Abstract
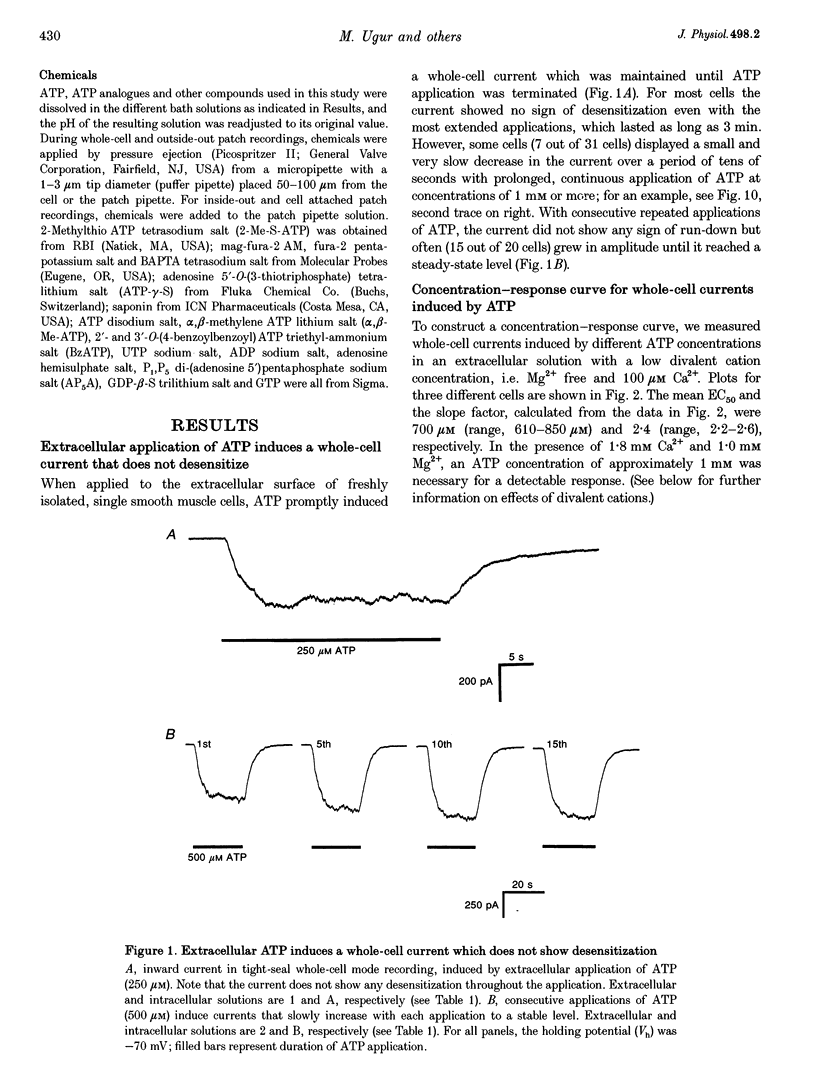
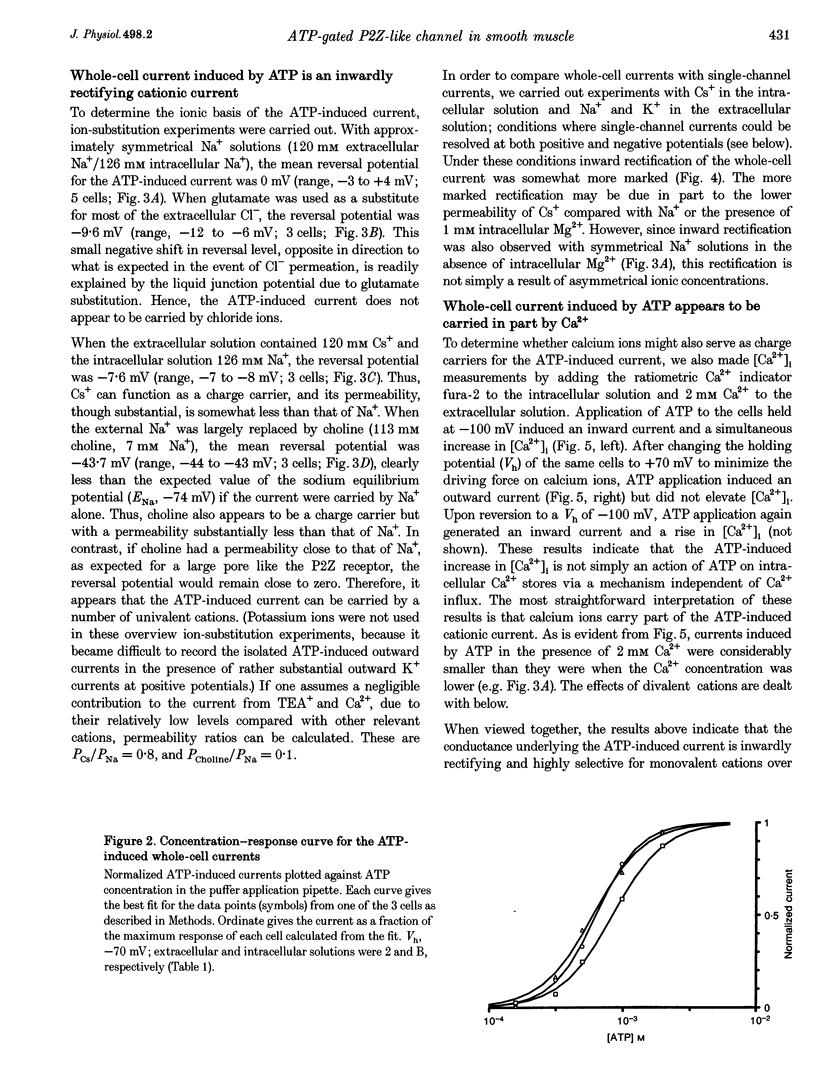
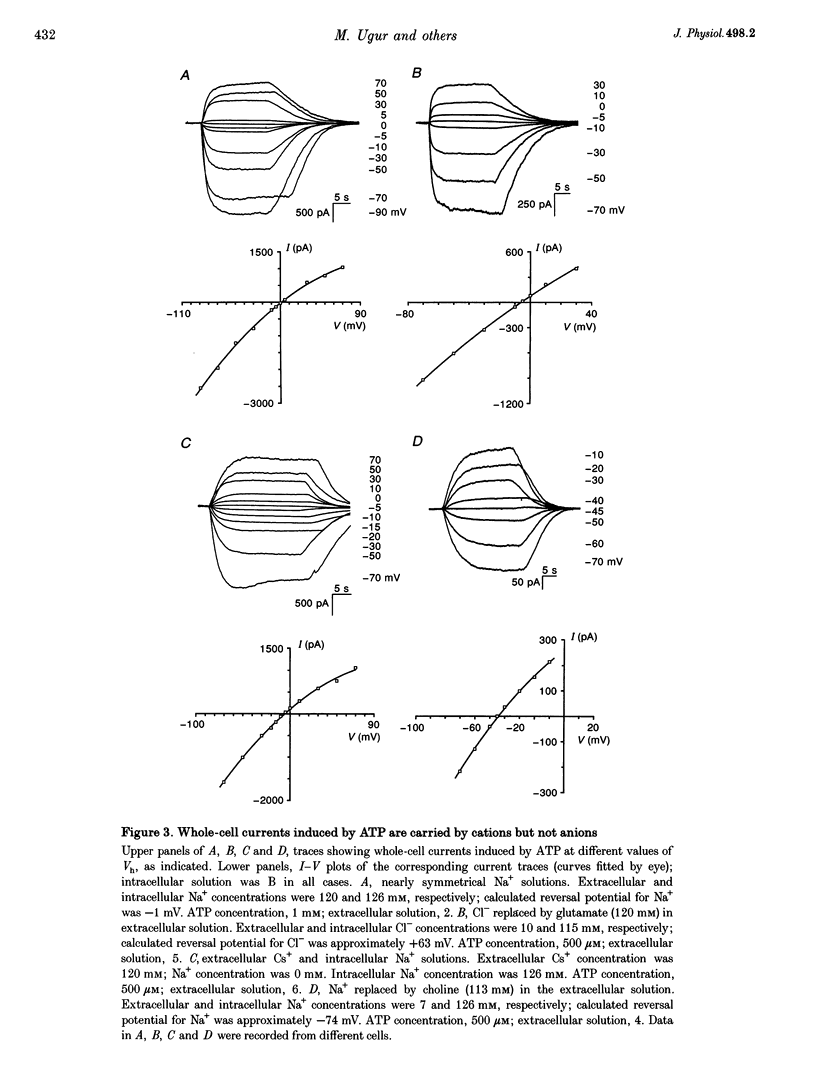
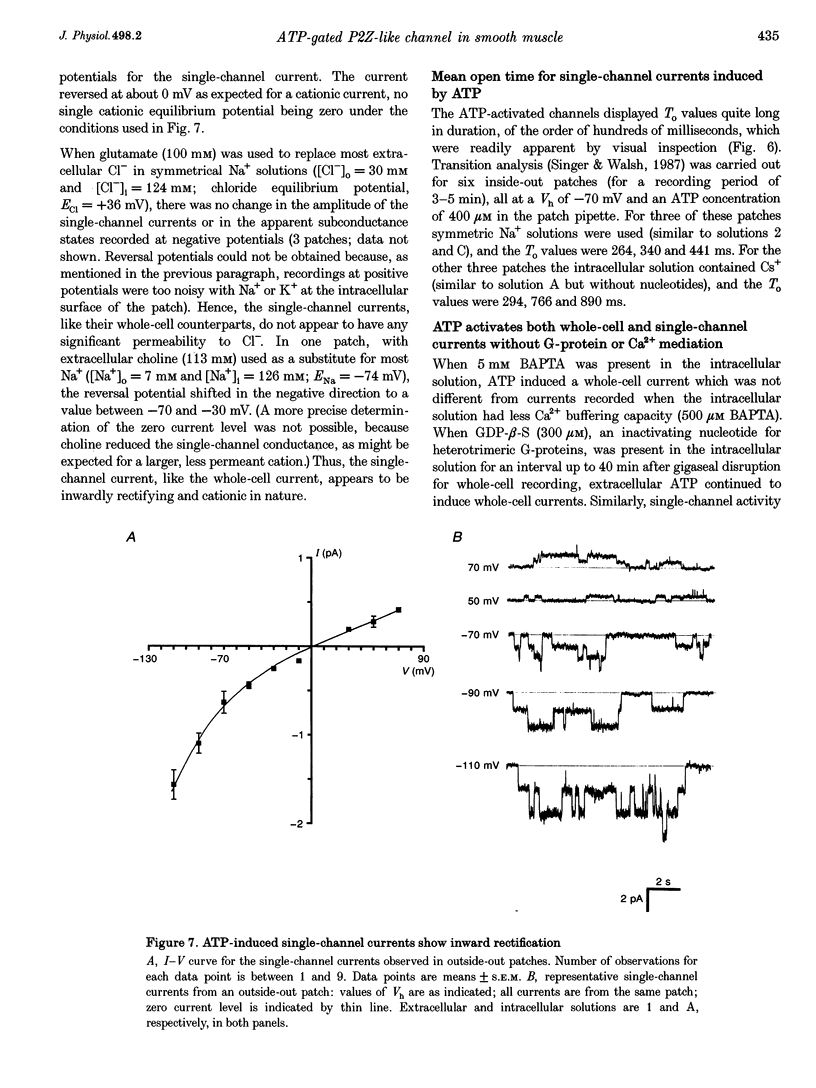
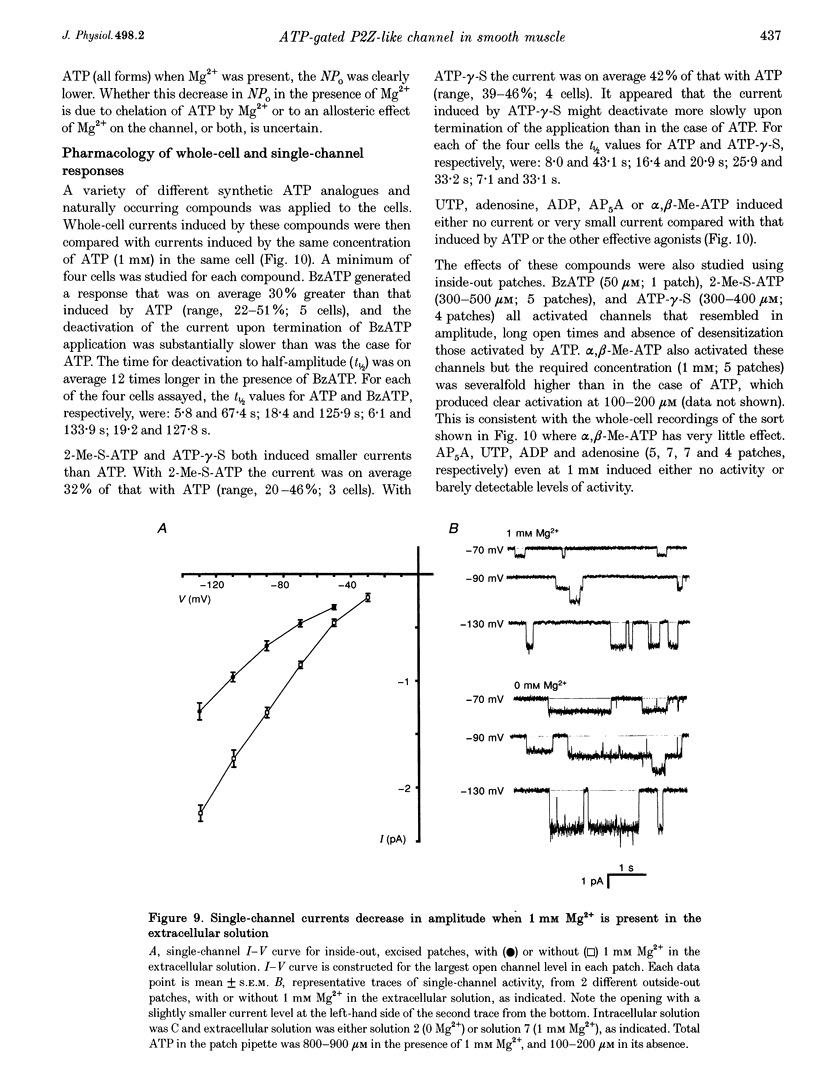
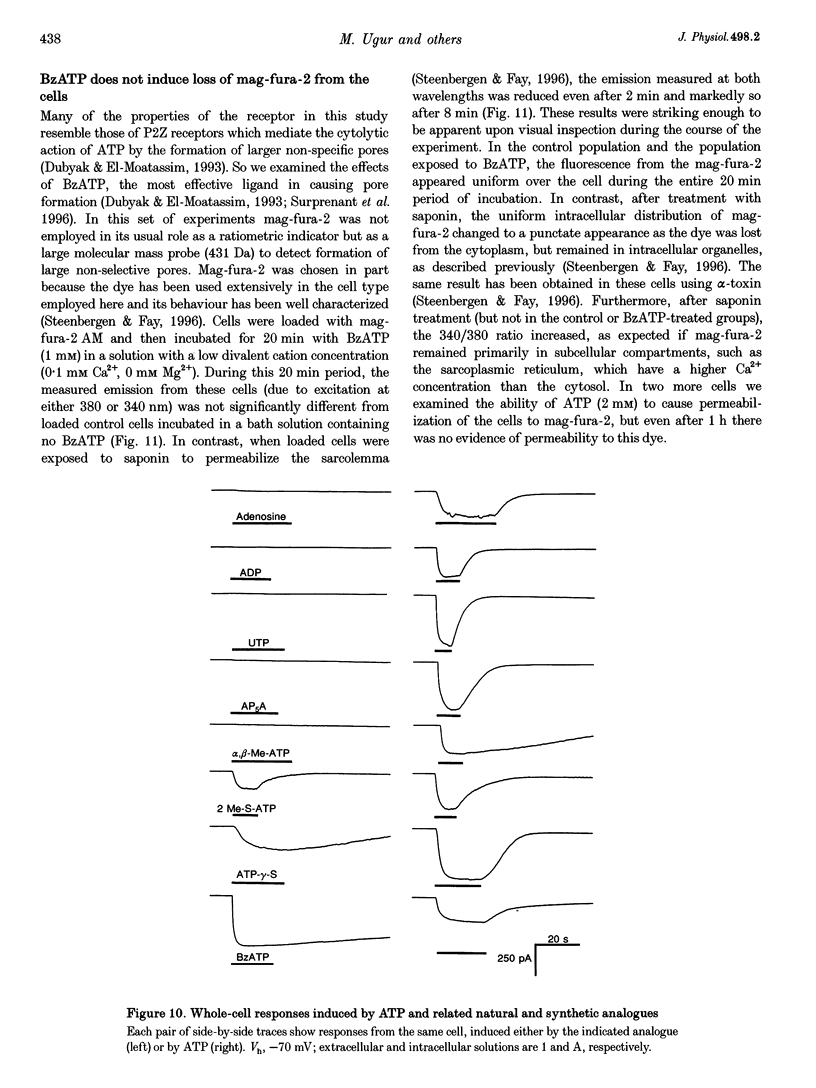
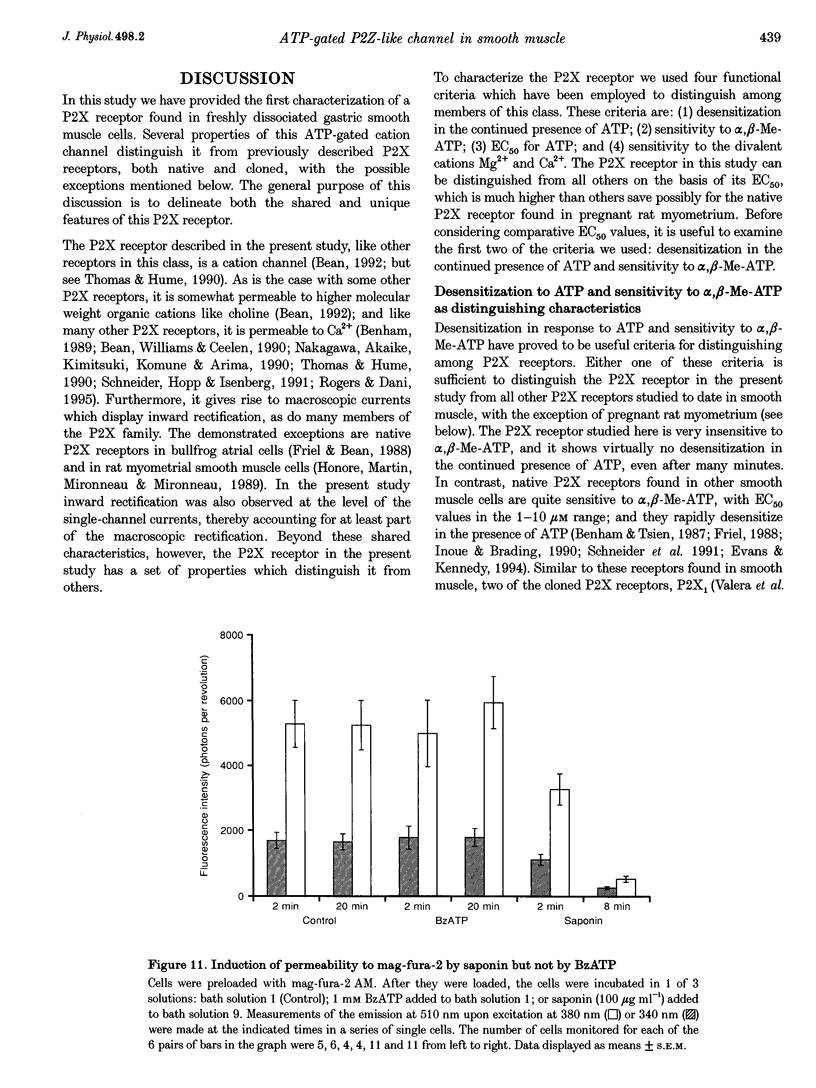
1. Whole-cell and single-channel currents elicited by extracellular ATP were studied in freshly dissociated smooth muscle cells from the stomach of the toad Bufo marinus using standard patch clamp and microfluorimetric techniques. 2. This ATP-gated cation channel shares a number of pharmacological and functional properties with native rat myometrium receptors, certain native P2Z purinoceptors and the recently cloned P2X7 purinoceptor. But, unlike the last two, the ATP-gated channel does not mediate the formation of large non-specific pores. Thus, it may represent a novel member of the P2X or P2Z class. 3. Extracellular application of ATP (> or = 150 microM) elicited an inward whole-cell current at negative holding potentials that was inwardly rectifying and showed no sign of desensitization. Na+, Cs+ and, to a lesser degree, the organic cation choline served as charge carriers, but Cl- did not. Ratiometric fura-2 measurements indicated that the current is carried in part by Ca2+. The EC50 for ATP was 700 microM in solutions with a low divalent cation concentration. 4. ATP (> or = 100 microM) at the extracellular surface of cell-attached or excised patches elicited inwardly rectifying single-channel currents with a 22 pS conductance. Cl- did not serve as a charge carrier but both Na+ and Cs+ did, as did choline to a lesser extent. The mean open time of the channel was quite long, with a range in hundreds of milliseconds at a holding potential of -70 mV. 5. Mg2+ and Ca2+ decreased the magnitude of the ATP-induced whole-cell currents. Mg2+ decreased both the amplitude and the activity of ATP-activated single-channel currents. 6. ADP, UTP, P1, P5-di-adenosine pentaphosphate (AP5A), adenosine and alpha, beta-methylene ATP (alpha, beta-Me-ATP) did not induce significant whole-cell current. ATP-gamma-S and 2-methylthio ATP (2-Me-S-ATP) were significantly less effective than ATP in inducing whole-cell currents, whereas benzoylbenzoyl ATP (BzATP) was more effective. BzATP, alpha, beta-Me-ATP, ATP-gamma-S and 2-Me-S-ATP induced single-channel currents, but a higher concentration of alpha, beta-Me-ATP was required. 7. BzATP did not induce the formation of large non-specific pores, as assayed using mag-fura-2 as a high molecular mass probe.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham C. D. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. J Physiol. 1989 Dec;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Brake A. J., Wagenbach M. J., Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994 Oct 6;371(6497):519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- Buell G., Lewis C., Collo G., North R. A., Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996 Jan 2;15(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Buisman H. P., Steinberg T. H., Fischbarg J., Silverstein S. C., Vogelzang S. A., Ince C., Ypey D. L., Leijh P. C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Akopian A. N., Sivilotti L., Colquhoun D., Burnstock G., Wood J. N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995 Oct 5;377(6548):428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Collo G., North R. A., Kawashima E., Merlo-Pich E., Neidhart S., Surprenant A., Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996 Apr 15;16(8):2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Evans R. J., Kennedy C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br J Pharmacol. 1994 Nov;113(3):853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D., Bean B. P. Two ATP-activated conductances in bullfrog atrial cells. J Gen Physiol. 1988 Jan;91(1):1–27. doi: 10.1085/jgp.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Honoré E., Martin C., Mironneau C., Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989 Aug;257(2 Pt 1):C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- Inoue R., Brading A. F. The properties of the ATP-induced depolarization and current in single cells isolated from the guinea-pig urinary bladder. Br J Pharmacol. 1990 Jul;100(3):619–625. doi: 10.1111/j.1476-5381.1990.tb15856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B. S., Humphrey P. P., Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J Physiol. 1995 Apr 15;484(Pt 2):385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassignal N. L., Singer J. J., Walsh J. V., Jr Multiple neuropeptides exert a direct effect on the same isolated single smooth muscle cell. Am J Physiol. 1986 May;250(5 Pt 1):C792–C798. doi: 10.1152/ajpcell.1986.250.5.C792. [DOI] [PubMed] [Google Scholar]
- Lewis C., Neidhart S., Holy C., North R. A., Buell G., Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995 Oct 5;377(6548):432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Latorre R., Miller C. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 1989 Oct 3;28(20):8092–8099. doi: 10.1021/bi00446a020. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Nakazawa K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci. 1994 Feb;14(2):740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P., Zanovello P., Bronte V., Di Virgilio F. Extracellular ATP causes lysis of mouse thymocytes and activates a plasma membrane ion channel. Biochem J. 1991 Feb 15;274(Pt 1):139–144. doi: 10.1042/bj2740139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Dani J. A. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys J. 1995 Feb;68(2):501–506. doi: 10.1016/S0006-3495(95)80211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J Physiol. 1993 May;464:197–212. doi: 10.1113/jphysiol.1993.sp019630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Talamo B. R. ATP activates a cation-permeable pathway in rat parotid acinar cells. Am J Physiol. 1992 Apr;262(4 Pt 1):C934–C940. doi: 10.1152/ajpcell.1992.262.4.C934. [DOI] [PubMed] [Google Scholar]
- Soto F., Garcia-Guzman M., Gomez-Hernandez J. M., Hollmann M., Karschin C., Stühmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen J. M., Fay F. S. The quantal nature of calcium release to caffeine in single smooth muscle cells results from activation of the sarcoplasmic reticulum Ca(2+)-ATPase. J Biol Chem. 1996 Jan 26;271(4):1821–1824. doi: 10.1074/jbc.271.4.1821. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Newman A. S., Swanson J. A., Silverstein S. C. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987 Jun 25;262(18):8884–8888. [PubMed] [Google Scholar]
- Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996 May 3;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Tatham P. E., Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990 Apr;95(4):569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S., Hussy N., Evans R. J., Adami N., North R. A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994 Oct 6;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Chen R., Jamieson G. P. The ATP4- receptor-operated channel (P2Z class) of human lymphocytes allows Ba2+ and ethidium+ uptake: inhibition of fluxes by suramin. Arch Biochem Biophys. 1993 Aug 15;305(1):54–60. doi: 10.1006/abbi.1993.1392. [DOI] [PubMed] [Google Scholar]


