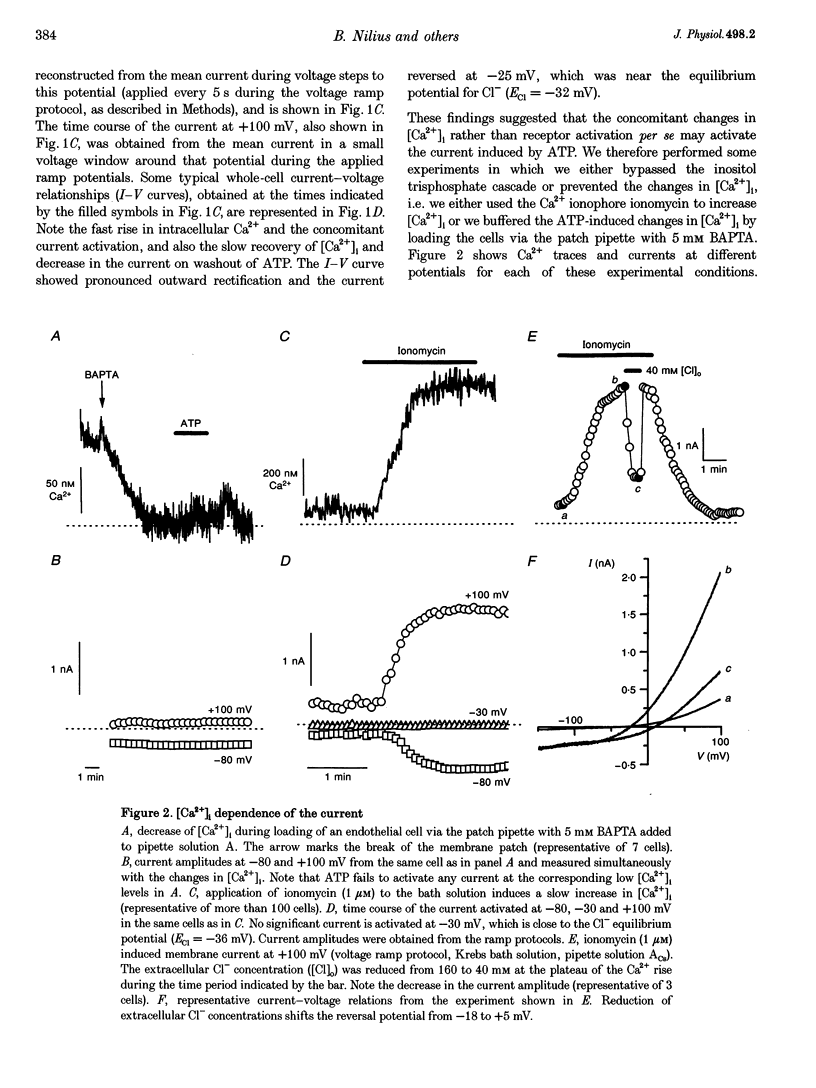
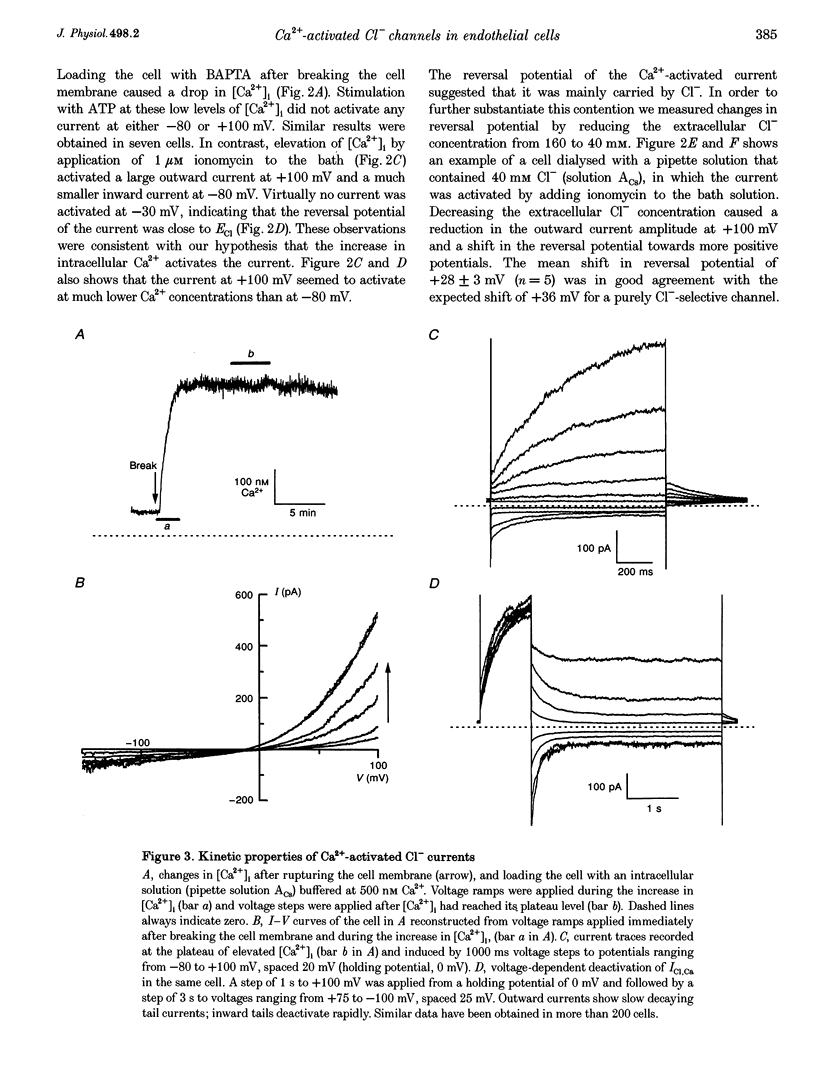
Abstract
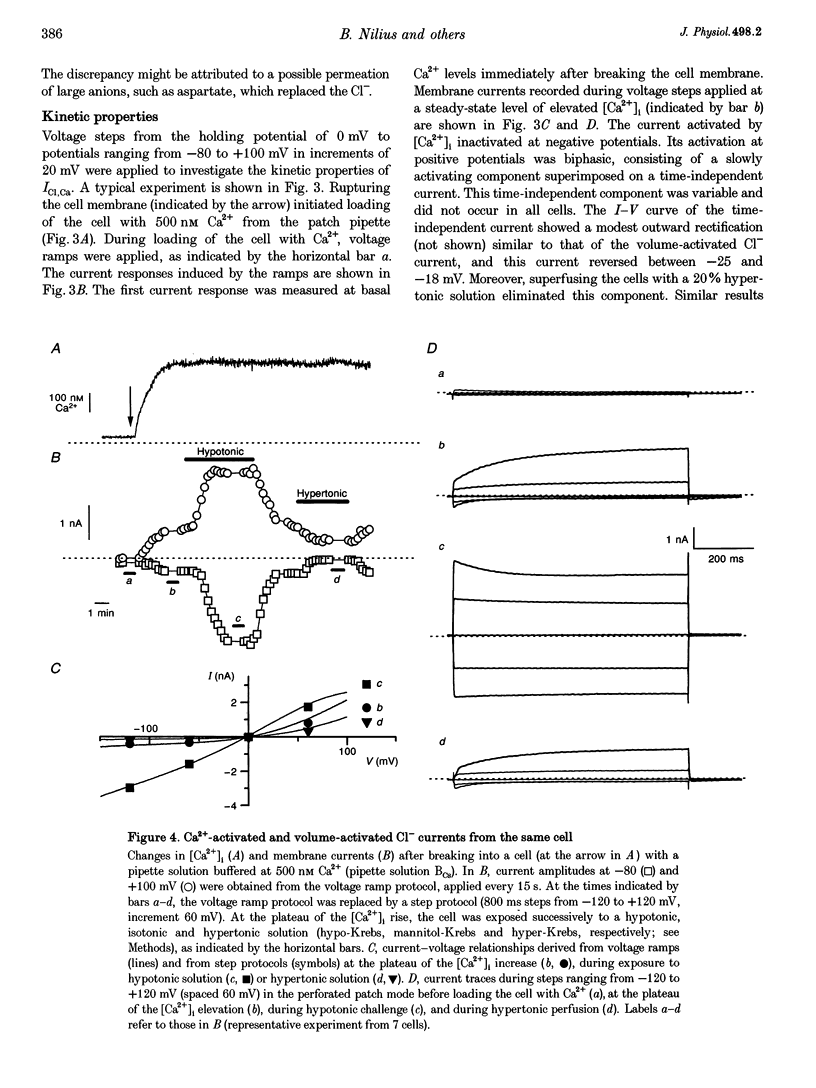
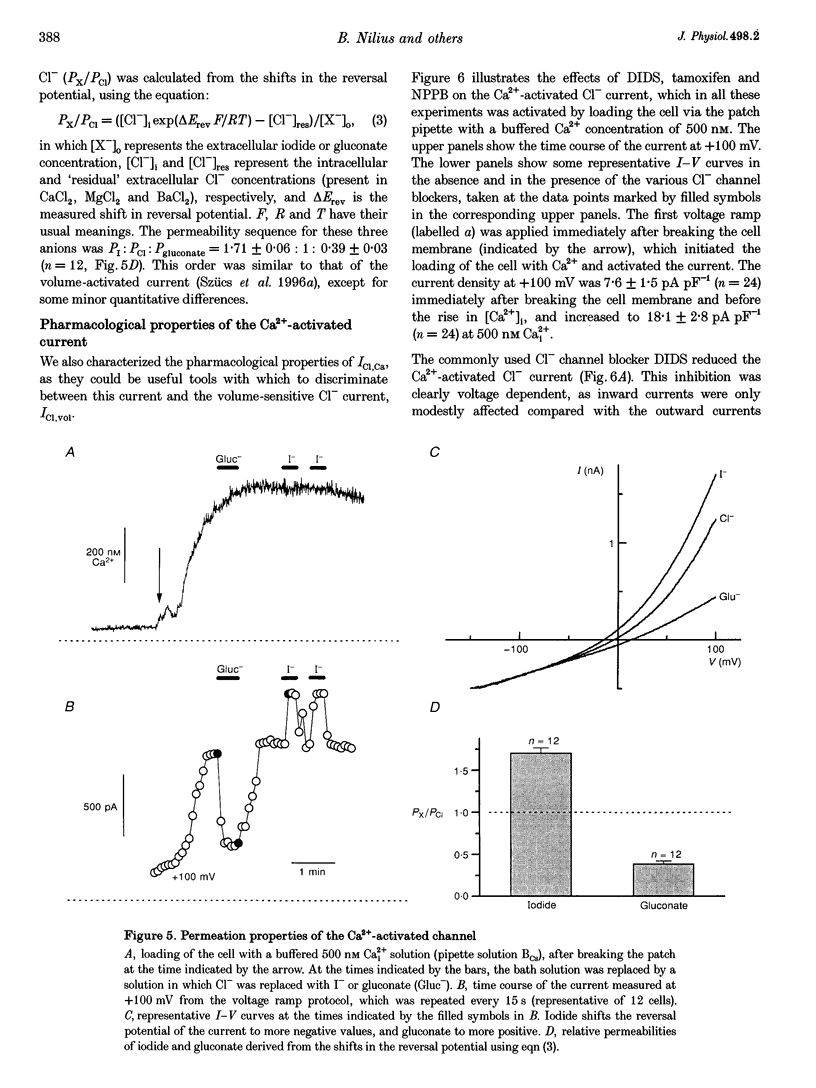
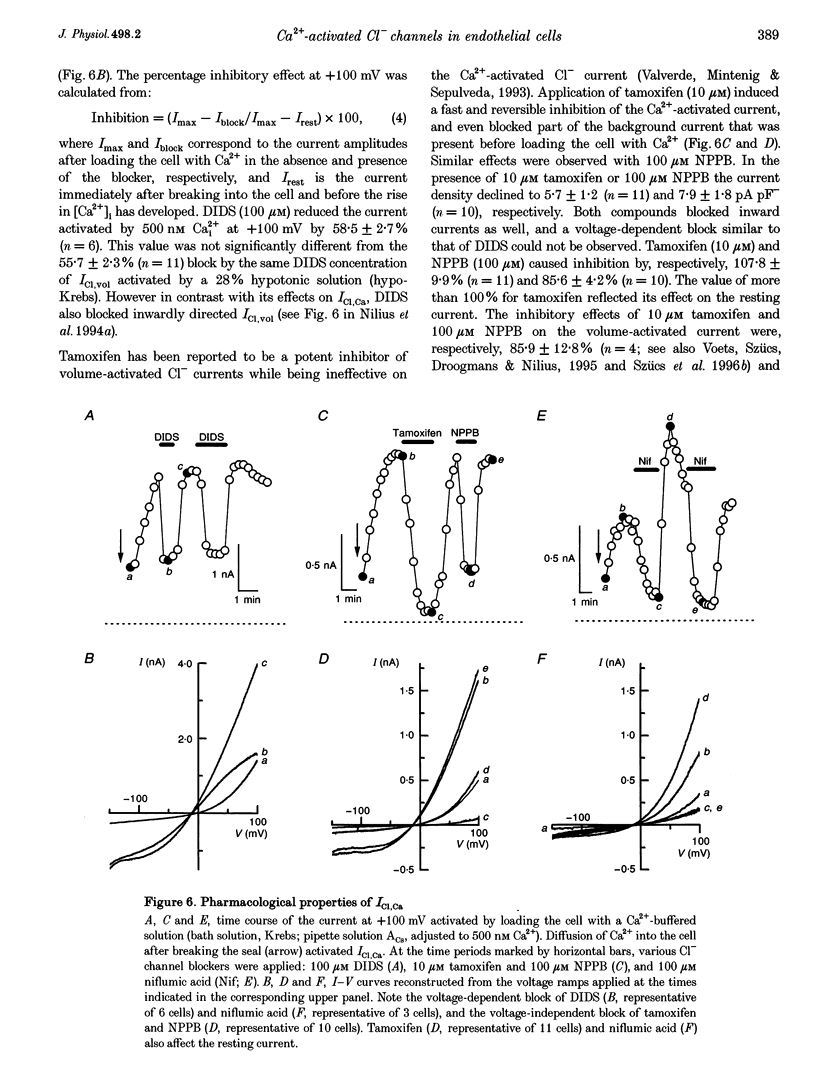
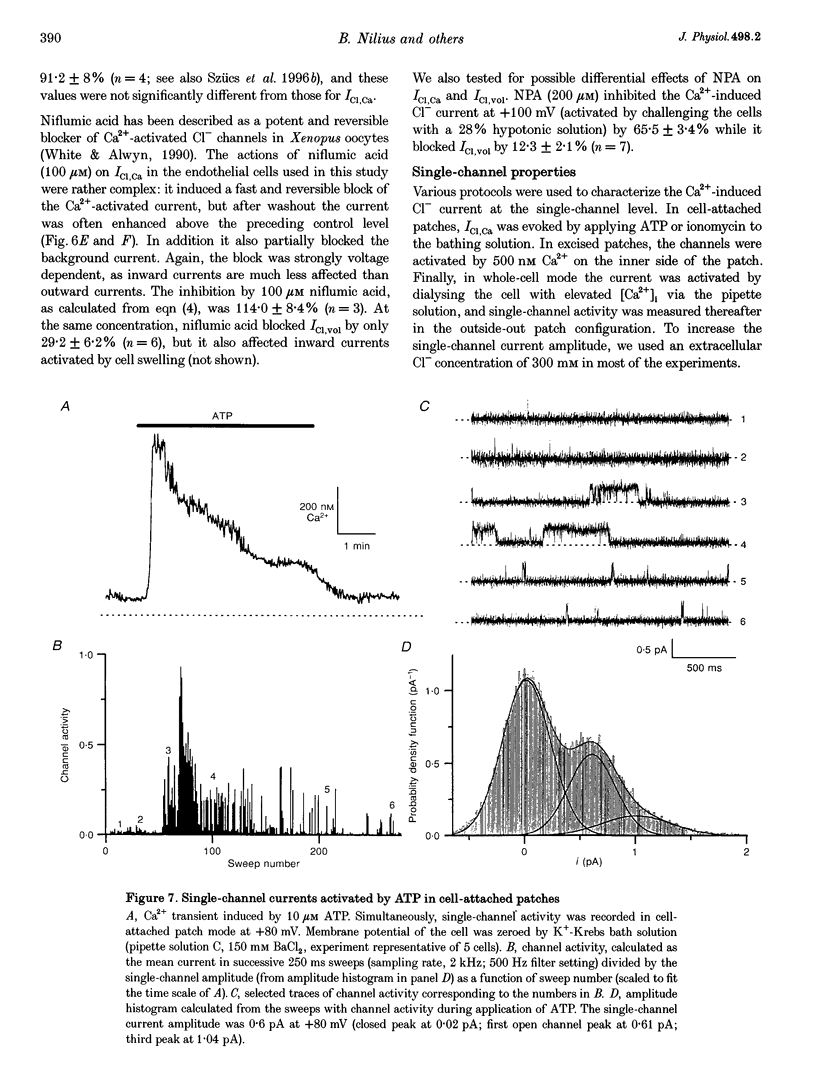
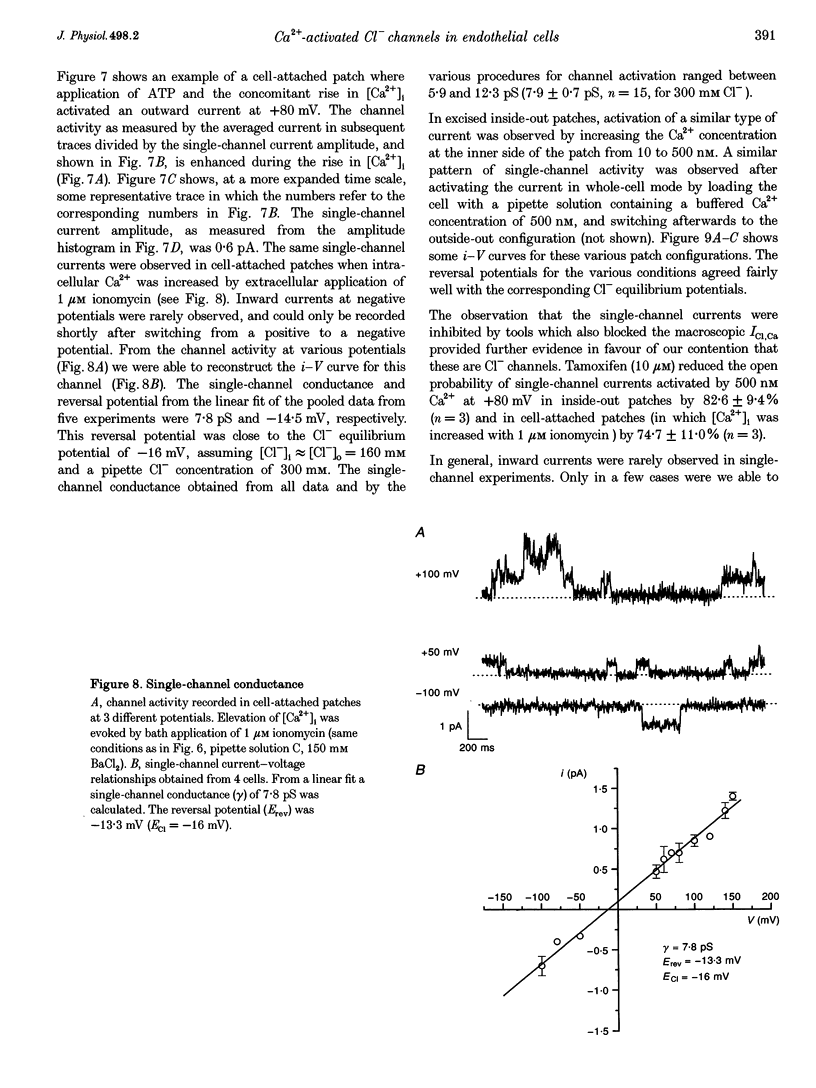
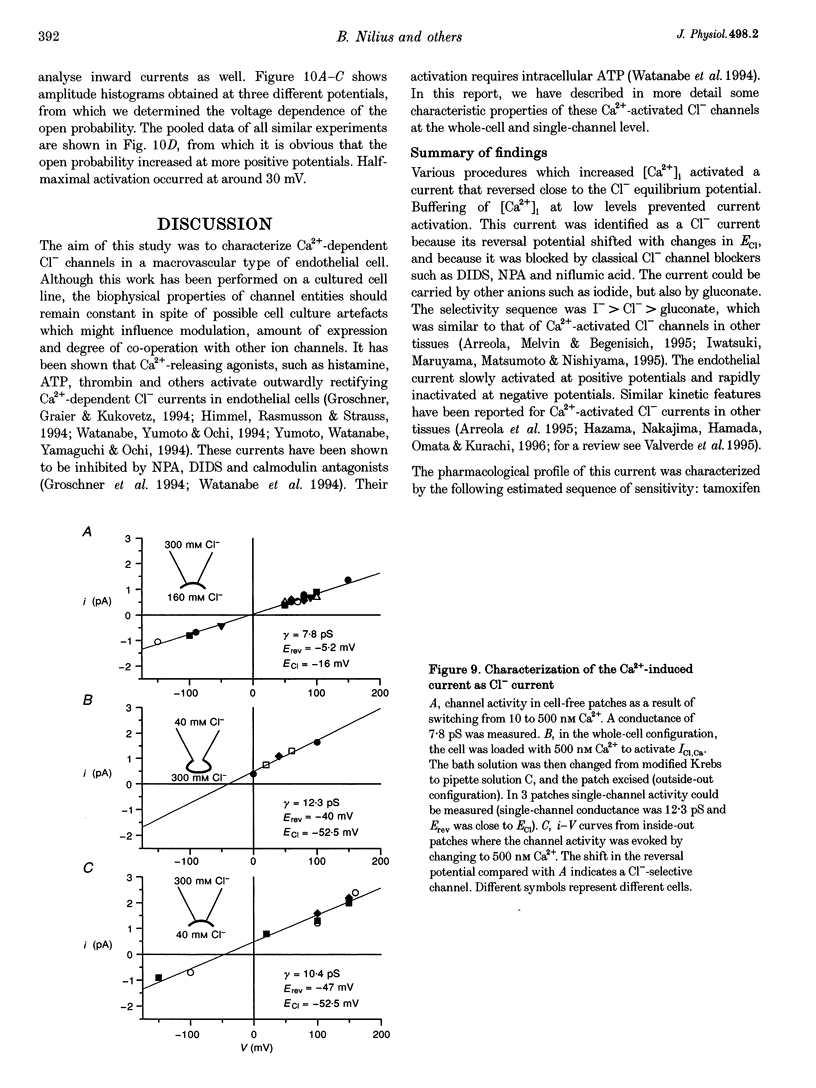
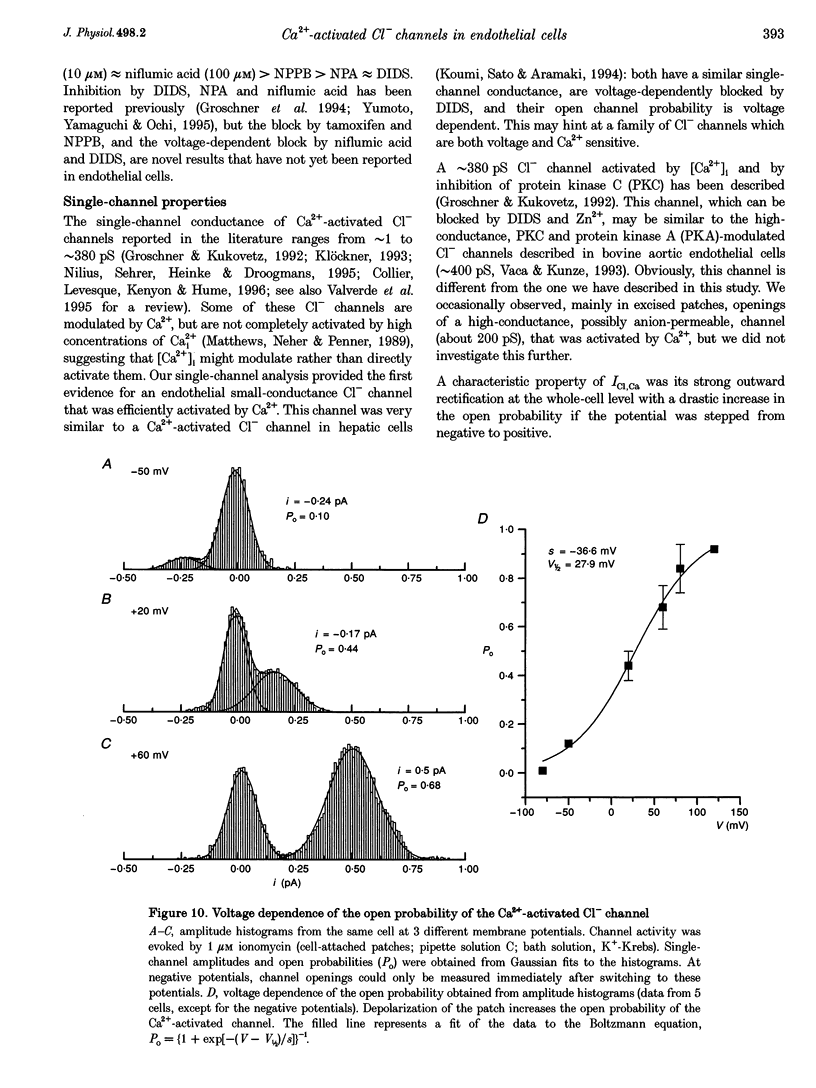
1. We characterized Ca(2+)-activated Cl- currents in calf pulmonary artery endothelial (CPAE) cells by using a combined patch clamp and fura-2 microfluorescence technique to simultaneously measure ionic currents and the intracellular Ca2+ concentration, [Ca2+]i. 2. Various procedures that increased [Ca2+]i, such as stimulation with ATP or ionomycin, or loading the cells with Ca2+ via the patch pipette, activated a strongly outwardly rectifying current with a reversal potential close to the Cl- equilibrium potential. Changing the extracellular Cl- concentration shifted this reversal potential as predicted for a Cl- current. Buffering Ca2+ rises with BAPTA prevented ATP from activating the current. 3. Ca(2+)-activated Cl- currents could be distinguished from volume-activated Cl- currents, which were sometimes coactivated in the same cell. The latter showed much less outward rectification, their activation was voltage independent, and they could be inhibited by exposing the cells to hypertonic solutions. 4. The permeability ratio for the Ca(2+)-activated conductance of the anions iodide:chloride: gluconate was 1.71 +/- 0.06:1:0.39 +/- 0.03 (n = 12). 5. This Ca(2+)-activated Cl- current, ICl, Ca, inactivated rapidly at negative potentials and activated slowly at positive potentials. Outward tail currents were slowly decaying, while inward tail currents decayed much faster. 6. 4,4'-Diisothiocyanatostilbene-2,2'-disulphonic-acid (DIDS) and niflumic acid inhibited Icl,Ca in a voltage-dependent manner, i.e. they exerted a more potent block at positive potentials. The block by N-phenylanthracilic acid (NPA), 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB) and tamoxifen was voltage independent. Niflumic acid and tamoxifen were the most potent blockers. 7. The single-channel conductance was 7.9 +/- 0.7 pS (n = 15) at 300 mM extracellular Cl-. The channel open probability was high at positive potentials, but very small at negative potentials. 8. It is concluded that [Ca2+]i activates small-conductance Cl- channels in endothelial cells, which coexist with the volume-activated Cl- channels described previously.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arreola J., Melvin J. E., Begenisich T. Volume-activated chloride channels in rat parotid acinar cells. J Physiol. 1995 May 1;484(Pt 3):677–687. doi: 10.1113/jphysiol.1995.sp020695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier M. L., Levesque P. C., Kenyon J. L., Hume J. R. Unitary Cl- channels activated by cytoplasmic Ca2+ in canine ventricular myocytes. Circ Res. 1996 May;78(5):936–944. doi: 10.1161/01.res.78.5.936. [DOI] [PubMed] [Google Scholar]
- Cunningham S. A., Awayda M. S., Bubien J. K., Ismailov I. I., Arrate M. P., Berdiev B. K., Benos D. J., Fuller C. M. Cloning of an epithelial chloride channel from bovine trachea. J Biol Chem. 1995 Dec 29;270(52):31016–31026. doi: 10.1074/jbc.270.52.31016. [DOI] [PubMed] [Google Scholar]
- Daut J., Standen N. B., Nelson M. T. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994 Feb;5(2):154–181. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Fuller C. M., Ismailov I. I., Keeton D. A., Benos D. J. Phosphorylation and activation of a bovine tracheal anion channel by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1994 Oct 28;269(43):26642–26650. [PubMed] [Google Scholar]
- Groschner K., Graier W. F., Kukovetz W. R. Histamine induces K+, Ca2+, and Cl- currents in human vascular endothelial cells. Role of ionic currents in stimulation of nitric oxide biosynthesis. Circ Res. 1994 Aug;75(2):304–314. doi: 10.1161/01.res.75.2.304. [DOI] [PubMed] [Google Scholar]
- Groschner K., Kukovetz W. R. Voltage-sensitive chloride channels of large conductance in the membrane of pig aortic endothelial cells. Pflugers Arch. 1992 Jun;421(2-3):209–217. doi: 10.1007/BF00374829. [DOI] [PubMed] [Google Scholar]
- Hazama H., Nakajima T., Hamada E., Omata M., Kurachi Y. Neurokinin A and Ca2+ current induce Ca(2+)-activated Cl(-) currents in guinea-pig tracheal myocytes. J Physiol. 1996 Apr 15;492(Pt 2):377–393. doi: 10.1113/jphysiol.1996.sp021315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S. Chloride channel blockers inhibit ACTH secretion from mouse pituitary tumor cells. Am J Physiol. 1991 Apr;260(4 Pt 1):E505–E512. doi: 10.1152/ajpendo.1991.260.4.E505. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hosoki E., Iijima T. Chloride-sensitive Ca2+ entry by histamine and ATP in human aortic endothelial cells. Eur J Pharmacol. 1994 Feb 15;266(3):213–218. doi: 10.1016/0922-4106(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Klöckner U. Intracellular calcium ions activate a low-conductance chloride channel in smooth-muscle cells isolated from human mesenteric artery. Pflugers Arch. 1993 Aug;424(3-4):231–237. doi: 10.1007/BF00384347. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Bolden A., Horn R. Control of action potentials and Ca2+ influx by the Ca(2+)-dependent chloride current in mouse pituitary cells. J Physiol. 1991 Aug;439:423–437. doi: 10.1113/jphysiol.1991.sp018674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S., Sato R., Aramaki T. Characterization of the calcium-activated chloride channel in isolated guinea-pig hepatocytes. J Gen Physiol. 1994 Aug;104(2):357–373. doi: 10.1085/jgp.104.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Neher E., Penner R. Chloride conductance activated by external agonists and internal messengers in rat peritoneal mast cells. J Physiol. 1989 Nov;418:131–144. doi: 10.1113/jphysiol.1989.sp017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Oike M., Zahradnik I., Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 1994 May;103(5):787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Droogmans G. Permeation properties and modulation of volume-activated Cl(-)-currents in human endothelial cells. Br J Pharmacol. 1994 Aug;112(4):1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Heinke S., Droogmans G. Ca2+ release and activation of K+ and Cl- currents by extracellular ATP in distal nephron epithelial cells. Am J Physiol. 1995 Aug;269(2 Pt 1):C376–C384. doi: 10.1152/ajpcell.1995.269.2.C376. [DOI] [PubMed] [Google Scholar]
- Oike M., Droogmans G., Nilius B. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2940–2944. doi: 10.1073/pnas.91.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Gericke M., Droogmans G., Nilius B. Calcium entry activated by store depletion in human umbilical vein endothelial cells. Cell Calcium. 1994 Nov;16(5):367–376. doi: 10.1016/0143-4160(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Revest P. A., Abbott N. J. Membrane ion channels of endothelial cells. Trends Pharmacol Sci. 1992 Nov;13(11):404–407. doi: 10.1016/0165-6147(92)90124-o. [DOI] [PubMed] [Google Scholar]
- Rugolo M., Mastrocola T., Whörle C., Rasola A., Gruenert D. C., Romeo G., Galietta L. J. ATP and A1 adenosine receptor agonists mobilize intracellular calcium and activate K+ and Cl- currents in normal and cystic fibrosis airway epithelial cells. J Biol Chem. 1993 Nov 25;268(33):24779–24784. [PubMed] [Google Scholar]
- Schumacher P. A., Sakellaropoulos G., Phipps D. J., Schlichter L. C. Small-conductance chloride channels in human peripheral T lymphocytes. J Membr Biol. 1995 Jun;145(3):217–232. doi: 10.1007/BF00232714. [DOI] [PubMed] [Google Scholar]
- Strange K., Jackson P. S. Swelling-activated organic osmolyte efflux: a new role for anion channels. Kidney Int. 1995 Oct;48(4):994–1003. doi: 10.1038/ki.1995.381. [DOI] [PubMed] [Google Scholar]
- Szücs G., Heinke S., De Greef C., Raeymaekers L., Eggermont J., Droogmans G., Nilius B. The volume-activated chloride current in endothelial cells from bovine pulmonary artery is not modulated by phosphorylation. Pflugers Arch. 1996 Feb;431(4):540–548. doi: 10.1007/BF02191901. [DOI] [PubMed] [Google Scholar]
- Ueda S., Lee S. L., Fanburg B. L. Chloride efflux in cyclic AMP-induced configurational change of bovine pulmonary artery endothelial cells. Circ Res. 1990 Apr;66(4):957–967. doi: 10.1161/01.res.66.4.957. [DOI] [PubMed] [Google Scholar]
- Vaca L., Kunze D. L. cAMP-dependent phosphorylation modulates voltage gating in an endothelial Cl- channel. Am J Physiol. 1993 Feb;264(2 Pt 1):C370–C375. doi: 10.1152/ajpcell.1993.264.2.C370. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Hardy S. P., Sepúlveda F. V. Chloride channels: a state of flux. FASEB J. 1995 Apr;9(7):509–515. doi: 10.1096/fasebj.9.7.7737459. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Mintenig G. M., Sepúlveda F. V. Differential effects of tamoxifen and I- on three distinguishable chloride currents activated in T84 intestinal cells. Pflugers Arch. 1993 Dec;425(5-6):552–554. doi: 10.1007/BF00374885. [DOI] [PubMed] [Google Scholar]
- Voets T., Szücs G., Droogmans G., Nilius B. Blockers of volume-activated Cl- currents inhibit endothelial cell proliferation. Pflugers Arch. 1995 Nov;431(1):132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Yumoto K., Ochi R. Indirect activation by internal calcium of chloride channels in endothelial cells. Jpn J Physiol. 1994;44 (Suppl 2):S233–S236. [PubMed] [Google Scholar]
- Yumoto K., Watanabe M., Yamaguchi H., Ochi R. ATP-induced chloride current and tonic increase of internal Ca2+ concentration in vascular endothelial cells. Jpn J Physiol. 1994;44 (Suppl 2):S241–S243. [PubMed] [Google Scholar]
- Yumoto K., Yamaguchi H., Ochi R. Depression of ATP-induced Ca2+ signalling by high K+ and low Cl- media in human aortic endothelial cells. Jpn J Physiol. 1995;45(1):111–122. doi: 10.2170/jjphysiol.45.111. [DOI] [PubMed] [Google Scholar]