Abstract
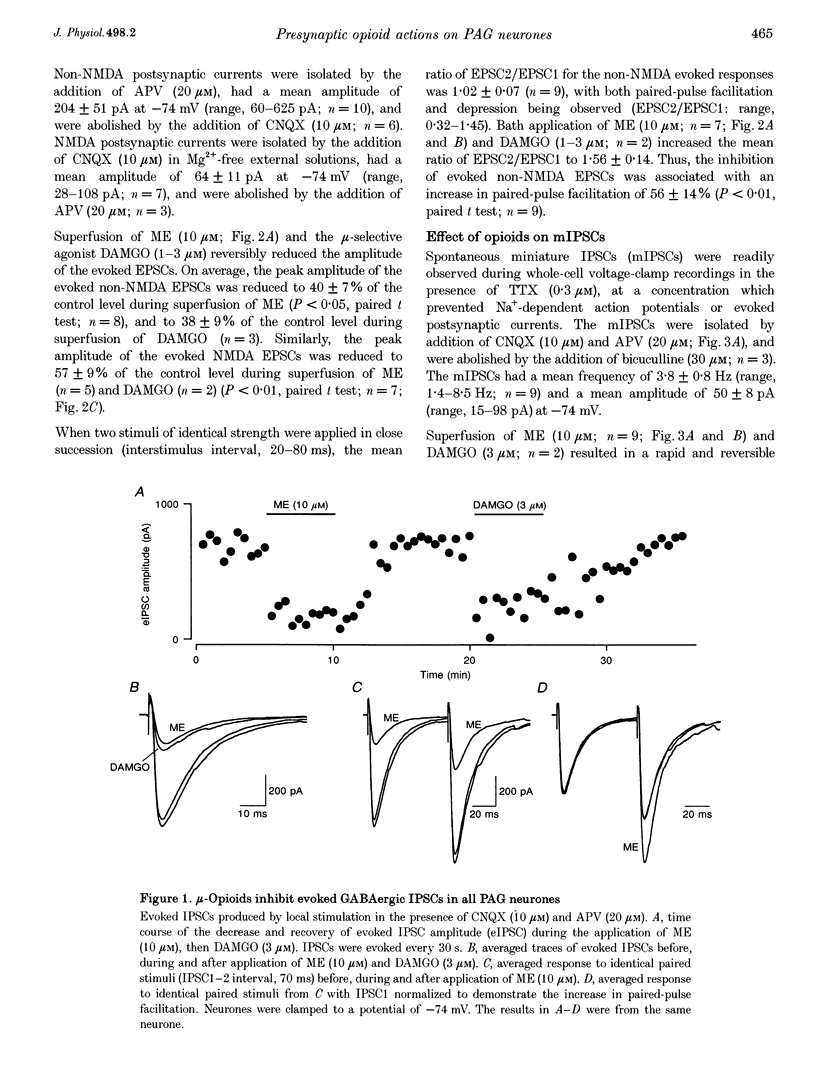
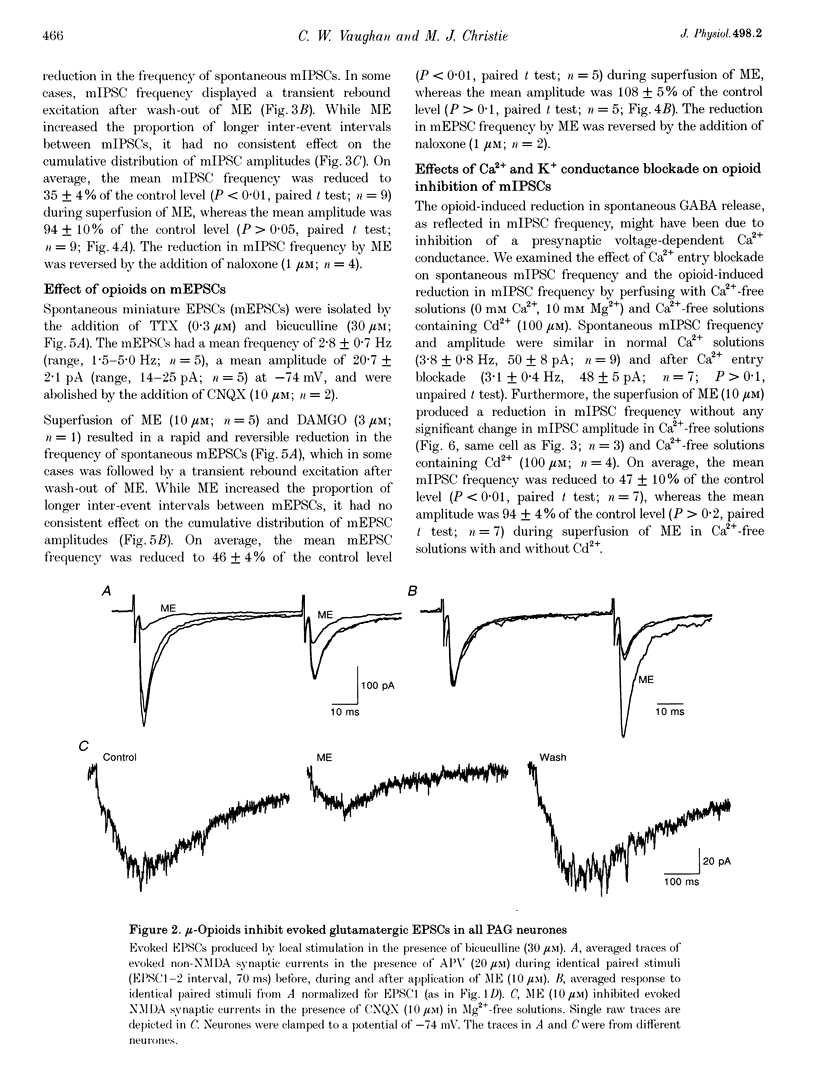
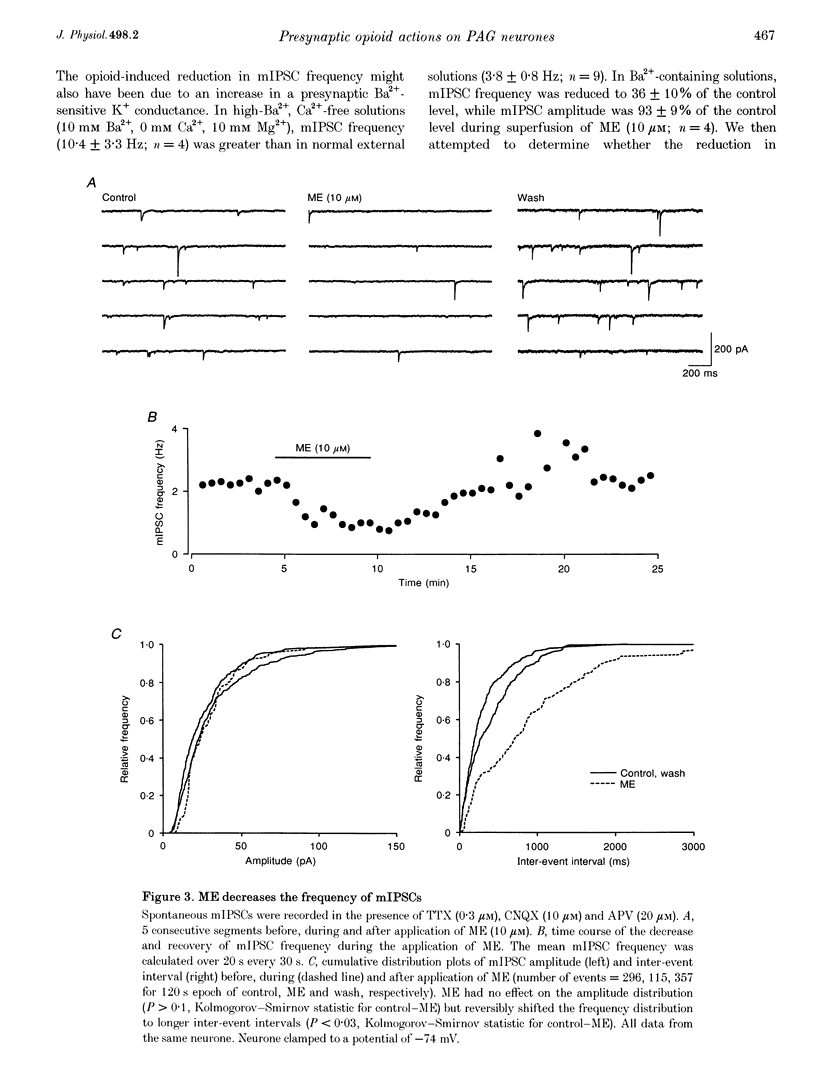
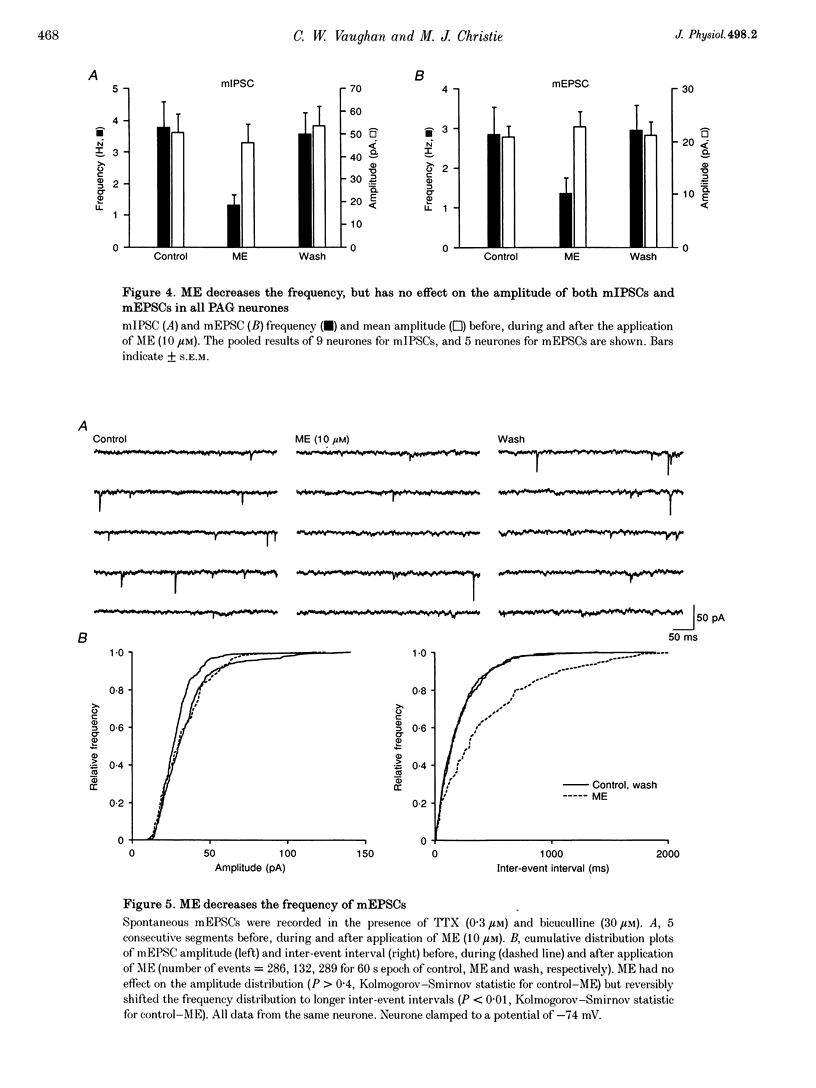
1. The actions of opioids on synaptic transmission in rat periaqueductal grey (PAG) neurones were examined using whole-cell patch-clamp recordings in brain slices. 2. Methionine enkephalin (ME; 10 microM) inhibited evoked GABAergic inhibitory postsynaptic currents (IPSCs) by 57%, non-NMDA excitatory postsynaptic currents (EPSCs) by 60%, and NMDA EPSCs by 43% in PAG neurones. This inhibition was associated with an increase in paired-pulse facilitation, was mimicked by the mu-agonist DAMGO (1-3 microM) and abolished by naloxone (1 microM). Neither the kappa-agonist U69593 (1-3 microM), nor the delta-agonist DPDPE (3-10 microM) had any specific actions on evoked PSCs. 3. ME decreased the frequency of spontaneous miniature, action potential-independent postsynaptic currents (mIPSCs by 65%, mEPSCs by 54%) in all PAG neurones, but had no effect on their amplitude distributions. The reduction in mIPSC frequency persisted in nominally Ca(2+)-free, high-Mg2+ (10 mM) solutions, which also contained Cd2+ (100 microM), or Ba2+ (10 mM). Opioid inhibition of mIPSC frequency is unlikely to be mediated by presynaptic Ca2+ or K+ conductances which are sensitive to extracellular Cd2+ or Ba2+. 4. In a subpopulation of PAG neurones, ME increased a Ba(2+)-sensitive K+ conductance at potentials below -97 mV. Opioids inhibited both GABAergic and glutamatergic synaptic transmission in all PAG neurones, independent of any postsynaptic opioid sensitivity. 5. These observations are consistent with, but only partially support, the opioid disinhibition model of PAG-induced analgesia. mu-Opioids also have the potential to modulate the behavioural and autonomic functions of the PAG via modulation of both inhibitory and excitatory presynaptic mechanisms, as well as postsynaptic mechanisms.
Full text
PDF

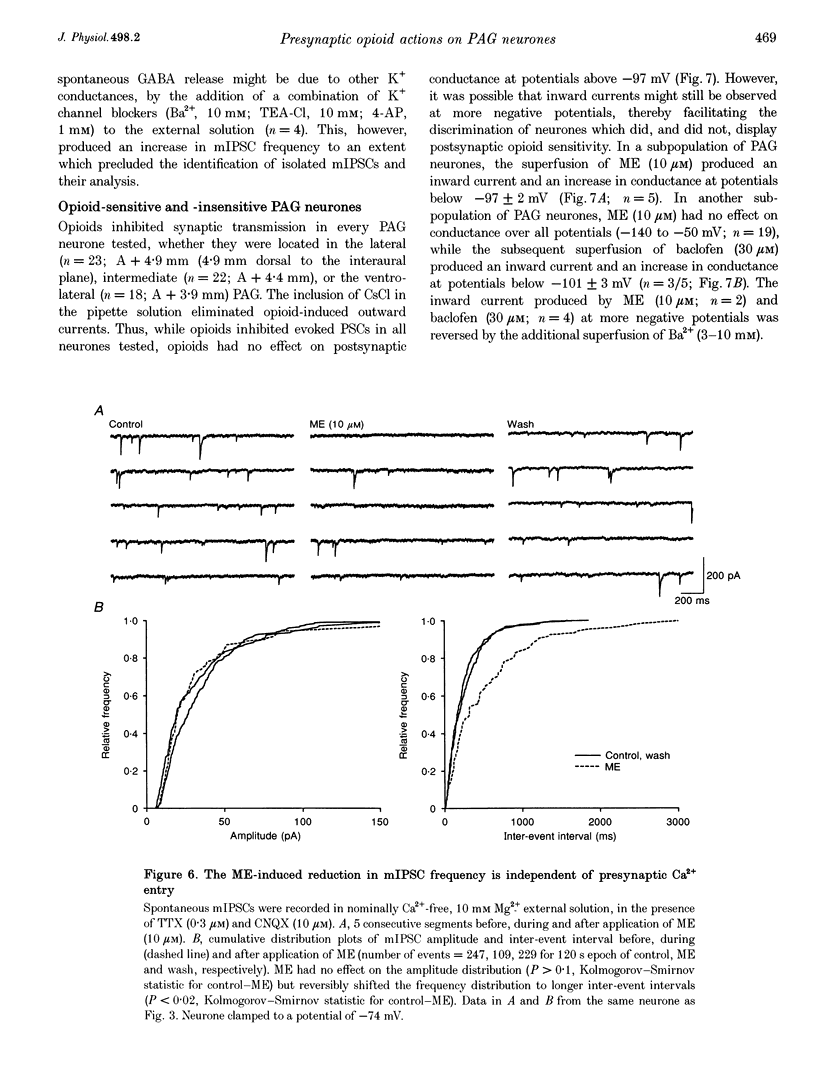
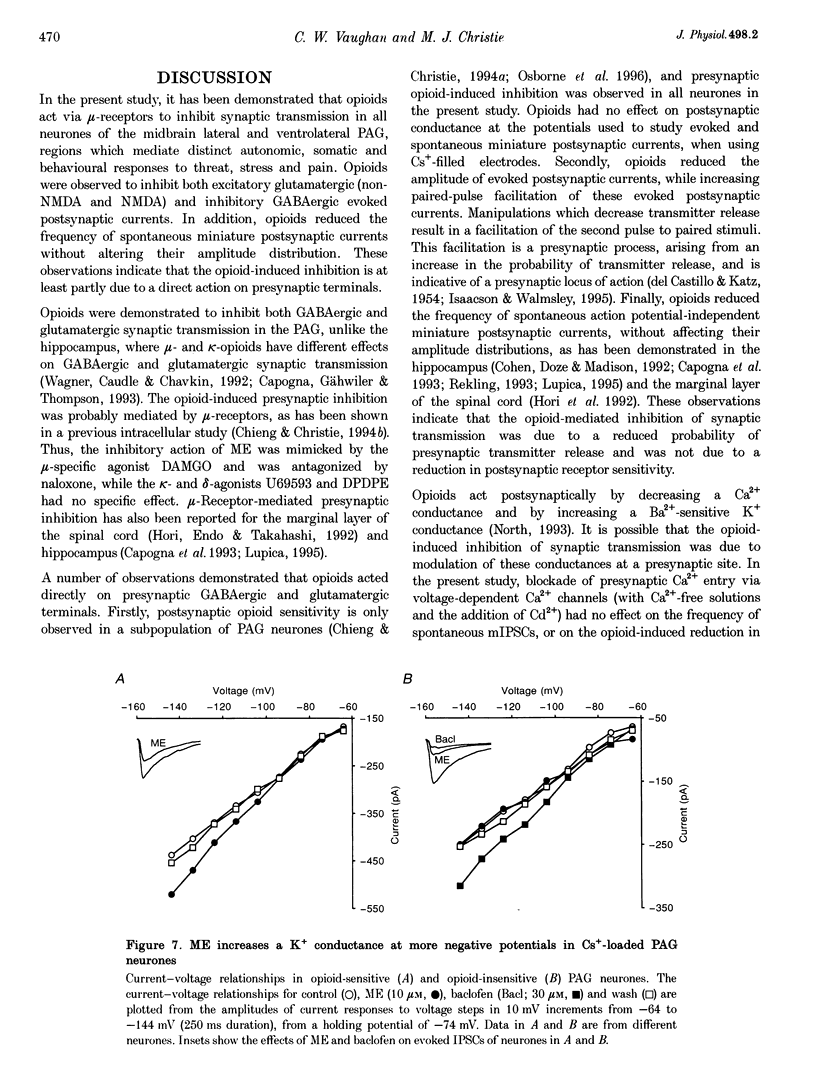


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandler R., Shipley M. T. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994 Sep;17(9):379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Fields H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Capogna M., Gähwiler B. H., Thompson S. M. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993 Oct;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994 Sep;113(1):121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B., Christie M. J. Inhibition by opioids acting on mu-receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994 Sep;113(1):303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. A., Doze V. A., Madison D. V. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992 Aug;9(2):325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y., Endo K., Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol. 1992 May;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J. S., Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995 Oct;15(4):875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993 May;40(5):631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Lupica C. R. Delta and mu enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995 Jan;15(1 Pt 2):737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne P. B., Vaughan C. W., Wilson H. I., Christie M. J. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol. 1996 Jan 15;490(Pt 2):383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling J. C. Effects of met-enkephalin on GABAergic spontaneous miniature IPSPs in organotypic slice cultures of the rat hippocampus. J Neurosci. 1993 May;13(5):1954–1964. doi: 10.1523/JNEUROSCI.13-05-01954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. J., Caudle R. M., Chavkin C. Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J Neurosci. 1992 Jan;12(1):132–141. doi: 10.1523/JNEUROSCI.12-01-00132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L., Al-Rodhan N. R., Jensen T. S. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371–394. doi: 10.1016/s0079-6123(08)62803-4. [DOI] [PubMed] [Google Scholar]


