Abstract
The minimal instrumentation of portable medical diagnostic devices for point-of-care applications is facilitated by using chemical heating in place of temperature-regulated electrical heaters. The main applications are for isothermal nucleic acid amplification tests (NAATs) and other enzymatic assays that require elevated, controlled temperatures. In the most common implementation, heat is generated by the exothermic reaction of a metal (e.g., magnesium, calcium, or lithium) with water or air, buffered by a phase-change material that maintains a near-constant temperature to heat the assay reactions. The ability to incubate NAATs electricity-free and to further to detect amplification with minimal instrumentation opens the door for fully disposable, inexpensive molecular diagnostic devices that can be used for pathogen detection as needed in resource-limited areas and during natural disasters, wars, and civil disturbances when access to electricity may be interrupted. Several design approaches are reviewed, including more elaborate schemes for multiple stages of incubation at different temperatures.
Keywords: chemical heating, self-heating, POC (point-of-care), diagnostics, isothermal nucleic acid amplification test (NAAT)
1. Introduction
This review surveys various approaches and implementations of chemical heating for minimally instrumented (electricity-free) POC medical diagnostic devices. Generally, these self-heating devices utilize a self-contained exothermic chemical reaction initiated at the time of use, along with a phase-change material, to produce controlled heating as needed in various steps of diagnostic assays, and particularly for molecular diagnostics, i.e., enzymatic nucleic acid amplification tests (NAATs).
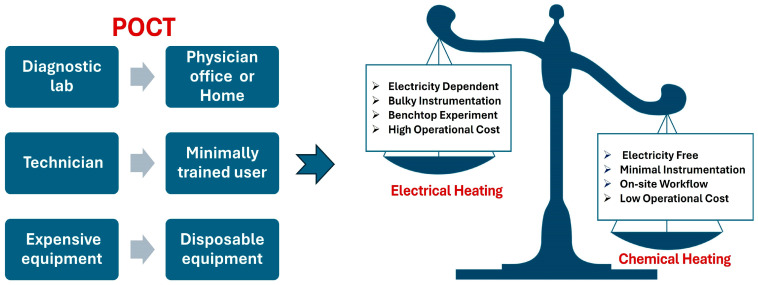
For comparison and as an ideal for other POC tests, lateral flow immunoassay strips, such as home pregnancy tests and similar rapid tests for infectious diseases, serve as the foremost example of pervasive, inexpensive, single-use (disposable), electricity-free diagnostic devices. These products are sold over the counter and can be used without any training or technical knowledge, are completely autonomous, and require no supporting instrument or equipment. In contrast, many other—if not most—POC diagnostic devices, especially those based on NAATs—require some form of temperature-regulated heating [1]. This implies a source of electricity, such as a battery or plug-in power supply, as electrical heating is the preferred means of temperature control. Since nucleic acid-based tests are generally more sensitive and specific than immunoassays [2], there is an incentive to make NAATs more like lateral flow strips with regard to simplicity, autonomous operation, and minimal instrumentation (Figure 1). One approach to this end is to use a chemical reaction to produce the required heat. This is no doubt inspired by so-called “meals ready-to-eat” (MRE) used by soldiers and outdoorsmen, wherein a packet of ‘fuel’ in powder form is mixed with water to bring food or beverages to near-boiling point temperatures [3], although there is not much temperature regulation other than being limited by the temperature of the boiling point of water. Another related consumer technology is hand warmers [4,5], which comprise a palm-sized pouch of sodium acetate that is melted by immersion in hot water and thereafter maintains a constant temperature (~50 °C) as the sodium acetate slowly (~30 min) recrystallizes.
Figure 1.
Electrical heating vs. chemical heating for point-of-care testing (POCT).
Chemical heating for diagnostics is promoted by the advent of isothermal NAATs, such as Loop-mediated Amplification (LAMP) and Recombinase Polymerase Amplification (RPA)—reviewed recently in [6]. Long-established laboratory methods based on PCR (polymerase chain reaction) need benchtop instruments that provide precise ( thermal cycling, peak cycle temperatures over 90 °C, and rapid heating and cooling (10 . In contrast, LAMP and RPA assays require merely constant-temperature incubation at approximately 65 °C (for LAMP) or 37–42 °C (for RPA) and thus are well suited for chemical heating [7,8,9].
The interest in electricity-free heating in POC tests stems from several considerations. There are an estimated 770 million people in the world without electricity, mostly in Sub-Saharan Africa [10], and in many areas of the world electric supply is unreliable with frequent outages. These resource-limited areas are where POC diagnostics are most needed due to the lack of medical infrastructure. Moreover, during natural disasters, civil disturbances, and wars, access to electricity may be interrupted. Chemical heating with phase-change materials also eliminates the need for a heater element, temperature sensor, and control electronics, considerably simplifying the device. Many proposed POC diagnostic systems utilize smartphone platforms for optical detection (cellphone cameras), computation, data logging, communication, GPS, and operator interfaces. However, electrical heating imposes an excessive demand on smartphone batteries, limiting the number of tests. Thus, smartphone-based diagnostics can also benefit from incorporating chemical heating to conserve battery power and reduce or eliminate peripheral instrumentation, such as electric heaters and temperature sensors [11,12].
The operational basis of chemical heating is to initiate an exothermic reaction with preloaded stored reagents (the ‘fuel’), typically in powder or solid form, by adding water or another solvent or exposing it to air. The reaction melts a phase-change material (PCM) that is an important medium for temperature control. The PCM dissipates its latent heat as it resolidifies and maintains a constant temperature, i.e., its melting point, for some prolonged time (~20 to 60 min) until it completely solidifies [13,14]. A sample in thermal contact with the PCM is thus incubated at the PCM melting point. Since a wide range of melting points are available with various phase-change materials, the incubation temperature can be adjusted accordingly. For LAMP, there is an incubation temperature window ranging from approximately 60 to 65 °C; however, the optimum amplification efficiency of LAMP may be sensitive within ±1 °C. RPA operates best in the range of 37 to 42 °C [8,15,16,17].
2. Chemical Heating Reactions
The main advantages of isothermal amplification assays over traditional molecular assays (such as PCR) are their constant incubation temperature, high amplification efficiency, and tolerance to contaminants and inhibitors. These factors reduce the complexity, cost, and power consumption of equipment, allowing diagnosis with minimal instrumentation [18]. The primary isothermal amplification assays used for point-of-care detection with chemical heating include the Recombinase Polymerase Assay (RPA) at approximately 37 °C, Helicase-Dependent Amplification (HAD) at around 65 °C, and Loop-Mediated Isothermal Amplification (LAMP) at about 65 °C [19].
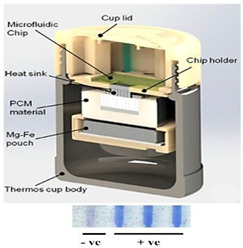
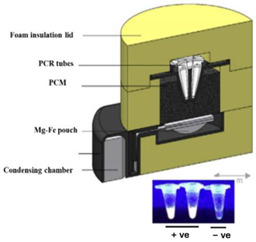
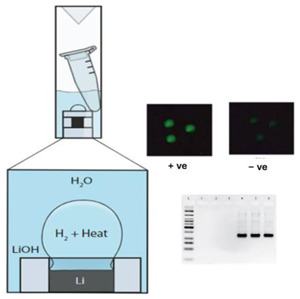
In one of the earliest reports of chemical heating for an NAAT, LaBarre et al. [20,21] employed a CaO (77 g) + H2O (18 g) reaction, along with a proprietary PCM (65 °C melting point), to heat a LAMP assay at 62 to 65 °C for 45 min. Three 200-µL sample tubes (with 50 µL of LAMP reaction) were embedded in ~14 cm3 of PMC, which was melted by the surrounding chemical heating reaction. They used a modified thermal bottle as a container. The heat transfer from the PCM to the reaction tubes was enhanced by a honeycomb aluminum structure. Singleton et al. [22] discussed the use of other exothermic reactants, including MgFe + H2O, iron + air, sodium acetate, and other PCMs with melting points in the 58 to 68 °C range from various manufacturers. An electricity-free point-of-care molecular diagnostic system for HIV-1 using RT-LAMP (reverse transcription LAMP) in PCR tubes incubated at 62 °C and heated with water added to MgFe, all of which were contained in a thermos bottle, has also been described [23,24,25].
A colorimetric tube-based LAMP test using Mg:Fe chemical heating was reported [26]. Buser et al. [27] described precision chemical heating using a smaller-volume system (insulating blocks in place of a thermos bottle) and a wick to control the addition of water to the exothermic reagents.
One of the most commonly used chemical heating reactions is based on Mg:Fe (1%) powder with a small amount of NaCl, which is initiated by the addition of water. The exothermic reaction is
The Fe component of the alloy reacts in a secondary galvanic corrosion reaction (with NaCl), preventing MgO formation that would otherwise inhibit the reaction [26,28]. (One caveat is that many online sources of MgFe pouches are military surplus and may well have been warehoused for more than ten years. Evidently, some premature oxidation may occur, perhaps due to packaging being permeable to air and moisture, and the fuel may lose much of its potency. Consistency of results can be obtained by first testing samples from suppliers and pooling the materials in large, well-mixed batches.)
Other reactions used for chemical heating include adding water to calcium oxide,
adding water to calcium chloride,
and exposing iron power to air,
The latter is used in some types of hand warmer pouches [5]. Huang et al. [29] employed a commercial ‘toe warmer’ composed of a mixture of iron powder, salt, activated carbon, cellulose, and vermiculite that, when exposed to air, maintained a 65 °C 2 °C temperature for 55 min to incubate a microfluidic chip hosting an isothermal helicase-dependent amplification (HDA) to detect C. difficile in stool samples. The heating reaction was performed in a Styrofoam cup, and the temperature and stability were controlled by the number of holes in the cup. Shaw et al. [30] employed an analogous exothermic Mg reaction with methanol (instead of water):
The temperature is buffered by the evaporation of methanol (boiling point 64.7 °C, latent heat of vaporization = 1100 J/g-MeOH) in place of including a solid PCM. Buser et al. [27] compared the ‘energy capacity’ [kJ/g] of various chemical heating reactions, ranked from highest to lowest (with a few additions): Li + H2O (32 Jg−1), iron filings exposed to air, Fe + 3O2 (30 kJ), Mg:Fe + H2O (15 kJ), CaO + H2O (1 kJ), CaCl (1 kJ), and Na+ + C2H3O2 (0.3 kJ). For comparison, Buser also includes two batteries: AA alkaline (0.4 kJ g−1) and lithium coin cells (0.7 kJ g−1).
In addition to the exothermic oxidation reactions listed above, the crystallization of salts from a melted phase can also be used for heating. (This type of heating could also be classified as a phase change, see below.) For example, in some types of hand warmer pouches, sodium acetate trihydrate (SAT) is heated by immersion in hot water. Upon cooling to room temperature, a supercooled liquid persists. A metal piece contained inside the pouch is flexed to spall metal particles that act as nucleation centers, precipitating crystallization and the release of latent heat to maintain the temperature at its melting point. Unfortunately, the melting point of SAT (57 °C) is a few degrees short of that typically used for LAMP, but it can otherwise be used for RPA [25]. A self-contained CRISPR-based SARS-CoV-2 diagnostic system employing incubation by way of a hand warmer has been described by Li et al. [31].
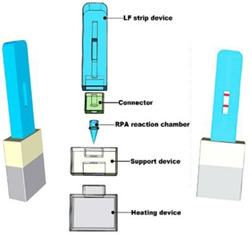
Miniature heaters using lithium metal molded in channels of a laser-machined acrylic piece were developed by Udugama et al. [32]. The acrylic piece was placed in a container with water, and the sample reaction tubes were immersed in a lithium-heated water bath. The temperature was controlled through modulating the lithium–water reaction kinetics by controlling the interface between the reactants (lithium and water) and products (hydrogen bubbles and lithium hydroxide) through a combination of channel dimensions and the addition of excipients. Temperatures between 37 and 65 °C were achieved by tuning the dimensions of the channels. The comparatively high heat of reaction between lithium and water allows for much smaller heaters, with faster heat-up times. Feng et al. [33] used a CaO-fueled chemical heater to incubate an RPA reaction, whereupon amplicons were detected on a lateral flow strip.
For applications requiring low incubation temperatures (<40 °C), such as RPA and related methods, chemical heating without a PCM may be viable. For example, Wang et al. [34] described a diagnostic heater that used a mixture of aluminum powder, CaO, CaCO3, CaOH, NaCO3, and NaOH wrapped in a fabric and sealed in a polypropylene film packet. To heat a vessel containing reaction tubes, the encapsulating film of the packet was removed and placed in a vessel to which water was added, sustaining the incubation temperature for approximately 30 min. As mentioned above, the latent heat of crystallization can also be used for heating. For example, sodium acetate solutions can be heated by immersion in hot water. During cooling, the temperature is retained (without exothermal chemical reactions), which can also be used to maintain high temperatures after heating. Vloemans et al. [35] demonstrated a compact system where sodium acetate crystallization was used to maintain steady incubation temperatures ranging between 37 and 42 °C.
3. Phase-Change Materials (PCMs)
The most common phase-change materials are paraffin wax and other similar organic compounds where the melting temperature is a function of molecular weight. Importantly, the PCM does not exhibit significant supercooling. Some feasible PMCs include Carbowax™ (polyethylene glycol, Dow Chemical), synthetic Polyesterwax (Electron Microscopy Sciences, Hatfield, PA, USA), palmitic acid [24], and various proprietary PCMs surveyed by Singleton et al. [30]. At least several groups have used PureTemp™ PCMs with latent heats of 200 J/g, available in over 200 formulations, with phase change transition temperatures ranging from −40 °C to 150 °C. These PCMs are made from agricultural sources and are biodegradable and nontoxic. One drawback of organic PCMs is their low thermal conductivity, which can be mitigated by adding high-conductivity fillers such as metal particles or carbon particles, but this may reduce the effective latent heat. Additionally, several non-instrumented POC diagnostic systems do not utilize a separate exothermic reaction. Instead, the phase-change material, enclosed in a plastic pouch, is heated in boiling or otherwise hot water, or by an electrical heater, and then contacted with the sample. For example, Lillis et al. [25] incubated RPA reactions in 0.2 µL PCR tubes by placing them in tubes filled with sodium acetate that had been immersed in 70 °C water.
4. Estimations
Some illustrative calculations provide estimates of the amount of fuel and PCM needed for chemically heating LAMP reactions. For adiabatic heating (no heat losses), a simple energy balance, assuming that the chemical heating reaction goes to completion, is given by
| (1) |
where is the mass of fuel (e.g., Mg powder), is the mass of water added, and is the mass of the sample(s) and container(s) and any other supporting parts. are the respective heat capacities, and is the heat of reaction (per gram of fuel). is the latent heat of melting of the PCM, and is the fraction melted of the PCM. is the increase in temperature from the ambient to the amplification temperature . The above energy balance indicates that the heat of reaction raises the temperature of the components and melts the PCM, assumes the reaction products have the same heat capacities as the reactants, and neglects gaseous products (e.g., hydrogen or CO2) that escape the reactor. The heat capacities for Mg and Mg oxides are about 1 J·g−1·C−1, 4.2 J·g−1·C−1 for H2O, and 2.5 J·g−1·C−1 for common phase-change materials. Plastics have heat capacities of about 1.5 J·g−1·C−1. A stoichiometric amount of water added to Mg (1 mole of Mg: 2 moles of H2O) implies 1.45 g (mL) of H2O added per 1 g of Mg: To speed up the heating, an excess of water may be used. The of the reaction is 350 kJ/mole-Mg = 1.4 × 104 J/g-Mg. The latent heat of typical PCMs is 200 J/g. The mass of the sample (mostly water) and container (tube or chip), plus other plastic fixtures, might range from 1 to several grams, depending on the design of the system.
The energy balance indicates the amount of fuel (grams of Mg) needed to heat the system to the target amplification temperature and melt a specified amount of PCM. Ideally, all of the PCM is melted ( More explicitly,
| (2) |
The amount of PCM needed to be melted is determined by the rate of heat loss and the required reaction incubation time, as the latent heat liberated by the solidification of the PCM must compensate for heat loss in order to maintain a constant amplification temperature.
The rate of the heat loss of the reactor due to thermal conduction [W = J·s−1] through the insulating layers is
| (3) |
where [W·cm−1 °C−1] is the thermal conductivity of the insulator, [cm2] is the area of insulation, [cm] is the thickness of the insulating layer(s), [°C] is the amplification temperature (65 °C for LAMP), and [°C] is the ambient temperature (20 °C). The rate of convective heat transfer [W] through air-exposed surfaces is
| (4) |
where [W·cm−2·C−1] is the convective heat transfer coefficient and [cm2] is the area of air-exposed surfaces. The total rate of heat loss is then
| (5) |
The total energy lost by conduction and convection during incubation time [s], e.g., 1 h = 3600 s, is supplied by the latent heat of melting of the PCM.
| (6) |
for Styrofoam insulation W·cm−1·°C−1. The convective heat transfer coefficient h is quoted over a wide range, from 0.01 to 1 [W·cm−2·°C−1], with typical values from 0.25 to 0.5. The parameters () are dependent on the design.
| (7) |
Assuming areas of = 10 cm2, = 25 cm2, d = 1 cm, and 3600 s (1 h), the mass of phase-change material is about 10 g. For 5 mL of water added, the estimated amount of Mg needed (Equation (1)) is about 0.25 g. This is close to values reported by Singleton et al. [22] (6 g PCM, 0.25 g Mg) and Liu et al. [36] (1.5 mL H2O, 0.36 Mg,0.5 g PCM), where a vacuum Thermos® bottle contained the heater, but lower than values given by Li et al. [37] (10 g PCM, 1 g Mg, and 5 mL H2O), where Styrofoam was used as insulation. Discrepancies from estimates might be due to higher heat losses, incomplete reactions, or Mg powder being partially oxidized prior to the test. This also suggests that with better insulation to reduce heat loss, there is some latitude for further miniaturization of the heater apparatus and a reduction in the amount of Mg powder and PCM needed.
5. Performance
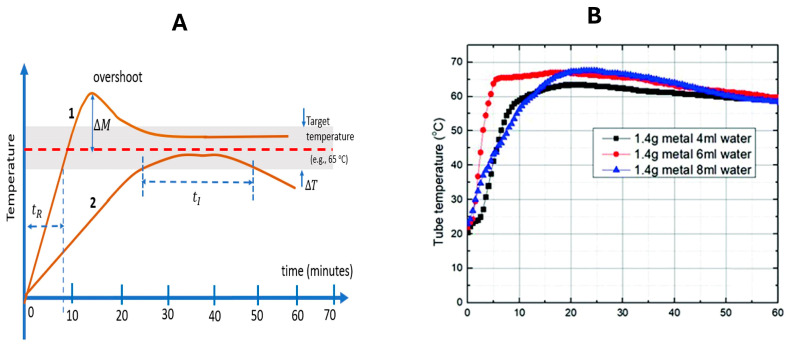
The time-temperature profile of chemical heating (Figure 2A), with a target incubation temperature (e.g., 65 °C for LAMP, as shown), suggests some figures of merit. The rise time is defined as the time needed to go from to . A rapid increase in temperature shortens the total test time and reduces nonspecific amplification. An excessive overshoot, , can denature enzymes and needs to be minimized. In the case of LAMP, temperatures in excess of 70 °C can significantly deactivate the polymerases [38]. The tolerance band is the deviation or variation of the plateau temperature about the target temperature, which should typically be approximately 5 °C or less, as the operating temperature range of LAMP is 60 °C to 65 °C [18,39]. The incubation time is the duration the temperature stays within the tolerance band before cooling. A minimum incubation time of 20 min, and perhaps as long as 60 min, may be needed for some applications. Infrared cameras can provide thermal images of heating, incubation, and cooling, showing temperature variations to identify hot spots and excessive heat leakage due to inadequate insulation. Figure 2B shows the experimental ‘tuning’ of a chemical heating system using Mg-Fe (‘metal’) mixed with powdered PCM for several amounts of water added. Another figure of merit is the robustness of the performance for different ambient temperatures and humidities. Finally, the shelf life of the product should probably be at least 1 year, aided, for example, by adequate packaging to prevent oxidation of the fuel.
Figure 2.
(A) Sample time–temperature profiles for chemical self heating showing various figures of merit: rise time , overshoot , tolerance band , and incubation time (time spent within tolerance temperature range). Two example cases: Curve 1 shows a fast ramp-up, but with overshoot and a long incubation time. Curve 2 shows a slow ramp-up and insufficient incubation time before cooling below the tolerance band. (B) Example of the optimization of the time–temperature in the system shown: 10.5 g of PCM, 1.4 g of Mg:Fe powder, and 4 mL, 6 mL, and 8 mL of water added [37].
6. Chemical Heating Formats
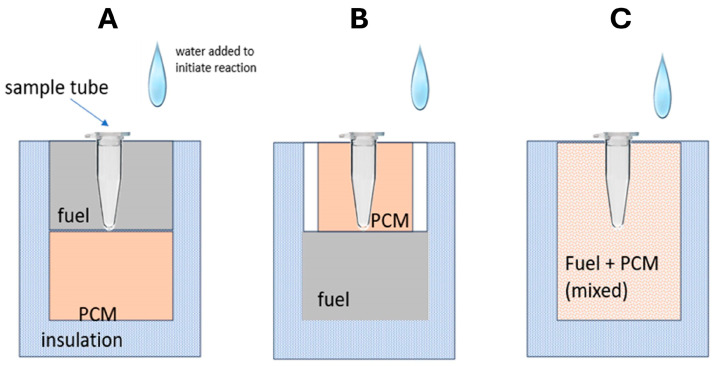
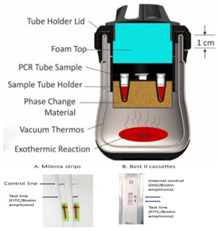
Chemical heating methods for ~100 µL reaction tubes, microfluidic chips, and lateral flow strips have been developed. Several configurations for heating a sample are shown in Figure 3. Initially, and most commonly, the reaction and PCM are separated but in close thermal contact. The sample can be surrounded by the reacting mixture or, more commonly, by the PCM. The reaction essentially melts the PCM, and as the reaction progresses, the slowly resolidifying PCM maintains the sample temperature. To moderate and prolong the reaction, water is continually delivered to the fuel, such as by seepage through a porous wick connecting the reaction chamber to a water reservoir.
Figure 3.
Three configurations for chemical heating with PCMs. (A) sample embedded in reactants and temperature buffered by thermal contact with phase-change material (PCM), (B) sample surrounded by PCM, which in turn is melted through contact with reactants, and (C) mixture of fuel and PCM powders (e.g., Li et al. [37]). Sample can be viewed (to monitor fluorescence of color change) either through tube lid, or from side through viewing port.
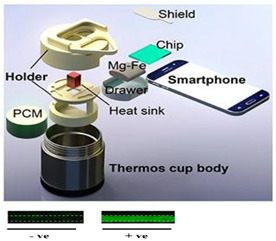
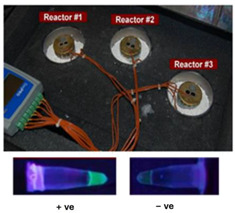
Liu et al. [36] regulated the exothermic reaction by using a porous material to wick water into the reaction mixture. Good thermal contact is needed between the PCM and the reaction. To improve the thermal conductivity of the PCM, carbon and metal particles have been added to the PCM. Composite paraffin-based PCMs containing expanded graphite and exhibiting improvements with respect to thermal conductivity, homogeneity, and latent heat were described by Ma et al. [40]. The Smart Cup [41] thermos chemical heater was developed into a Smart-Connected Cup for POC Zika virus detection [11]. A smartphone seamlessly couples to the smart cup via a holder bracket. A credit-card-sized microfluidic chip (with three parallel LAMP reaction chambers) is incubated by chemical heating. The LAMP reaction includes a bioluminescent reporter (BART, Bioluminescence Assay in Real Time), obviating the need for an excitation source. Luminescence (as a positive test) is detected by a smartphone camera. The smartphone provides signal monitoring and analysis, target quantification, data sharing, and spatiotemporal disease mapping through its GPS.
In an alternate format [37], the Mg/Fe powder is well mixed with PureTemp™ PCM powder (PureTemp LLC, Minneapolis, MN, USA) (with a granule size of 0.4 to 0.6 mm) and packed into a 2.5 cm centrifuge tube with a cutoff length of approximately 5 cm. The tube is placed in a hole formed in a block of Styrofoam. The reaction tubes are inserted into the powder mix with their caps above the surface of the powder mix, and then water is added to initiate the reaction. As water slowly percolates through the powder mix, heat evolves. The formation of hydrogen bubbles also provides a path for water to seep down into the powder mix. The advantage here is simplicity; water is added to the mixture in one dose rather than being dribbled in by wicking, as described above. Moreover, the heat transfer between the reaction and phase-change material is enhanced due to the large interfacial area between the MgFe and PCM granules. The performance was optimized by varying the ratio of MgFe powder to PCM, the total volume of the powder mixture, and the amount of water added. Goertz et al. [42] showed that the ramp-up rate was also sensitive to the NaCl concentration (0 to 1.5%).
Fu et al. [43] described a multistep assay using CaO chemical heating and three PCMs for immunomagnetic separation (37 °C), bacterial lysis and DNA extraction (100 °C), and LAMP (65 °C) in a Thermos®-type vacuum bottle, thus demonstrating the chemical heating capability for various sample processing steps. To maintain constant temperatures, chemical-heated systems should be well insulated. Most simply, the system can be packed in Styrofoam, which can be easily cut to shape, leaving access for inserting the sample and ports for visual or optical detector (e.g., cellphone camera) monitoring. Thermos bottles were also used to insulate the reactions. Cup chambers, sample holders, and other structural components can be 3D printed.
More sophisticated applications of chemical heating, including diagnostic assays, utilize phase change partitions. A reaction vessel can be compartmentalized by layers of phase-change materials (various paraffins with distinct melting points) that separate reaction zones. As the temperature increases, the successive partition layers melt, resulting in mixing and the initiation of reactions in stages. Thus, a sequence of reactions and mixing steps can be ‘programmed’ as the heating temperature is slowly increased. Goertz et al. [43] described a multilayered reaction in a PCR tube where the NaCl concentration in the MgFe chemical heating reaction could be controlled and varied to change the temperature ramp from approximately 0.6 °C per minute (0.1% NaCl) to >15 °C per minute (1.5% NaCl); thus, the time between the temperature and activation of PCM melting could be tailored. This work expands the functionality of chemical heating to include initiating mixing and processing, which widens the scope of applications and expands the hands-free automation of diagnostic tests. Table 1 provides a survey of chemical heating methods used for pathogen detection, along with fuel types, reactants, amplification product detection methods, and assay sensitivities.
Table 1.
Chemical heating for POC molecular detection of pathogens.
| Isothermal Amplification Assay | Pathogen (s) | Fuel | Reactants | Time to Chemical Heating | Amplification Time |
Amplified Product Detection | Analytical Sensitivity | The Used Device | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1—LAMP | Zika virus | Magnesium Iron | Mg-Fe + H2O | 10 min | 60 min | Bioluminescence | 5 PFU/sample |
|
[11] |
| 2—LAMP | Zika virus | Magnesium Iron | Mg-Fe + H2O | 10 min | 40 min | Colorimetric detection—intercalating dye Leco Crystal Violet (LCV) |
5 PFU/sample |
|
[12] |
| 3—LAMP | Plasmodium falciparum malaria | Calcium oxide | CaO + H2O | 15 min | 45 min | Turbid visual detection and fluorescent (Calcein) detection | 50 copies/µL |
|
[20] |
| 4—LAMP | Plasmodium falciparum malaria | Calcium oxide | CaO + H2O | 15 min | 60 min | Turbid visual detection and fluorescent (Calcein) detection | 50 copies/µL |

|
[21] |
| 5—LAMP | HIV-1 | Magnesium Iron | Mg-Fe + H2O | 12 min | 60 min | Nucleic acid lateral flow detection | 75 copies/reaction |
|
[23] |
| 6—LAMP | HIV-1 | Magnesium Iron | Mg-Fe + H2O | 11 min | 60 min | BHQ1-labeled quencher probe | 3115 copies/mL |
|
[24] |
| 7—RPA | HIV-1 | Sodium acetate | Na+ + C2H3O2 | 1 min | 20–30 min | Nucleic acid lateral flow detection | 10 copies/µL |
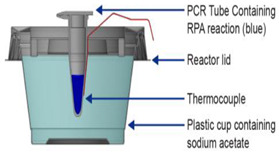
|
[25] |
| 8—LAMP | Filarial parasites (B. malayi, O. volvulus, and W. bancrofti) | Magnesium Iron | Mg-Fe + H2O | 15 min | 40–70 min | Colorimetric detection—proton detection (pH-sensitive indicator dyes) |
B. malayi 1.0 pg DNA/reaction, O. volvulus 0.01 ng DNA/reaction, and W. bancrofti 0.1 pg DNA/reaction |
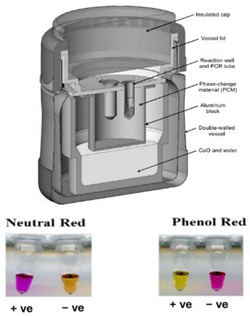
|
[26] |
| 9—HDA | Clostridium difficile (C. difficile) | Iron filings | Fe + 3 O2 | 22 min | 30 min | Gel electrophoresis analysis | 1.25 × 10−2 pg/reaction |
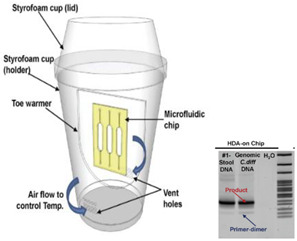
|
[29] |
| 10—RPA/CRISPR | SARS-CoV-2 | Iron filings | Fe + 3 O2 | 2 min | 15 min | Nucleic acid lateral flow detection | 100 copies/reaction |
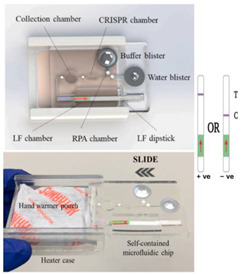
|
[31] |
| 11—RPA | T4 bacteriophage | Lithium | Li + H2O | 1 min | 15 min | - Fluorescent test (Taqman probe) - Gel electrophoresis |
NA |
|
[32] |
| 12—RPA | Enterohemorrhagic Escherichia coli O157:H7 | Calcium oxide | CaO + H2O | 9 min | 15 min | Nucleic acid lateral flow detection | 36.23 CFU/mL |
|
[33] |
| 13—LAMP | Cronobacter species | Calcium oxide | CaO + H2O | 7 min | 60 min | HNB (Hydroxy naphthol blue)—Metal indicator | 4.2 × 102 cfu/g |
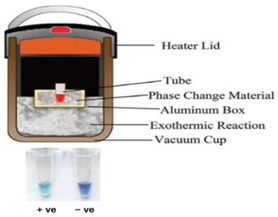
|
[43] |
| 14—LAMP | Herpes simplex virus type 2 (HSV-2) | Magnesium Iron | Mg-Fe + H2O | 10 min | 60 min | Fluorescent intercalating dyes | 4.2 PFU/sample |
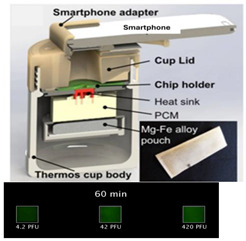
|
[41] |
| 15—LAMP | SARS-CoV-2 | Magnesium Iron | Mg-Fe + H2O | 3 min | 30 min | - pH-sensitive indicator dyes (Phenol red) - Fluorescent intercalating dyes |
10 copies/reaction |
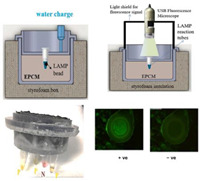
|
[37] |
| 16—LAMP | HIV-1 | Calcium oxide | CaO + H2O | 10–15 min | 60 min | Fluorescence detection using labeled primer along with a quencher probe | HIV- 1 DNA: 10 copies/reaction HIV-1 RNA: 140 copies/reaction |
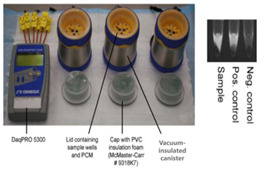
|
[44] |
NA = Not applicable.
7. Conclusions and Future Directions
This review highlights different strategies and applications of chemical heating in POC medical diagnostic devices that function without electricity. Chemical heating has proven effective for operating molecular diagnostic assays, such as isothermal amplification techniques (like RPA or LAMP) and CRISPR-based tests. Chemical heating offers several benefits over electrical heating for POCT, including the ability to operate without electricity, minimal instrumentation requirements, support for on-site workflows, and low operational costs. Although chemical heating offers several key benefits over electrical heating in POCT, it also has drawbacks. It tends to be relatively slow and can generate hazardous chemical waste, raising concerns about environmental pollution.
Based on the prices for MgFe fuels (as per commercial products sold as Meals Ready-to-Eat) and the amount of PCMs used, the total material cost is about USD 0.10 per test. We also note that many approaches heat a much larger mass of material than the reaction volume, so there is ample room for continued miniaturization, especially with microfluidic formats. The use of phase-change materials as temperature-controlled barriers for partitioning allows multi-stage reactions at several temperatures.
The current challenges in using chemical heating include the miniaturization of chemical heating for palm-sized microfluidic chips or cassettes and the creation of more automated methods of adding water to the reaction, e.g., by depressing pouches integrated in the chip or by other means of providing self-actuated water reservoirs.
Based on this review, developing a two-stage nested isothermal amplification method that utilizes RPA at 42 °C, followed by LAMP at 60–65 °C to enhance the sensitivity by ~10-fold [45,46,47], enabled by chemical heating, should be feasible and of practical interest. Additionally, integrating a streamlined nucleic acid extraction method with chemical heating-based isothermal amplification could create an efficient molecular screening test.
Author Contributions
Conceptualization, M.G.M. and M.E.-T.; writing—original draft preparation, M.G.M. and M.E.-T., writing—review and revision, M.E.-T., F.A. and M.G.M.; figures preparation, M.G.M. and M.E.-T.; manuscript revision and supervision, M.G.M. and M.E.-T.; funding acquisition, M.E.-T. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by a grant awarded by the Higher Colleges of Technology, Interdisciplinary research grant-212335, United Arab Emirates. F.A. was partly supported by a grant from the Science for Africa Foundation to the Developing Excellence in Leadership, Training and Science in Africa (DELTAS Africa) Programme [DEL-22-014], with support from Wellcome Trust and the UK Foreign, Commonwealth & Development Office.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Miralles V., Huerre A., Malloggi F., Jullien M.C. A review of heating and temperature control in microfluidic systems: Techniques and application. Diagnostics. 2013;3:33–67. doi: 10.3390/diagnostics3010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hema M., Charan K.N. Recent developments in detection and diagnosis of plant viruses. In: Viswanath B., editor. Recent Developments in Applied Microbiology and Biochemistry. Volume 2. Academic Press; Cambridge, MA, USA: 2021. pp. 163–180. [Google Scholar]
- 3.Scott D., Meadows R. Hot Meals. ChemMatters. 1992;10:12–13. [Google Scholar]
- 4.Sands W.A., Kimmel W.L., Wurtz B.R., Stone M.H., McNeal J.R. Comparison of commercially available disposable chemical hand and foot warmers. Wilderness Environ. Med. 2009;20:33–38. doi: 10.1580/08-WEME-OR-243.1. [DOI] [PubMed] [Google Scholar]
- 5.Alp F.B., Balköse D. Hand warmer types, silica and zeolite 4A as potential hand warmers. Sigma J. Eng. Nat. Sci. 2024;42:356–365. doi: 10.14744/sigma.2024.00035. [DOI] [Google Scholar]
- 6.Srivastava P., Prasad D. Isothermal nucleic acid amplification and its uses in modern diagnostic technologies. 3 Biotech. 2023;13:200. doi: 10.1007/s13205-023-03628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notomi T., Okayama H., Masubuchai H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notomi T., Mori Y., Tomita N., Kanda H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015;53:1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 9.Euler M., Wang Y., Otto P., Tomaso H., Escudero R., Anda P., Hufert F.T., Weidmann M. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J. Clin. Microbiol. 2012;50:2234–2238. doi: 10.1128/JCM.06504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Energy Outlook 2019. International Energy Agency (IEA) IEA; Paris, France: 2019. [Google Scholar]
- 11.Song J., Pandian V., Mauk M.G., Bau H.H., Cherry S., Tisi L.C., Liu C. Smartphone-based mobile detection platform for molecular diagnostics and spatiotemporal disease mapping. Anal. Chem. 2018;90:4823–4831. doi: 10.1021/acs.analchem.8b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J., Mauk M.G., Hackett B.A., Cherry S., Bau H.H., Liu C. Instrument-Free Point-of-Care Molecular De-tection of Zika Virus. Anal. Chem. 2016;88:7289–7294. doi: 10.1021/acs.analchem.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babaev B.D. Principles of heat accumulation and heat-accumulating materials in use. High Temp. 2014;52:736–751. doi: 10.1134/S0018151X14050010. [DOI] [Google Scholar]
- 14.Gong J., Wang Q., Sun J. Thermal analysis of nickel cobalt lithium manganese with varying nickel content used for lithium ion batteries. Thermochim. Acta. 2017;655:176–180. doi: 10.1016/j.tca.2017.06.022. [DOI] [Google Scholar]
- 15.Zhang X., Lowe S.B., Gooding J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP) Biosens. Bioelectron. 2014;61:491–499. doi: 10.1016/j.bios.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Macdonald J., Von Stetten F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2019;144:31–67. doi: 10.1039/C8AN01621F. [DOI] [PubMed] [Google Scholar]
- 17.Özay B., McCalla S.E. A review of reaction enhancement strategies for isothermal nucleic acid amplification reactions. Sens. Actuators Rep. 2021;3:100033. doi: 10.1016/j.snr.2021.100033. [DOI] [Google Scholar]
- 18.El-Tholoth M., Bau H.H. Molecular Detection of Respiratory Tract Viruses in Chickens at the Point of Need by Loop-Mediated Isothermal Amplification (LAMP) Viruses. 2024;16:1248. doi: 10.3390/v16081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon Y.-J., Lee S.-Y., Oh S.-W. A Review of Isothermal Amplification Methods and Food-Origin Inhibitors against Detecting Food-Borne Pathogens. Foods. 2022;11:322. doi: 10.3390/foods11030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBarre P., Gerlach J., Wilmoth J., Beddoe A., Singleton J., Weigl B. Non-instrumented nucleic acid amplification (NINA): Instrument-free molecular malaria diagnostics for low-resource settings; Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology; Buenos Aires, Argentina. 31 August–4 September 2010; pp. 1097–1099. [DOI] [PubMed] [Google Scholar]
- 21.LaBarre P., Hawkins K.R., Gerlach J., Wilmoth J., Beddoe A., Singleton J., Boyle D., Weigl B. A simple, inexpensive device for nucleic acid amplification without electricity-toward instrument-free molecular diagnostics in low-resource settings. PLoS ONE. 2011;6:e19738. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton J., Zentner C., Buser J., Yager P., LaBarre P., Weigl B.H. Instrument-free exothermic heating with phase change temperature control for paper microfluidic devices. Proc. SPIE Int. Soc. Opt. Eng. 2013;8615:86150R. doi: 10.1117/12.2005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton J., Osborn J.L., Lillis L., Hawkins K., Guelig D., Price W., Johns R., Ebels K., Boyle D., Weigl B., et al. Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PLoS ONE. 2014;9:e113693. doi: 10.1371/journal.pone.0113693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis K.A., Rudolph D.L., Morrison D., Guelig D., Diesburg S., McAdams D., Burton R.A., LaBarre P., Owen M. Single-use, electricity-free amplification device for detection of HIV-1. J. Virol. Methods. 2016;237:132–137. doi: 10.1016/j.jviromet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lillis L., Lehman D., Singhal M.C., Cantera J., Singleton J., Labarre P., Toyama A., Piepenburg O., Parker M., Wood R., et al. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS ONE. 2014;9:e108189. doi: 10.1371/journal.pone.0108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole C.B., Li Z., Alhassan A., Guelig D., Diesburg S., Tanner N.A., Zhang Y., Evans T.C., Jr., LaBarre P., Wanji S., et al. Colorimetric tests for diagnosis of filarial infection and vector surveillance using non-instrumented nucleic acid loop-mediated isothermal amplification (NINA-LAMP) PLoS ONE. 2017;12:e0169011. doi: 10.1371/journal.pone.0169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buser J.R., Diesburg S., Singleton J., Guelig D., Bishop J.D., Zentner C., Burton R., LaBarre P., Yager P., Weigl B.H. Precision chemical heating for diagnostic devices. Lab Chip. 2015;15:4423–4432. doi: 10.1039/C5LC01053E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sergev S.S., Black S.A., Jenkins J.F. Supercorroding Galvanic Cell Alloys for Generation of Heat and Gas. 4264362. US Patent. 1981 April 28;
- 29.Huang S., Do J., Mahalanabis M., Fan A., Zhao L., Jepeal L., Singh S.K., Klapperich C.M. Low cost extraction and isothermal amplification of DNA for infectious diarrhea diagnosis. PLoS ONE. 2013;8:e60059. doi: 10.1371/journal.pone.0060059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw S.A., Balasubramanian B., Bonacorsi S., Cortes J.C., Cao K., Chen B.C., Dai J., Decicco C., Goswami A., Guo Z., et al. Synthesis of Biologically Active Piperidine Metabolites of Clopidogrel: Determination of Structure and Analyte Development. J. Org. Chem. 2015;80:7019–7032. doi: 10.1021/acs.joc.5b00632. [DOI] [PubMed] [Google Scholar]
- 31.Li Z., Ding X., Yin K., Avery L., Ballesteros E., Liu C. Instrument-free, CRISPR-based diagnostics of SARS-CoV-2 using self-contained microfluidic system. Biosens. Bioelectron. 2022;199:113865. doi: 10.1016/j.bios.2021.113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udugama B., Kadhiresan P., Chan W.C.W. Tunable and precise miniature lithium heater for point-of-care applications. Proc. Natl. Acad. Sci. USA. 2020;117:4632–4641. doi: 10.1073/pnas.1916562117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng F., Yuan Y., Fu Q., Cao F., Kong R., Ji D., Liu H. An integrated self-heating recombinase polymerase amplification lateral flow strip biosensor for quantification of enterohemorrhagic Escherichia coli O157: H7. Microchem. J. 2024;199:109979. doi: 10.1016/j.microc.2024.109979. [DOI] [Google Scholar]
- 34.Wang X.F., Chen W.Q., Guo J.L., Peng C., Chen X.Y., Xu X.L., Wei W., Yang L., Ca J., Xu J.F. A Fast, Visual, and Instrument-free Platform Involving Rapid DNA Extraction, Chemical Heating, and Recombinase Aided Amplification for On-Site Nucleic Acid Detection. Front Bioeng. Biotechnol. 2021;9:764306. doi: 10.3389/fbioe.2021.764306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vloemans D., Dal Dosso F., Verboven P., Nicolai B., Lammertyn J. Exploiting phase change materials in tunable passive heating system for low-resource point-of-care diagnostics. Appl. Therm. Eng. 2020;173:115269. doi: 10.1016/j.applthermaleng.2020.115269. [DOI] [Google Scholar]
- 36.Liu C., Mauk M.G., Hart R., Qiu X., Bau H.H. A self-heating cartridge for molecular diagnostics. Lab Chip. 2011;11:2686. doi: 10.1039/c1lc20345b. [DOI] [PubMed] [Google Scholar]
- 37.Li R.J., Mauk M.G., Seok Y., Bau H.H. Electricity-free chemical heater for isothermal nucleic acid amplification with applications in COVID-19 home testing. Analyst. 2021;146:4212–4218. doi: 10.1039/d1an00309g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oscorbin I., Filipenko M. Bst polymerase—A humble relative of Taq polymerase. Comput. Struct. Biotechnol. J. 2023;21:4519–4535. doi: 10.1016/j.csbj.2023.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becherer L., Borst N., Bakheit M., Frischmann S., Zengerle R., von Stetten F. Loop-mediated isothermal amplification (LAMP)–review and classification of methods for sequence-specific detection. Anal. Methods. 2020;12:717–746. doi: 10.1039/C9AY02246E. [DOI] [Google Scholar]
- 40.Ma C., Zhang Y., Chen X., Song X., Tang K. Experimental Study of an Enhanced Phase Change Material of Paraffin/Expanded Graphite/Nano-Metal Particles for a Personal Cooling System. Materials. 2020;13:980. doi: 10.3390/ma13040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao S.-C., Peng J., Mauk M.G., Awasthi S., Song J., Friedman H., Bau H.H., Liu C. Smart cup: A minimally-instrumented, smartphone-based point-of-care molecular diagnostic device. Sens. Actuators B Chem. 2016;229:232–238. doi: 10.1016/j.snb.2016.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goertz J.P., Colvin K.M., Lippe A.B., Daristotle J.L., Kofinas P., White I.M. Multistage Chemical Heating for Instrument-Free Biosensing. ACS Appl. Mater. Interfaces. 2018;10:33043–33048. doi: 10.1021/acsami.8b11611. [DOI] [PubMed] [Google Scholar]
- 43.Fu S., Jiang Y., Qin X., Yang T., Chen S., Yang X., Zhang W., Qu Y., Man C. Electricity-free amplification and visual detection of Cronobacter species in powdered infant formula. J. Dairy Sci. 2020;103:6882–6893. doi: 10.3168/jds.2019-17661. [DOI] [PubMed] [Google Scholar]
- 44.Curtis K.A., Rudolph D.L., Nejad I., Singleton J., Beddoe A., Weigl B., LaBarre P., Owen S.M. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS ONE. 2012;7:e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song J., Liu C., Mauk M.G., Rankin S.C., Lok J.B., Greenberg R.M., Bau H.H. Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin. Chem. 2017;63:714–722. doi: 10.1373/clinchem.2016.263665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Tholoth M., Anis E., Bau H.H. Two stage, nested isothermal amplification in a single tube. Analyst. 2021;146:1311–1319. doi: 10.1039/D0AN01835J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seok Y., Yin Q., Bai H., Bau H.H. Sensitive, Single-Pot, Two-Stage Assay for Hepatitis Viruses. Anal. Chem. 2022;94:1778–1786. doi: 10.1021/acs.analchem.1c04480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.