Abstract
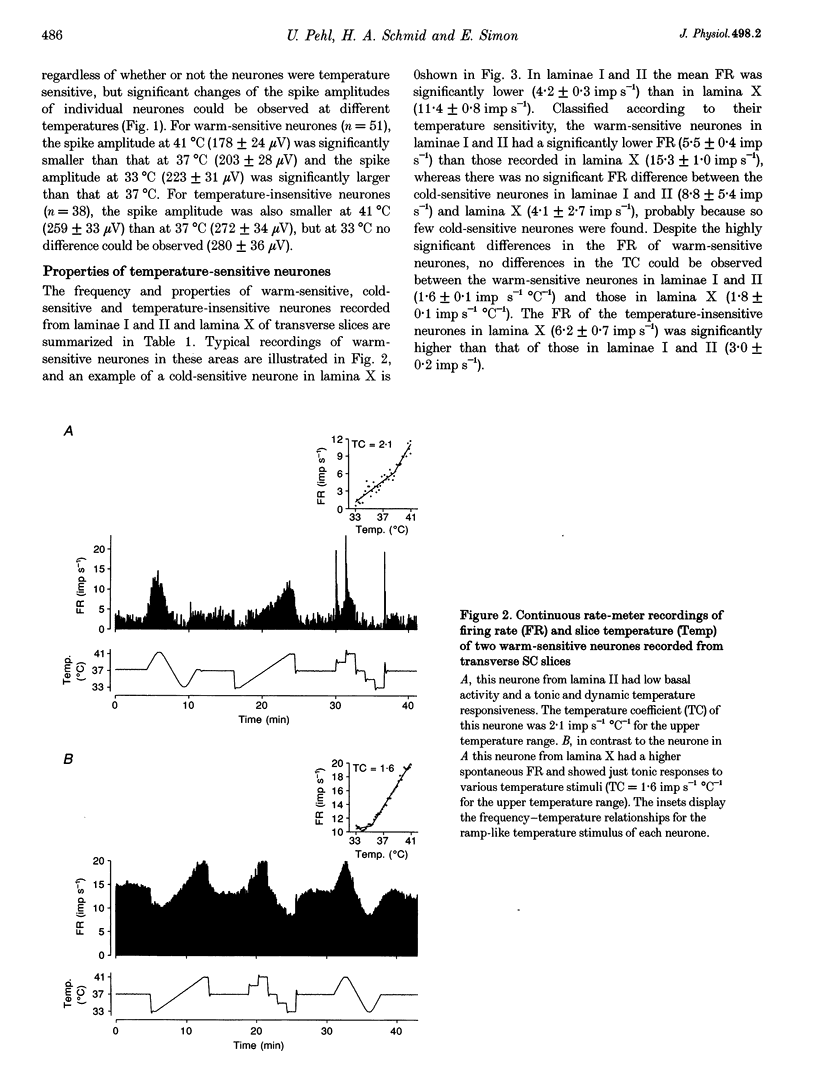
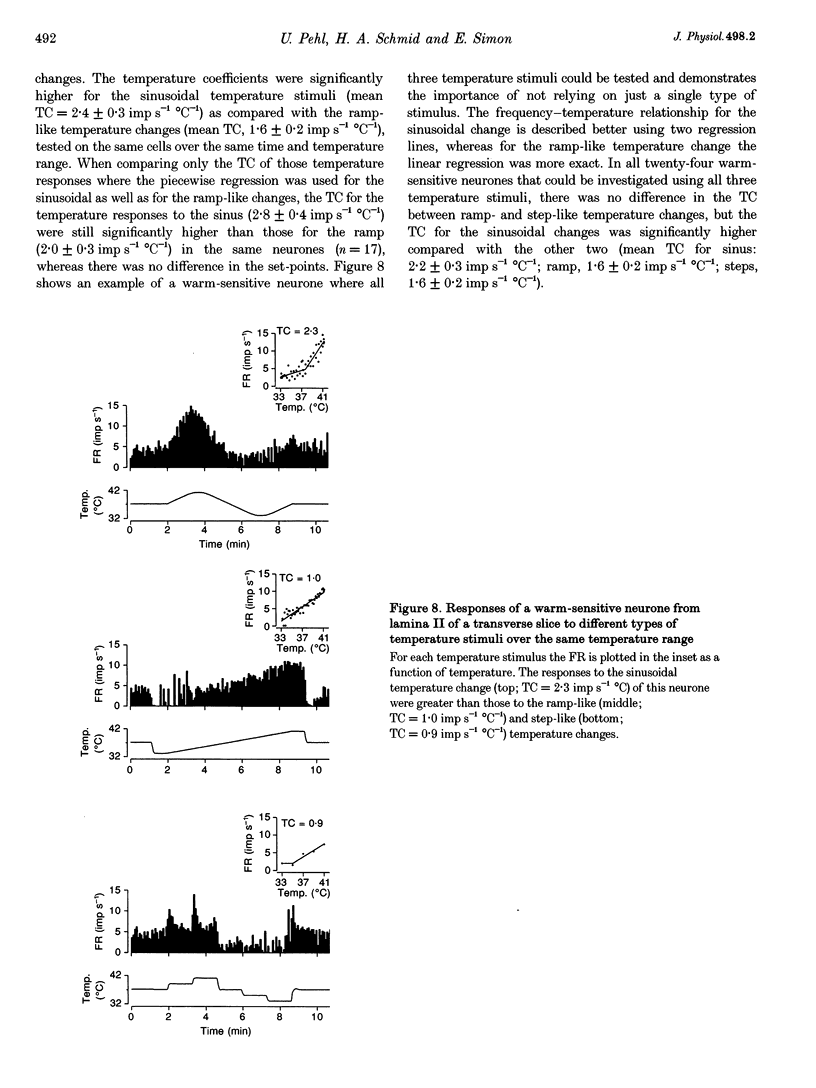
1. The inherent temperature sensitivity of 343 spontaneously active neurones recorded from rat spinal cord (SC) slices was investigated electrophysiologically. Recordings were made from 321 neurons from transverse and 22 neurons from longitudinal slices and their thermosensitivity was determined by relating changes in firing rate to changes in slice temperature. 2. Of the neurones from transverse slices, 53% were warm sensitive, 2% were cold sensitive and 45% were temperature insensitive. In longitudinal slices, 68% were warm sensitive and the remaining neurones were temperature insensitive. 3. When classified according to their recording sites in transverse slices, warm-sensitive neurones in laminae I and II had the same mean temperature coefficient compared with those recorded from lamina X, despite the fact that the latter had a significantly higher spontaneous activity. 4. The intrinsic temperature sensitivity of the majority of warm-sensitive neurones was confirmed by blocking their synaptic input. 5. A transient overshoot in activity, i.e. a dynamic response characteristic following rapid temperature stimuli (0.4 degree C s-1) was observed in 73% of the warm-sensitive and 59% of the temperature-insensitive neurones in laminae I and II in response to rapid warming, but only rarely (< 10%) in lamina X. 6. Temperature-sensitive SC neurones share response characteristics with temperature-sensitive neurones in the preoptic and anterior hypothalamic (PO/AH) area and with peripheral temperature receptors. Functionally, these neurones may represent the cellular basis for the temperature sensory function of the spinal cord that has been well characterized in vivo in homeothermic species.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulant J. A., Dean J. B. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Brown A. G. The dorsal horn of the spinal cord. Q J Exp Physiol. 1982 Apr;67(2):193–212. doi: 10.1113/expphysiol.1982.sp002630. [DOI] [PubMed] [Google Scholar]
- Dean J. B., Boulant J. A. Delayed firing rate responses to temperature in diencephalic slices. Am J Physiol. 1992 Sep;263(3 Pt 2):R679–R684. doi: 10.1152/ajpregu.1992.263.3.R679. [DOI] [PubMed] [Google Scholar]
- Granum S. L. The spinothalamic system of the rat. I. Locations of cells of origin. J Comp Neurol. 1986 May 8;247(2):159–180. doi: 10.1002/cne.902470204. [DOI] [PubMed] [Google Scholar]
- Hammel H. T. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- Hancock M. B., Peveto C. A. A preganglionic autonomic nucleus in the dorsal gray commissure of the lumbar spinal cord of the rat. J Comp Neurol. 1979 Jan 1;183(1):65–72. doi: 10.1002/cne.901830106. [DOI] [PubMed] [Google Scholar]
- Honda C. N., Perl E. R. Functional and morphological features of neurons in the midline region of the caudal spinal cord of the cat. Brain Res. 1985 Aug 12;340(2):285–295. doi: 10.1016/0006-8993(85)90925-4. [DOI] [PubMed] [Google Scholar]
- Honda C. N. Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J Neurophysiol. 1985 Apr;53(4):1059–1078. doi: 10.1152/jn.1985.53.4.1059. [DOI] [PubMed] [Google Scholar]
- Hori T. An update on thermosensitive neurons in the brain: from cellular biology to thermal and non-thermal homeostatic functions. Jpn J Physiol. 1991;41(1):1–22. doi: 10.2170/jjphysiol.41.1. [DOI] [PubMed] [Google Scholar]
- Hori T., Nakashima T., Kiyohara T., Shibata M. Comparison of anterior hypothalamic and preoptic thermosensitive neurons in vitro. Neurosci Lett. 1982 Aug 31;31(3):283–288. doi: 10.1016/0304-3940(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Hori T., Nakashima T., Kiyohara T., Shibata M., Hori N. Effect of calcium removal on thermosensitivity of preoptic neurons in hypothalamic slices. Neurosci Lett. 1980 Nov;20(2):171–175. doi: 10.1016/0304-3940(80)90141-x. [DOI] [PubMed] [Google Scholar]
- Jessen C., Simon-Oppermann C. Production of temperature signals in the peripherally denervated spinal cord of the dog. Experientia. 1976 Apr 15;32(4):484–485. doi: 10.1007/BF01920811. [DOI] [PubMed] [Google Scholar]
- Kelso S. R., Boulant J. A. Effect of synaptic blockade on thermosensitive neurons in hypothalamic tissue slices. Am J Physiol. 1982 Nov;243(5):R480–R490. doi: 10.1152/ajpregu.1982.243.5.R480. [DOI] [PubMed] [Google Scholar]
- Kiyohara T., Miyata S., Nakamura T., Shido O., Nakashima T., Shibata M. Differences in Fos expression in the rat brains between cold and warm ambient exposures. Brain Res Bull. 1995;38(2):193–201. doi: 10.1016/0361-9230(95)00093-t. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J Comp Neurol. 1978 Feb 1;177(3):417–434. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Price R. H., Williams F. G., Mayer B., Beitz A. J. Nitric oxide synthase is found in some spinothalamic neurons and in neuronal processes that appose spinal neurons that express Fos induced by noxious stimulation. Brain Res. 1993 Apr 16;608(2):324–333. doi: 10.1016/0006-8993(93)91474-7. [DOI] [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lin M. T., Yin T. H., Chai C. Y. Effects of heating and cooling of spinal cord on CV and respiratory responses and food and water intake. Am J Physiol. 1972 Sep;223(3):626–631. doi: 10.1152/ajplegacy.1972.223.3.626. [DOI] [PubMed] [Google Scholar]
- Meurer K. A., Jessen C., Iriki M. Kältezittern während isolierter Kühlung des Rückenmarkes nach Durchschneidung der Hinterwurzeln. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;293(3):236–255. [PubMed] [Google Scholar]
- Necker R. Temperature-sensitive ascending neurons in the spinal cord of pigeons. Pflugers Arch. 1975;353(3):275–286. doi: 10.1007/BF00584289. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Klee M. R., Klussmann F. W. Effects of local hypo- and hyperthermia on mammalian spinal motoneurones. Fed Proc. 1969 May-Jun;28(3):1006–1009. [PubMed] [Google Scholar]
- Pierau F. R., Klee M. R., Klussmann F. W. Effect of temperature on postsynaptic potentials of cat spinal motoneurones. Brain Res. 1976 Sep 10;114(1):21–34. doi: 10.1016/0006-8993(76)91004-0. [DOI] [PubMed] [Google Scholar]
- Schmid H. A., Jansky L., Pierau F. K. Temperature sensitivity of neurons in slices of the rat PO/AH area: effect of bombesin and substance P. Am J Physiol. 1993 Feb;264(2 Pt 2):R449–R455. doi: 10.1152/ajpregu.1993.264.2.R449. [DOI] [PubMed] [Google Scholar]
- Schmid H. A., Pierau F. K. Temperature sensitivity of neurons in slices of the rat PO/AH hypothalamic area: effect of calcium. Am J Physiol. 1993 Feb;264(2 Pt 2):R440–R448. doi: 10.1152/ajpregu.1993.264.2.R440. [DOI] [PubMed] [Google Scholar]
- Simon E., Iriki M. Ascending neurons highly sensitive to variations of spinal cord temperature. J Physiol (Paris) 1971 May;63(3):415–417. [PubMed] [Google Scholar]
- Simon E., Iriki M. Ascending neurons of the spinal cord activated by cold. Experientia. 1970 Jun 15;26(6):620–622. doi: 10.1007/BF01898724. [DOI] [PubMed] [Google Scholar]
- Simon E., Iriki M. Sensory transmission of spinal heat and cold sensitivity in ascending spinal neurons. Pflugers Arch. 1971;328(2):103–120. doi: 10.1007/BF00592439. [DOI] [PubMed] [Google Scholar]
- Simon E., Pierau F. K., Taylor D. C. Central and peripheral thermal control of effectors in homeothermic temperature regulation. Physiol Rev. 1986 Apr;66(2):235–300. doi: 10.1152/physrev.1986.66.2.235. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974;(71):1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature signals from skin and spinal cord converging on spinothalamic neurons. Pflugers Arch. 1972;337(4):323–332. doi: 10.1007/BF00586649. [DOI] [PubMed] [Google Scholar]
- Strack A. M., Sawyer W. B., Marubio L. M., Loewy A. D. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988 Jul 5;455(1):187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Lee C. L., Perl E. R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986 Oct 17;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Vieth E. Fitting piecewise linear regression functions to biological responses. J Appl Physiol (1985) 1989 Jul;67(1):390–396. doi: 10.1152/jappl.1989.67.1.390. [DOI] [PubMed] [Google Scholar]
- Yakimova K., Sann H., Schmid H. A., Pierau F. K. Effects of GABA agonists and antagonists on temperature-sensitive neurones in the rat hypothalamus. J Physiol. 1996 Jul 1;494(Pt 1):217–230. doi: 10.1113/jphysiol.1996.sp021486. [DOI] [PMC free article] [PubMed] [Google Scholar]


