Simple Summary
Genetic diversity of the different origin genotypes is one of the most important topics to evaluate the desired properties and select high-yield genotypes. Coriander (Coriandrum sativum L.) is an annual plant native to the Mediterranean region, Western Europe, and Asia that belongs to the Apiaceae (Umbelliferae) family. The fruits and essential oils of coriander are used for spice, folk remedies, perfumery, food, tobacco, soft and alcoholic beverages, and pharmaceutical industries in the different parts of the world. The yield and some quality characteristics of coriander genotypes of different origins should be investigated for the breeding program. In this study, both phenotypic, morphological, and yield values showed wide variations. Also, some analyses, such as cluster, heat map, and PCA analyses, revealed important results for the coriander genotypes.
Keywords: coriander, morphology, yield, fixed oil, essential
Abstract
In this study, 119 different coriander genotypes (38 different countries), including 114 genotypes and five cultivars, were undertaken to characterize the genotypes based on phenotypic, morphological, yield, and International Union for the Protection of New Varieties of Plants (UPOV) properties, along with some chemical properties. The yield components were between 1.34 and 21.49 g for thousand-grain weight, 0.02–9.58 g/plant for fruit yield, 0.01–50.78 g/plant for biological yield, and 8.48–73.36% for harvest index. Similarly, the results of this study revealed significant variations in essential oil (0.05–1.86%v/w) and fixed oil content (10.22–34.03%v/w). The main components of the essential oil were determined as linalool (3.13–45.70%v/v), p-cymene (0.10–15.77%v/v), ɣ-terpinene (0.04–13.80%v/v), while the fixed oil main acids were determined as petroselinic (24.47–87.70%v/v), palmitic (7.13–23.04%v/v), elaidic (1.55–47.44%v/v), and behenic acids (3.17–12.56%v/v). The cluster, heat map, correlation, and principal coordinate (PCA) analyses were conducted to determine the genetic diversity and relationship among the genotypes based on the examined properties. The cluster and heat map analyses showed differences in the same origin genotypes. Petroselinic acid was the major contributing factor for PCA. As a result of this study, Ames 13900 and Ames 18595 genotypes had high values for fruit yield, fixed oil content, and essential oil content.
1. Introduction
Coriander (Coriandrum sativum L.) is a medicinal and aromatic plant belonging to the Apiaceae family. It is cultivated in different parts of the world, and about 2.3 million tons of anise, badian, coriander, cumin, fennel, and juniper fruits were produced, along with 2.3 million ha in 2021 in the world, and 204 tons of coriander cultivation was produced in 2022 in 157.1 ha of land in Türkiye [1,2]. Between 2019 and 2021, India, Colombia, China, Saint Lucia, Mali, Russia, and Ghana exported the most coriander in the world, respectively. Coriander exports from Türkiye have been over USD 46.7 million in 2022 [2].
Coriander has a high commercial value in the global market, which is crucial for both the national economy and export opportunities. This requires the production of high-quality products that meet established standards. To grow such quality products, it is essential to select appropriate varieties and employ effective cultivation techniques tailored to the specific ecological conditions.
Dried fruits of coriander are used widely as a condiment, flavoring in sauces, liquor, cocoa, chocolate industries, and meat, bakery, and confectionery products. In addition, it is a mixed major ingredient with many curry powders as a standard matter. The aroma and flavor of coriander fruits are attributable to the essential oil present in oil glands in the mericarp. It contains up to 1%v/w essential oil content (EOC), and the main essential oil composition classified as monoterpenoid is linalool. Traditionally, coriander fruits are used to cure bed colds, spasmolytic, digestive, galactagogue, and stomach disorders and are also used as a drug for indigestion, against worms, rheumatism, and pain in the joints [3]. In addition to the medicinal uses, the essential oils of coriander are used in the flavoring of many food products and soap production. Moreover, this plant is superior to other oils of its class, an advantage due to being more stable and retaining its agreeable odor longer [4].
Climate change, a global concern, also affects coriander production due to irregular rainfall, increased water demand, and increased biotic and abiotic stresses. However, these changes increase CO2 concentration, thus increasing photosynthesis and accelerating the ripening process. To prepare for climate change, it is necessary the identification of heat, flooding, and drought-tolerant genotypes and the development of nutrient-efficient cultivars. Therefore, it will be essential to develop coriander genotypes that are resistant to stresses such as drought, moisture stress, salinity, and high temperatures. This requires high-priority research to address the impact of climate change. Based on all these explanations, our strategy in this study is to conduct adaptation studies with coriander genotypes from different countries and compare them with existing local genotypes/cultivars.
Fruit yield of plants is affected by some genetic factors connected to the environment. Furthermore, the coriander’s fruit composition was different under different ecological conditions and applications. The essential oil content and its compositions changed in different maturation periods, different parts of the coriander, different climatic conditions, geographical regions, agronomical applications, and genetic (genotype) factors [5,6,7,8]. In addition, fixed oil acids showed variability during fruit maturation periods, maturity stages, and geographical areas [9,10]. Thus, breeding studies on plant production are necessary to construct on the less affected by the environment for suitable selection criteria [11].
In recent years, there has been a clear upward trend in studies on coriander. There are studies on coriander around the world, but research on the agricultural and quality characteristics of coriander populations from different origins is not yet at a satisfactory level. However, these studies generally cover the local genotypes of the country studied [12,13,14]. Our study has genotypes of different origins from many countries, as well as local genotypes and cultivars.
Hence, the distinguishing and superior feature of this planned study compared to previous research was its comprehensive approach. It encompassed a broad range of coriander varieties from diverse climates, geographies, and topographies, covering nearly all coriander-growing regions globally. This extensive variation was characterized by morphological and agronomic traits and by the biochemical properties of local varieties and genotypes. Additionally, this study incorporated UPOV (International Union for the Protection of New Varieties of Plants) criteria, enhancing the depth and scope of the analysis. Therefore, assessing quality characteristics such as fruit yield, essential oil yield, and linalool content in coriander populations of foreign origin, alongside evaluating the quality of these coriander seeds, will provide significant benefits.
To our knowledge, this study is the first to determine the different origin coriander genotypes collected from overseas based on the morphological and yield values with UPOV criteria, in addition to essential oil, fixed oil, and their compositions in some promising outstanding in terms of efficiency coriander genotypes.
2. Materials and Methods
2.1. Plant Material
In this study, the fruits of 114 coriander genotypes obtained from the USDA (United States Department of Agriculture) in 2019, 2 cultivars (Arslan and Gürbüz) obtained from Ankara University Faculty of Agriculture, and 3 cultivars (Erbaa, Gamze, and Pelmus) obtained from the Black Sea Agricultural Research Institute were used. Agricultural and some quality characteristics of a total of 119 genotypes were examined in Bolu ecological conditions, Bolu, Türkiye. All of them were grown in the 2019 growing season and, except 3 genotypes (Ames 14364, Ames 19032, and Ames 20047), adapted to Bolu ecological conditions. The list of genotypes and cultivars used in the research is given in Table 1.
Table 1.
The general information on coriander genotypes and cultivars used in the study.
| No | ID | Plant Name | Collected Place | No | ID | Plant Name | Collected Place |
|---|---|---|---|---|---|---|---|
| 1 | Ames 4998 | 896 | Türkiye | 61 | Ames 23632 | CORI 88 | Oman |
| 2 | Ames 10234 | Ames 10234 | Israel | 62 | Ames 23634 | CORI 90 | Oman |
| 3 | Ames 10235 | Ames 10235 | Minnesota, USA | 63 | Ames 23635 | CORI 91 | Oman |
| 4 | Ames 12778 | 2410 | Nepal | 64 | Ames 23639 | CORI 95 | Oman |
| 5 | Ames 13899 | USSR 90-08-07 | Tajikistan | 65 | Ames 23640 | CORI 96 | Oman |
| 6 | Ames 13900 | USSR 90-08-08 | Tajikistan | 66 | Ames 23641 | CORI 97 | Oman |
| 7 | Ames 14363 | Ames 14363 | Egypt | 67 | Ames 23642 | CORI 133 | Pakistan |
| 8 | Ames 14364 | Ames 14364 | Egypt | 68 | Ames 24907 | ISN 187 | Bulgaria |
| 9 | Ames 18559 | 880558 | N. Rhine-Westphalia, Germany | 69 | Ames 24909 | Coriander Long Standing | USA |
| 10 | Ames 18560 | 880566 | N. Rhine-Westphalia, Germany | 70 | Ames 24917 | A008 | Portugal |
| 11 | Ames 18561 | 880589 | Quebec, Canada | 71 | Ames 24921 | A012 | USA |
| 12 | Ames 18563 | 880780 | Calvados, France | 72 | Ames 24923 | A014 | Georgia |
| 13 | Ames 18564 | 880850 | Maine-et-Loire, France | 73 | Ames 24926 | A017 | Azerbaijan |
| 14 | Ames 18565 | 880885 | Berlin, Germany | 74 | Ames 25170 | CO94INC 1121 | USA |
| 15 | Ames 18566 | 880886 | South Moravia, Czech Republic | 75 | Ames 27391 | Z023 | Uzbekistan |
| 16 | Ames 18567 | 880972 | Madrid, Spain | 76 | Ames 27392 | Z075 | Uzbekistan |
| 17 | Ames 18568 | 883197 | Tuscany, Italy | 77 | Ames 27770 | CO SA 3-02 | Palestine |
| 18 | Ames 18569 | 883216 | Ibaraki, Japan | 78 | Ames 27771 | CO SA 3-03 | Palestine |
| 19 | Ames 18570 | 883217 | Ibaraki, Japan | 79 | Ames 27772 | CO SA 3-04 | Palestine |
| 20 | Ames 18571 | 883218 | Ibaraki, Japan | 80 | Ames 27870 | CO SA 3-05 | Palestine |
| 21 | Ames 18572 | 883258 | North Holland, Netherlands | 81 | Ames 29172 | GSMO 1-2 | Georgia |
| 22 | Ames 18573 | 883260 | Gelderland, Netherlands | 82 | Ames 29173 | GSMO 2-8 | Georgia |
| 23 | Ames 18574 | 883261 | Central Bohemia, Czech Republic | 83 | Ames 29174 | GSMO 8-3 | Georgia |
| 24 | Ames 18575 | 883296 | Leningrad, Russia | 84 | PI 170319 | No. 2348 | Türkiye |
| 25 | Ames 18577 | 883297 | Leningrad, Russia | 85 | PI 170320 | Türkiye | |
| 26 | Ames 18578 | 883298 | Leningrad, Russia | 86 | PI 171592 | TU2_Ic | Türkiye |
| 27 | Ames 18580 | 883300 | Leningrad, Russia | 87 | PI 172808 | Maydonoz Gili; Kinzi | Türkiye |
| 28 | Ames 18581 | 891044 | Germany | 88 | PI 174129 | TU4_IIIb | Türkiye |
| 29 | Ames 18582 | 891121 | Arhus, Denmark | 89 | PI 193493 | Ethiopia | |
| 30 | Ames 18583 | 891124 | Seine-Maritime, France | 90 | PI 193769 | Dimbelal | Ethiopia |
| 31 | Ames 18585 | 891139 | South Moravia, Czech Republic | 91 | PI 193770 | Dimbelal | Ethiopia |
| 32 | Ames 18587 | 891161 | Gelderland, Netherlands | 92 | PI 196843 | Ethiopia | |
| 33 | Ames 18588 | 891280 | Warszawa, Poland | 93 | PI 249115 | India | |
| 34 | Ames 18589 | 891281 | Poland | 94 | PI 253146 | Gashniz | Iran |
| 35 | Ames 18590 | 891282 | Poland | 95 | PI 256061 | AF1_IIIb | Afghanistan |
| 36 | Ames 18591 | 891358 | Saxony-Anhalt, Germany | 96 | PI 268378 | Gashniz | Afghanistan |
| 37 | Ames 18592 | 901014 | England | 97 | PI 269470 | Pakistan | |
| 38 | Ames 18594 | 901016 | England | 98 | PI 269472 | Pakistan | |
| 39 | Ames 18595 | 901050 | Romany | 99 | PI 274290 | 1180 | India |
| 40 | Ames 18596 | 901069 | Canada | 100 | PI 478378 | O 21 | China |
| 41 | Ames 19032 | Ames 19032 | Kazakhstan | 101 | PI 483232 | CHL1_IIIb | Chile |
| 42 | Ames 19089 | Ames 19089 | Kazakhstan | 102 | PI 502320 | AR-235 | Uzbekistan |
| 43 | Ames 20046 | VIR 79 | Azerbaijan | 103 | PI 531293 | CSILLAG | Hungary |
| 44 | Ames 20047 | VIR 97 | Armenia | 104 | PI 531296 | 441 | Hungary |
| 45 | Ames 20048 | VIR 101 | Kazakhstan | 105 | PI 633685 | CDC Major | Canada |
| 46 | Ames 21105 | IC 67145 | India | 106 | PI 664512 | TJK 2006:074 | Tajikistan |
| 47 | Ames 21108 | IC 67155 | India | 107 | PI 669959 | IC 33013 | India |
| 48 | Ames 21655 | G18171 | Russia | 108 | PI 669960 | IC 33667 | India |
| 49 | Ames 23614 | CORI 189 | Saarland, Germany | 109 | PI 669961 | IC 34513 | India |
| 50 | Ames 23616 | CORI 220 | Russia | 110 | PI 669962 | IC 33728 | India |
| 51 | Ames 23618 | CORI 115 | Sudan | 111 | PI 669963 | IC 62485 | India |
| 52 | Ames 23619 | CORI 127 | Bhutan | 112 | PI 669964 | IC 67148 | India |
| 53 | Ames 23620 | CORI 131 | Baluchistan, Pakistan | 113 | PI 669965 | IC 67153 | India |
| 54 | Ames 23621 | CORI 82 | Syria | 114 | PI 669966 | IC 67161 | India |
| 55 | Ames 23622 | CORI 84 | Syria | 115 | Arslan | Cultivar | Türkiye |
| 56 | Ames 23623 | CORI 86 | Syria | 116 | Erbaa | Cultivar | Türkiye |
| 57 | Ames 23624 | CORI 87 | Syria | 117 | Gürbüz | Cultivar | Türkiye |
| 58 | Ames 23625 | CORI 98 | China | 118 | Pelmus | Cultivar | Türkiye |
| 59 | Ames 23626 | CORI 117 | Sudan | 119 | Gamze | Cultivar | Türkiye |
| 60 | Ames 23627 | CORI 119 | Sudan |
2.2. Experimental Details
The experiment was established on 30 April 2019 at Bolu Abant İzzet Baysal University, Faculty of Agriculture, Research and Application area, as 6 blocks in a single row, according to the augmented trial design. The experiment was conducted with 1 m distance between blocks, 3.5 m length of the blocks, and a plot size of 24.6 m × 8 m with a spacing of 0.3 m between rows and 0.1 m between plants. There are 24 rows in each block, and each block is 25.2 m2. The soil properties of the experimental area were as follows: low in phosphorus (P) (0.50 kg/ha), rich in potassium (K) (1083.1 kg/ha) and organic matter (3.71%), clayey and slightly alkaline (pH = 7.56), medium-lime (1.14%), and low salinity (0.04%). Average climatic data were recorded between 8.3 and 19.5 °C for temperature, 6.3 and 138.6 mm for rainfall, and 70.0 and 80.9% for humidity during the vegetation period of 2019 from April to October [15]. In the experiment, the fruits were sown on 30 April 2019, and sowing was conducted with an excessive number of fruits (approximately 50), enabling thinning to be performed 10–15 days after emergence to achieve the desired plants in each row. With the planting in the experimental area, 60 kg/ha of diammonium phosphate (DAP) and 25 kg/ha of ammonium sulfate (21% N) were applied as base fertilizers; at the beginning of flowering, 25 kg/ha of ammonium sulfate was applied again as fertilizer. After the seedling days, watering was performed every day as much as the plants needed using the drip irrigation method, and weed control was performed by hand every two days. After 10 randomly selected plants for the morphological and yield values were taken from each row in the blocks, the entire row was harvested between 27 July and 30 September 2019.
2.3. Essential Oil Content (%v/w)
Essential oil contents were determined volumetrically with a Clevenger apparatus according to the water distillation method in dried fruits at 35 °C. Approximately 20 g of sample from the dried fruit prepared for analysis was weighed. The weighed sample was placed in a glass Clevenger flask. Approximately 200 mL of pure water was added to the sample. It was subjected to hydrodistillation for approximately 4 h. Then, the essential oil sample, which accumulates in the graduated section and creates a phase difference with water, was read, and the result is recorded in ml. Then, based on the weighing amount, the amount of essential oil was calculated as a percentage mL/100 g (%v/w) based on dry matter.
2.4. Essential Oil Components (%v/v)
Essential oil components were prepared in the Central Laboratory of Bolu Abant İzzet Baysal University. Samples were diluted 1:100 with methanol for analysis. Essential oil component analysis of the samples was carried out using a GC/GC-MS (gas chromatography (Thermo Scientific Trace 1300) mass detector (Thermo Scientific ISQ QD, Waltham, MA, USA)) device and a capillary column (TG-624; 30.0 m × 0.25 mm × 1.4 μm). In the analysis, helium was used as the carrier gas at a flow rate of 1.00 mL/min, and the samples were injected into the device at 1 μL. The injector temperature was kept at 220 °C, and the column temperature program was set as 70 °C (2 min), 70 °C to 200 °C at 3 °C/minute, and 200 °C (15 min) in splitless mode. In line with this temperature program, the total analysis time was 60 min. For the mass detector, a scanning range (m/z) of 40–650 atomic mass units and electron bombardment ionization of 70 eV, a transfer line temperature of 250 °C, and an ion source temperature of 220 °C were used. Data from WILEY and NIST libraries were used to identify the components of the essential oil. MS was used to identify component percentages and components of the results.
2.5. Fixed Oil Contents (%v/w)
The fixed oil content (%v/w) was determined in the hexane (C6H14) extraction of the samples taken from each row according to the Soxhlet method for 8 hours. Fruits of coriander genotypes, 10 g, were used and extracted with a Soxhlet extractor at 60 °C for 8 h using n-hexane as the solvent. After oil extraction, the solvent was removed with a rotary evaporator.
2.6. Fixed Acids (%)
To determine the fixed acids, oil samples were esterified according to the principles given in Anonymous [16]. Esters were injected into the gas chromatograph, and the fixed acids were determined as a percentage. Fixed acids were performed using automatic sampling (Shimadzu-AOC20i) and GC (Shimadzu-2010 Plus) (Shimadzu, Kyoto, Japan) with flame ionization detector (FID) and Rtx-2330 Capillary Column (60 m, 0.25-mm inner diameter, 0.2 μm film thickness). The detector temperature was set at 240 °C. Meanwhile, the oven temperature was kept at 140 °C for 5 min. Afterwards, it was increased by 4 °C every minute, brought up to 260 °C, and kept for 20 min. The sample amount was 1 μL, and the carrier gas He control was ensured at 1 mL/min. Fixed acids were identified by comparing the arrival times of the standard 37-component FAME mixture.
2.7. Morphological Characteristics and UPOV Criteria
The morphological characteristics in the study were determined as follows: Days to 50% seedlings, flowering, and fruit setting were recorded as the number of days from sowing until 50% of the plants in a net plot produced seedlings, flowers, and fruits as determined by visual observation, respectively. Plant height was measured in centimeters (cm) for ten randomly selected plants, from ground level to the apex, at the time of physiological maturity in the net plot area. The average number of primary branches that emerged directly from the main shoot was counted for ten randomly selected plants at physiological maturity. The number of umbels from 10 randomly selected plants in each row was counted, and the average number of umbels per plant was calculated. The number of umbelletes from 10 randomly selected plants in each row was counted, and the average number of umbelletes per plant was calculated. Thousand fruit weights were calculated from the portion of fruits separated as pure seed from each row; four samples of 100 fruits were weighed using a precision scale. The average weight from these four measurements was then multiplied by 10 to determine the thousand grain weight in grams. After harvest, the fruits from the threshed plants were weighed, and the fruit yield per plant was recorded in g/plant. Biological yield per plant (g) was calculated on the dry weight of the harvested plants. The harvest index was calculated by dividing the biological yield by the fruit yield and multiplying the result by 100 using the following formula.
| Harvest index = (Fruit yield/Biological yield) × 100 |
UPOV criteria examined in coriander genotypes and cultivars were determined according to UPOV [17]. The examined criteria, notes, and explanations are given in Table 2.
Table 2.
UPOV criteria of the different origin coriander genotypes.
| No | Criteria | Note | Explanation | No | Criteria | Note | Explanation |
|---|---|---|---|---|---|---|---|
| 1 | Anthocyanin coloration in the flower |
1 | Absent | 7 | Number of leaflets in basal leaf |
1 | Three |
| 9 | Present | 2 | Five | ||||
| 2 | Intensity of anthocyanin coloration in the flowers |
3 | Weak | 8 | Size of terminal leaflet in leaf |
3 | Small |
| 5 | Medium | 5 | Medium | ||||
| 7 | Strong | 7 | Large | ||||
| 3 | Anthocyanin coloration of hypocotyl in seedling |
1 | Absent/very weak | 9 | Structure of feathering in basal leaf |
1 | Fine |
| 3 | Weak | 2 | Medium | ||||
| 5 | Medium | 3 | Coarse | ||||
| 7 | Strong | 10 | Density of incisions on margin in leaflet |
3 | Sparse | ||
| 9 | Very strong | 5 | Medium | ||||
| 4 | Cotyledon shape | 1 | Narrow elliptic | 7 | Dense | ||
| 2 | Elliptic | 11 | Fruit shape | 1 | Rounded | ||
| 3 | Broad elliptic | 2 | Elongated | ||||
| 5 | Density of foliage in plant |
3 | Sparse | 3 | Elliptic | ||
| 5 | Medium | 12 | Intensity of brown color in fruit |
3 | Light | ||
| 7 | Dense | 5 | Medium | ||||
| 6 | Coloration in foliage | 1 | Yellowish green | 7 | Dark | ||
| 3 | Green | ||||||
| 5 | Dark green |
2.8. Statistical Analysis
The data obtained in the study were subjected to variance analysis according to the augmented trial design, separately for each property, and the significance tests were made using the AVCI statistical program according to the F test and the difference grouping of the means according to the least significant difference (LSD) method [18]. As a result of variance analysis, among genotypes, the following were calculated: 1- Two corrected applications (lines) in different blocks; 2- Corrected application (line) and control (std) difference; 3- Two corrected applications (lines) in the same block; and 4- Four control (std) variances and LSD values. The least significant difference was found by calculation, according to Peterson [19]. In statistics, letters “A” in uppercase, “z” in lowercase, and numbers were used to denote statistical differences among the examined properties, respectively. In addition, cluster analysis was performed between coriander cultivars and genotypes in terms of morphology, yield, and UPOV criteria using the JMP 14 statistical program, and genetic differences were determined. Heat maps and PCA analyses were conducted based on the fixed oil content, major essential oil compositions, and major fixed acids. Also, correlation analysis was carried out to determine the relationship among the morphological and yield values.
3. Results and Discussion
3.1. Phenotypic Properties
The results of the growing season indicated that different origin coriander genotypes and cultivars showed statistically significant differences in terms of phenotypic properties at a 5% level. The first 10 genotypes and cultivars that stood out in terms of the days to seedling (50%), 50% flowering, and 50% fruit setting days are given in Table 3.
Table 3.
Fifty percent seedling, flowering, and fruit setting days of the first top 10 coriander genotypes with cultivars.
| Genotypes/Cultivars | 50% SD | G/C | %50 FD | G/C | %50 FSD |
|---|---|---|---|---|---|
| Ames 18574 | 24.37 S | Ames 18570 | 52.40 mn | Ames 18567 | 61.40 gh |
| Ames 18568 | 24.97 S | Ames 18563 | 52.40 mn | Ames 18561 | 61.40 gh |
| Ames 23624 | 25.57 S | Ames 19089 | 53.00 lmn | Ames 18572 | 62.40 fg |
| Ames 18569 | 25.97 Q–S | Ames 18573 | 53.40 klm | Ames 18569 | 62.40 fg |
| Ames 18570 | 26.97 P–S | Ames 18572 | 53.40 klm | Ames 12778 | 62.40 fg |
| Ames 18595 | 29.37 O–Q | Ames 18568 | 53.40 k–n | Ames 18571 | 62.40 fgh |
| Ames 18580 | 29.37 O–Q | Ames 10234 | 53.4 klm | Ames 10234 | 63.40 efg |
| Ames 18575 | 29.37 O–Q | Ames 18588 | 54.00 j–m | PI 274290 | 65.00 d–g |
| Ames 18588 | 29.37 O–R | Ames 12778 | 54.40 ı–m | Ames 23626 | 65.40 c–g |
| Ames 23618 | 29.57 N–Q | Ames 23623 | 54.60 h–m | Ames 23618 | 65.40c–g |
| Arslan | 36.57 B–F | Arslan | 56.20 d–m | Arslan | 67.17 Z–g |
| Erbaa | 36.57 B–F | Erbaa | 55.33 f–m | Erbaa | 78.50 G–R |
| Gamze | 36.57 B–F | Gamze | 61.50 N–V | Gamze | 75.67 K–Y |
| Gürbüz | 33.17 F–N | Gürbüz | 59.17 S–f | Gürbüz | 73.17 O–c |
| Pelmus | 33.33 F–M | Pelmus | 59.00 T–g | Pelmus | 78.50 G–R |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, SD: seedling days, FD: flowering days, FSD: fruit setting days.
Days to seedling (50%) differ significantly in all the coriander genotypes, ranging from 24.37 to 40.97 days (Table S1). The Ames 18573 genotype showed the minimum days, followed by the Ames 18567 and Ames 23627. The Ames 18559 and Ames 18589 genotypes were found to take maximum days to seedling. The cultivars (average 33.37 days) compared to genotypes had the earliest seedling days from 64 genotypes. Previous studies reported that seedling days changed between 19.46 and 20.80 days [20] and 15 and 20 days [21]. The obtained seedling day values were found to be different from the previous studies. The differences can be explained by the different sowing times, genotype differences, climate, and growing conditions. Also, it can be noted that low temperature effected the coriander seedling positively due to promoting the breakdown of existing proteins in fruits to specific amino acids, which are necessary for the growth of the embryo [22].
The 50% flowering days are one of the most important properties to selection for the earliness in plant breeding programs. In this study, coriander genotypes showed wide variations in relation to 50% flowering days and ranged from 52.40 to 72.20 days among the coriander genotypes (Table 3). The early 50% flowering was observed in Ames 18569 and Ames 18561 genotypes, followed by Ames 18596, Ames 18571, Ames 18567, Ames 10234, and Ames 18572 (Table 3). The latest 50% flowering days were found from Ames 24907 and Ames 24909 genotypes. Gökduman [23] reported a range of 51 to 79 days for coriander under Isparta ecological conditions, and Moniruzzaman et al. [24] noted that 50% flowering time of 14 coriander genotypes changed between 57.33 and 134.30 days.
In addition, the 50% fruit setting days of coriander genotypes showed statistical difference and changed between 61.40 and 96.20 days (Table S1). The earliest fruit setting days were obtained from Ames 18567 and Ames 18561 genotypes, followed by Ames 12778, Ames 18569, Ames 18572, and Ames 18572 genotypes (Table 3). The latest values were found in PI 170319 and Ames 24909 genotypes. When the genotypes were compared with the cultivars (average 74.60 days), it was observed that 59 genotypes set fruit late and 52 genotypes set fruit early. No previous study has been found on the 50% fruit setting days of coriander genotypes. So, this study is the first for the determination of the 50% fruit setting days of coriander.
In fact, a positive association between days to seedling (50%) and days to flowering (50%) is expected. However, in our study, the early seedling days could not show a meaningful and significant relationship with the early flowering days. Differences in early seedling days and early flowering days among coriander genotypes have been attributed to variations in temperature, light intensity, and competition for resources between reproductive and vegetative tissues [25]. In addition, genes for phenology and plant development, their interactions with each other, and the environment may affect the late or early flowering [26]. It was determined that 50% flowering and 50% fruit setting days were found partially similar among the genotypes. The differences can be explained by reducing pollination and fruit setting for insect-pollinated coriander genotypes that flowered earlier [27].
3.2. Plant Height and Branch Number
The results revealed that statistical differences were found for the plant heights among the coriander genotypes (Table S2). The first 10 genotypes and cultivars that stood out in terms of the plant height and branch number values are given in Table 4. The plant height value showed wide variations, and it changed between 25.44 and 84.30 cm. The highest plant height was found in the PI 124179 genotype, followed by Ames 21655, Ames 29172, and Ames 18578 genotypes (Table 4). Plant height values of sixteen coriander genotypes were found higher than coriander cultivars. The lowest plant height values were obtained from Ames 23626, Ames 14363, and PI 274290 genotypes. Compared to the obtained values, there is a 30.18% difference between the highest and the lowest plant heights. Previous studies reported that the plant height of coriander changed between 19.7 and 35.8 cm [14], 33.77–67.86 cm [28], 40.2–69 cm [29], 66.86–84.78 cm [30], and 54.8–64.3 cm [31]. The results of the plant height in this study were in correspondence with the findings of reported previous studies.
Table 4.
The first top 10 genotypes for plant height, branch number, umbel number, and umbellet number values in different origin coriander genotypes and cultivars.
| G/C | PH | G/C | BN | G/C | UN | G/C | UmbltN |
|---|---|---|---|---|---|---|---|
| PI 174129 | 84.30 A | PI 193769 | 15.10 A | Ames 13900 | 72.68 A | Ames 13900 | 380.19 A |
| Ames 21655 | 83.24 AB | Ames 13900 | 12.74 B | Ames 13899 | 62.61 B | PI 193769 | 315.31 B |
| Ames 29172 | 81.10 A–C | Ames 23623 | 11.62 BC | PI 193769 | 52.41 C | Ames 13899 | 200.19 C |
| Ames 18578 | 77.52 A–D | PI 269472 | 11.51 B–D | Ames 10235 | 43.61 CD | Ames 24909 | 156.79 CD |
| Ames 20046 | 76.24 A–E | Ames 23642 | 11.42 B–D | Ames 24909 | 40.57 DE | Ames 10235 | 153.19 C–E |
| Ames 20046 | 76.24 A–E | Ames 13899 | 11.14 B–E | Ames 20046 | 35.65 DEF | Ames 18595 | 148.55 D–F |
| PI 193769 | 75.90 A–F | Ames 18595 | 11.06 B–F | Ames 18568 | 34.41 D–G | Ames 19089 | 138.91 D–G |
| Ames 18581 | 73.92 A–G | Ames 21655 | 11.02 B–F | PI 664512 | 32.77 E–H | Ames 23642 | 130.19 D–H |
| Ames 23621 | 73.84 A–G | Ames 18581 | 10.66 B–G | Ames 23642 | 32.17 E–I | PI 170320 | 126.11 D–H |
| Ames 19089 | 73.64 A–G | PI 174129 | 10.50 B–H | Ames 20048 | 32.05 E–I | Ames 24907 | 98.79 G–P |
| Pelmus | 58.52 G–c | Pelmus | 6.33 T–l | Pelmus | 11.47 Y–q | Pelmus | 38.57 V–l |
| Gürbüz | 59.37 G–b | Gürbüz | 7.50 L–d | Gürbüz | 12.90 T–p | Gürbüz | 53.43 O–k |
| Gamze | 65.63 C–R | Gamze | 7.63 L–b | Gamze | 18.87 N–d | Gamze | 68.20 M–e |
| Erbaa | 70.63 A–L | Erbaa | 7.47 M–d | Erbaa | 20.47 K–Z | Erbaa | 85.10 H–Y |
| Arslan | 49.45 R–m | Arslan | 7.17 N–e | Arslan | 11.97 Y–q | Arslan | 40.27 T–l |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, PH: plant height, BN: branch number, UN: umbel number, UmbltN: umbellet number.
Branch number per plant is so important for the breeding of the coriander due to directly impacting fruit yield [11]. Statistically significant differences were found among the coriander genotypes for branch number (Table 4). The branch number per plant showed high variability and ranged from 2.82 to 15.10 numbers. The most branch number was found in the PI 193769 genotype, followed by Ames 13900, Ames 23623, and PI 269472 genotypes. Ames 23626 had the lowest branch number, followed by Ames 23625 and Ames 23627 genotypes. Forty-nine genotypes were found to have higher branch numbers compared to cultivars. The positive relationship was detected between the plant height and branch number values in coriander genotypes. Previous studies reported that branch number of coriander changed between 4.67 and 15.20 number [28] and 5.13–7.33 number [32]. The obtained branch number values in this study (2.82–15.10 number) were found partly similar with previous studies.
3.3. Number of Umbels per Plant and Number of Umbellets per Plant
There were statistically significant differences for different coriander genotypes with respect to the number of umbels in the experimental year. The differences between the highest and the lowest values showed that the genotypes contained in the present study are quite various (Table S2). The first 10 genotypes and cultivars that stood out in terms of the umbel number and umbellet number values are given in Table 4. The greatest number of umbels was obtained from Ames 13900 (72.68), followed by Ames 13899 (62.61), PI 193769 (52.41), and Ames 10235 (43.61), whereas the lowest number of umbels was noted for the PI 172808 (2.81) and PI 170319 (3.61) genotypes. Twenty-eight genotypes had higher umbel numbers compared to cultivars. Arslan had the highest umbel number, and Gamze had the lowest value among the coriander cultivars. In earlier studies, Hongal et al. [32] reported that variability of yield and quality parameters in different coriander genotypes showed a wide range in umbel number per plant (9.37–18.03). Similarly, Devi and Sharangi [33] reported that the umbel number of coriander genotypes under Gangetic alluvial soils ranged from 17.34 to 29.98 per plant. A study conducted by Santha et al. [14] determined the morphological and seed yield traits of coriander local land races of Tamil Nadu and reported the number of umbels per plant changed between 4 and 34.6 under irrigated and rain-fed conditions. The previous studies showed that the number of umbels in coriander can show variability depending on the genotypes, growing and soil conditions, or geographical regions. With these possibilities, our results on the number of umbels were found to be partly similar to the previous studies.
The number of umbellets per plant was changed among the coriander genotypes and cultivars (Table S2). The existence of a wide difference between the highest and the lowest values was quite diverse in the vegetation period. The different origin genotypes were Ames 13900 (originating from Tajikistan) and PI 193769 (originating from Ethiopia), and the property making them distinct was the highest number of umbellets (380.19 and 315.31, respectively) (Table 4), in contrast with other genotypes showing a number of umbellets in the range of 0.19–200.19 (Table S2). However, the lowest number of umbellets were found from different origin genotypes, such as Ames 12778, Ames 18567, and Ames 18572, with 0.19 per plant. Eighteen genotypes had a greater number of umbellets per umbel over than all other remaining genotypes, which is considered very high fruit yield. Especially, the Ames 13900 genotype had the highest number of umbellets, and this property directly affected positively the fruit and biological yield values of Ames 13900.
The obtained number of umbellets per plant results (0.19–380.19 number) in this study were compared to previous studies; Joble et al. [34] reported between 141.23 and 239.63 number/umbellet per plant; Bhandari and Gupta [35] noted between 7.38 and 174.31 number/umbellet per plant; and Qureshi et al. [36] found between 121 and 336 number/umbellet per plant. The results were found to be partly similar with previous studies. The differences can be explained by the growing conditions, soil properties, ecological conditions, and genotype differences.
3.4. Yield and Yield Attribute Properties
One of the yield attributes of coriander is the thousand fruit weight as a quantitative property. This property can be used for a successful selection breeding program. Among the yield components, 1000 fruit weight seems to be the most important. High yielding ability in genotypes could be attributed to significantly higher thousand fruit weight. Especially, significant differences were found among the coriander genotypes for the thousand fruit weight, and it varied between 1.34 and 21.49 g (Table S3). The first 10 genotypes and cultivars that stood out in terms of the 1000 fruit weight, fruit yield, biological yield, and harvest index are given in Table 5. The lowest thousand fruit weight was detected in Ames 14363 genotype, and the highest thousand fruit weight was determined in Ames 18569 genotype. When the genotypes were compared with the cultivars (average 10.32 g), it was observed that 38 genotypes had high values and 73 genotypes had low values. While the genotypes with the highest values in terms of thousand fruit weight were Ames 18569 (21.49 g), Ames 18572 (20.74 g), Ames 18585 (19.42 g), Ames 18571 (17.62 g), and Ames 23635 (17.06 g), the genotypes with the lowest values were Ames 14363 (1.34 g), Ames 10235 (3.22 g), Ames 29173 (3.23 g), PI 170320 (4.48 g), and Ames 24923 (4.54 g). Previous studies reported that the 1000 fruit weight of the coriander changed between 8.00 and 12.00 g [24] and between 9.33 and 13.82 g [37]. The obtained results from this study were found to be partly similar with the previous studies.
Table 5.
The first top 10 genotypes for 1000 fruit weight, fruit yield, biological yield, and harvest index of different coriander genotypes and cultivars.
| G/C | 1000 FW | G/C | FY | G/C | BY | G/C | HI |
|---|---|---|---|---|---|---|---|
| Ames 18569 | 21.49 A | Ames 13900 | 9.58 A | Ames 13900 | 50.78 A | Ames 23642 | 73.36 A |
| Ames 18572 | 20.74 A | Ames 10234 | 6.58 B | PI 193769 | 26.21 B | Ames 24921 | 48.52 B |
| Ames 18585 | 19.42 AB | Ames 18573 | 6.39 BC | Ames 18573 | 24.34 BC | Ames 18595 | 46.27 BC |
| Ames 18571 | 17.62 BC | Ames 18581 | 6.13 B–D | Ames 18581 | 24.30 BC | Ames 18572 | 45.29 B–D |
| Ames 23635 | 17.06 CD | Ames 24909 | 6.04 B–E | Ames 13899 | 23.86 B–D | PI 478378 | 43.32 B–E |
| Ames 23641 | 16.89 CD | Ames 10235 | 6.04 B–E | Ames 10235 | 22.12 B–E | Ames 19089 | 42.45 B–F |
| Ames 18596 | 16.50 CD | Ames 13899 | 5.92 B–F | PI 172808 | 20.78 B–F | Ames 18588 | 42.14 B–G |
| Ames 23614 | 16.38 C–E | Ames 27391 | 5.84 B–G | Ames 10234 | 20.42 B–G | Ames 18571 | 41.79 B–H |
| PI 274290 | 15.24 D–F | PI 172808 | 5.17 B–H | Ames 23640 | 19.46 B–H | Ames 18585 | 41.66 B–H |
| PI 193769 | 15.11 D–F | PI 633685 | 4.85 B–I | PI 170319 | 17.61 B–I | Ames 21108 | 41.50 B–I |
| Pelmus | 7.29 ı–2 | Pelmus | 1.41 N–e | Pelmus | 5.61 O–d | Pelmus | 25.42 V–o |
| Gürbüz | 10.33 N–a | Gürbüz | 2.84 H–c | Gürbüz | 8.22 I–d | Gürbüz | 34.39 E–V |
| Gamze | 9.08 T–l | Gamze | 2.98 H–b | Gamze | 9.86 I–c | Gamze | 31.46 J–e |
| Erbaa | 7.54 h–z | Erbaa | 3.78 B–Q | Erbaa | 13.87 E–Q | Erbaa | 26.56 Q–o |
| Arslan | 17.37 BC | Arslan | 2.99 G–b | Arslan | 8.90 I–d | Arslan | 34.37 E–V |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, 1000 FW: 1000 fruit weight, FY: fruit yield, BY: biological yield, HI: harvest index.
The fruit yield is a very important property in terms of selecting the high yield genotype for breeding purposes. It also has commercial importance in the spice industry. Among the 114 genotypes and five cultivars tested, the Ames 13900 genotype recorded the highest fruit yield per plant, which was followed by the Ames 10234 and Ames 18573 genotypes (Table 5). The lowest values were recorded in Ames 18567 and Ames 24926 genotypes. Seventeen genotypes had the higher fruit yield compared to coriander cultivars. While the Erbaa cultivar had a higher value, the Pelmus cultivar had the lowest fruit yield value among the coriander cultivars. Devi and Sharangi [29] stated that fruit yield per plant ranged from 1.15 g to 6.17 g. It was reported that the precipitation and temperatures impact the yield and yield product of the coriander [38]. Moreover, different sowing times of coriander decreased the fruit yield between 30.6 and 76.4% [39]. Delaying in sowing time decreased the both fruit yield and biomass yield of coriander by 76.4 and 74.7, respectively [40]. In addition, many more studies reported that productivity of coriander decreased as sowing was postponed to the latest dates [41,42,43].
There were statistically significant differences in coriander genotypes for the biological yield, and it changed between 0.01 and 50.78 g per plant (Table S3). The Ames 13900 and PI 193769 genotypes demonstrated the highest biological yield values compared to other genotypes (Table 5). The lowest biological yield values were observed in Ames 12778, Ames 14363, and Ames 18567 genotypes lower than 1 g. Generally, seventeen coriander genotypes had higher biological yield compared to cultivars, and the Erbaa cultivar had the highest biological yield among the cultivars. Rashed and Darwesh [44] reported that a significant positive relationship was observed between the biological yield and average minimum temperature in the vegetation period of coriander, and the biological yield increased with the increasing of the average minimum temperature. Thakur and Thakur [45] reported that the biological yield of the coriander changed between 31.54 and 60.61 g/plant under growth water stress conditions. The obtained results were found to be partly similar to the findings of Thakur and Thakur [45]. In this study, it is thought that high average rainfall (69.4 kg/m2), high humidity (73.1%), and low temperature (16.1 °C) values cause a decrease in the biological yield of coriander genotypes.
Statistically significant differences were found among the coriander genotypes for the harvest index (Table S3). The harvest index ranged from 7.79% to 73.36%, and Ames 23640, Ames 24921, and Ames 18595 genotypes had the highest values (Table 5 and Table S3). The PI 669964 and PI 193769 genotypes showed the lowest values for harvest index. Thirty-two coriander genotypes were found to have lower values compared to coriander cultivars. The Gürbüz cultivar had the highest value, and the Pelmus cultivar had the lowest value among the coriander cultivars. In earlier studies, Nagappa et al. [46] reported that variability of combined analysis results on harvest index values of thirty coriander genotypes showed a wide range between 21.02 and 57.75%, and Kassu et al. [47] reported that the coriander showed variability harvest index between 18.90 and 42.10% depending on the different sowing dates. The present results comply with the mentioned results with respect to harvest index.
3.5. Essential Oil and Essential Oil Components
The secondary metabolites of the plants may change in quality and quantity based on the genotype, tissue, growth period, geographical area, and stress factors as abiotic and biotic factors [48]. For the efficacy and the informative value of the released essential oils of coriander fruits, the composition of terpenes is crucial, influenced by the different origin genotypes and growing conditions. In total, essential oil contents were determined in 91 coriander genotypes (86 coriander genotypes and five cultivars) selected as promising based on the high yield and other properties according to the sufficient fruit quantity to obtain essential oil contents (Table 6). Similarly, essential oil compositions and fixed acids were determined in 40 coriander genotypes (35 coriander genotypes and five cultivars) selected as promising on the basis of the high yield and, most importantly, other properties, such as umbels/plant, branches/plant, 1000 fruit weight, early flowering, and early fruit setting days, which directly or indirectly affected fruit yield (Table 3, Table 4 and Table 5).
Table 6.
Essential oil contents of different origin high-yielding coriander genotypes.
| G/C | EOC (%v/w) | G/C | EOC (%v/w) | G/C | EOC (%v/w) |
|---|---|---|---|---|---|
| PI 669963 | 0.40 Y–d | Ames 27771 | 0.24 ıj | Ames 18588 | 0.41 V–c |
| PI 669959 | 0.50 P–S | Ames 27770 | 0.24 ıj | Ames 18587 | 0.61 J–M |
| PI 664512 | 0.50 P–S | Ames 27392 | 0.49 Q–T | Ames 18585 | 0.36 b–f |
| PI 633685 | 0.60 K–N | Ames 27391 | 0.94 F | Ames 18583 | 0.56 M–P |
| PI 531296 | 1.20 E | Ames 25170 | 0.39 a–e | Ames 18582 | 0.31 fgh |
| PI 531293 | 1.65 C | Ames 24926 | 0.49 Q–T | Ames 18581 | 0.96 F |
| PI 502320 | 1.75 B | Ames 24923 | 0.24 ıj | Ames 18580 | 0.51 O–R |
| PI 483232 | 0.20 ıjk | Ames 24921 | 0.44 T–a | Ames 18578 | 0.31 fgh |
| PI 478378 | 0.80 GH | Ames 24917 | 0.49 Q–T | Ames 18577 | 0.66 IJK |
| PI 269472 | 0.10 lm | Ames 24909 | 0.14 l | Ames 18575 | 0.46 R–Y |
| PI 269470 | 0.50 P–S | Ames 24907 | 0.99 F | Ames 18574 | 0.56 M–P |
| PI 268378 | 0.51 O–R | Ames 23639 | 0.25 hı | Ames 18573 | 0.56 M–P |
| PI 256061 | 0.51 O–R | Ames 23635 | 0.34 efg | Ames 18572 | 0.30 gh |
| PI 196843 | 0.21 ıj | Ames 23634 | 0.69 I | Ames 18571 | 0.30 gh |
| PI 193770 | 0.46 R–V | Ames 23632 | 0.34 efg | Ames 18570 | 0.60 LMN |
| PI 193493 | 0.10 lm | Ames 23624 | 0.05 m | Ames 18569 | 0.35 d–g |
| PI 174129 | 0.41 U–b | Ames 23622 | 0.53 OPQ | Ames 18568 | 0.45 S–Z |
| PI 171592 | 0.21 ıj | Ames 23621 | 0.06 m | Ames 18567 | 0.35 d–g |
| PI 170320 | 0.81 G | Ames 23616 | 0.55 NOP | Ames 18566 | 0.65 I–L |
| Pelmus | 0.60 LMN | Ames 23614 | 0.20 ıjk | Ames 18565 | 0.60 LMN |
| Gürbüz | 0.35 c–g | Ames 21655 | 0.46 R–U | Ames 18564 | 0.80 GH |
| Gamze | 0.55 M–P | Ames 20048 | 0.55 NOP | Ames 18563 | 0.80 GH |
| Erbaa | 0.55 M–P | Ames 20046 | 0.40 Z–d | Ames 18561 | 0.20 jk |
| Arslan | 0.15 kl | Ames 19089 | 1.50 D | Ames 18560 | 0.65 I–L |
| Ames 4998 | 0.65 I–L | Ames 18596 | 0.51 O–R | Ames 18559 | 0.75 H |
| Ames 23640 | 0.25 hı | Ames 18595 | 1.51 D | Ames 13900 | 1.86 A |
| Ames 29174 | 0.76 GH | Ames 18594 | 0.51 O–R | Ames 13899 | 0.65 I–L |
| Ames 29173 | 0.66 IJ | Ames 18592 | 1.16 E | Ames 10235 | 0.53 OPQ |
| Ames 29172 | 0.51 O–R | Ames 18591 | 0.76 GH | Ames 10234 | 0.40 Z–d |
| Ames 27870 | 0.31 fg | Ames 18590 | 0.66 IJK | ||
| Ames 27772 | 0.56 MNO | Ames 18589 | 0.76 GH |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, EOC: essential oil content.
Essential oil contents (EOCs) of the coriander genotypes were isolated in 86 genotypes and five cultivars depending on the fruit yield values. EOCs of the coriander genotypes ranged from 0.05%v/w to 1.86%v/w. The highest EOC was found in Ames 13900, followed by PI 502320 and PI 531293 genotypes. The lowest EOC were observed from Ames 23624, Ames 23621, PI 269472, and PI 193493 genotypes. In general, 30 genotypes were found to be higher than all cultivars in terms of essential oil content, while five genotypes were found to be lower than all cultivars. It was determined that more than 50% of the genotypes whose essential oil contents were determined due to their fruit yield values had essential oil contents above 0.50%v/w.
The essential oil content of the coriander showed high different variabilities depending on the country, region, growing conditions, or other factors. Ebrahimi et al. [12] conducted a study to determine the essential oil compositions of 19 coriander accessions in Iran, and it was noted that the essential oil content of the dried seeds changed between 0.1 and 0.36%v/w. Fattahi et al. [49] found the essential oil content of dried shoot sample between 0.18 and 0.31%v/w under cadmium and lead stress conditions.
The international standard for coriander essential oil varies by country. According to the European Pharmacopoeia, the essential oil value of coriander should not be less than 0.2%v/w in Indian origin, 0.8–1%v/w and 0.5%v/w in Russian origin. In addition, according to the Turkish Food Codex Spice Communiqué, the essential oil content of unground coriander spice is required to be not less than 0.4%v/w [29]. The result of the study revealed that five genotypes and Arslan cultivars had lower than 0.2% essential oil contents, and these genotypes were found to have lower values than European Pharmacopoeia standards.
In total, forty-three essential oil compositions were detected in different coriander genotypes. Some differences in the quantity of the main components of essential oils extracted from fruit of the different origin coriander genotypes and cultivars. The total oil compositions of fruits were found to be in the range of 77.23–93.85%v/v, with linalool, camphor, ɣ-terpinene, p-cymene, and β-pinene being the most abundant compounds, constituting around 18.94 to 71.13%v/v of the investigated total concentration of essential oils (Table 7). The first major essential oil composition was linalool, whose content varied from 3.13 to 45.70%v/v. It was observed that 11 genotypes had high values and 24 genotypes had low values compared to cultivar means (13.03%). The genotypes with the highest values in terms of linalool ratio were Ames 29174 (45.70%v/v), Ames 23635 (35.00%v/v), Ames 18585 (33.87%v/v), Ames 24907 (30.25%v/v), and Ames 24921 (25.81%v/v) genotypes. The genotypes with the lowest values were Ames 18577 (3.13%v/v), Ames 18590 (5.56%v/v), Ames 18573 (5.73%v/v), Ames 18591 (6.08%v/v), and Ames 18569 (6.29%v/v) genotypes. The second major essential oil composition was ɣ-terpinene. The values of the ɣ-terpinene ranged from 0.05 to 15.60%v/v. Ames 29174 and Ames 24907 genotypes had the lowest and highest ɣ-terpinene values, respectively. The ɣ-terpinene means of the cultivars were found higher than 17 coriander genotypes. The third major essential oil composition was camphor, which changed between 0.05 and 11.18%v/v (Table 7). The highest camphor contents were found in the Pelmus cultivar, followed by the Ames 33640 genotype. The lowest value was noted in the Ames 20048 genotype. It was noted that seven genotypes had high values and 28 genotypes had low values compared to cultivars means (5.51%v/v). p-cymene was the fourth essential oil composition of the coriander genotypes, and it changed between 0.02 and 6.90%v/v. The lowest p-cymene ratio was detected in Ames 29174 genotype, and the highest p-cymene ratio was detected in Ames 23634 genotype. When genotypes were compared with cultivars (average 4.02%), it was observed that 19 genotypes had high values and 16 genotypes had low values. The last major essential oil composition was noted as β-Pinene. It ranged from 0.10 to 2.68%, and Ames 18587 and Ames 10235 genotypes showed the lowest and highest β-Pinene contents, respectively. When the genotypes were compared with the cultivars (0.69% on average), it was noted that 24 genotypes had high values and 11 genotypes had low values. The genotypes with the highest β-pinene content were Ames 10235 (2.68%v/v), Ames 29174 (2.07%v/v), PI 193493 (1.94%v/v), PI 478378 (1.94%v/v), and Ames 18590 (1.86%v/v); the lowest genotypes are Ames 18587 (0.10%v/v), Ames 10234 (0.19%v/v), Ames 23635 (0.36%v/v), Ames 24921 (0.37%v/v), and Ames 18568 (0.39%v/v).
Table 7.
Major essential oil compositions of selected high-yielding coriander genotypes.
| G/C/EOCom | Linalool (%v/v) | Camphor (%v/v) | γ-Terpinene (%v/v) | p-Cymene (%v/v) | β-Pinene (%v/v) | Total |
|---|---|---|---|---|---|---|
| RT (min) | 23.63 | 25.95 | 21.17 | 20.4 | 18.48 | |
| PI 664512 | 6.48 YZ | 6.32 F | 8.34 H | 5.07 A–D | 1.03 N | 25.15 |
| PI 633685 | 6.45 YZa | 4.35 N | 6.56 S | 3.31 C–F | 1.17 M | 29.09 |
| PI 483232 | 10.60 Q | 5.28 H | 8.32 H | 4.50 A–E | 1.59 FG | 21.19 |
| PI 478378 | 7.80 ST | 4.09 OP | 6.92 P | 4.37 A–F | 1.94 C | 28.20 |
| PI 269472 | 13.92 M | 3.75 RS | 9.66 C | 2.68 D–G | 0.55 R | 22.37 |
| PI 193493 | 6.68 VY | 4.28 NO | 7.05 O | 4.97 A–D | 1.94 C | 34.54 |
| PI 174129 | 11.81 P | 0.97 a | 4.60 b | 1.58 FG | 0.98 N | 29.05 |
| PI 171592 | 6.42 YZa | 4.55 LM | 6.04 V | 3.61 B–F | 1.84 D | 26.26 |
| Pelmus | 7.29 TU | 11.18 A | 8.68 G | 4.13 A–F | 0.88 O | 26.46 |
| Gürbüz | 7.12 UV | 4.40 LMN | 6.24 T | 3.65 B–F | 0.74 P | 29.18 |
| Gamze | 29.54 E | 4.36 MN | 7.88 J | 3.26 C–F | 0.25 V | 42.24 |
| Erbaa | 15.29 K | 3.80 QR | 5.06 a | 3.77 B–F | 0.34 U | 40.02 |
| Arslan | 5.89 abc | 3.80 QR | 6.57 S | 5.30 A–D | 1.26 L | 30.56 |
| Ames 29174 | 45.70 A | 4.89 K | 0.05 d | 0.02 G | 2.07 B | 71.13 |
| Ames 24921 | 25.81 F | 4.57 L | 7.69L | 3.97 B–F | 0.37 STU | 54.97 |
| Ames 24909 | 12.95 N | 5.95 G | 8.93F | 6.24 AB | 1.25 L | 41.54 |
| Ames 24907 | 30.25 D | 6.83 D | 15.60A | 4.08 A–F | 0.54 R | 54.49 |
| Ames 23640 | 7.74 ST | 9.37 B | 9.34 E | 5.21 A–D | 0.60 Q | 19.75 |
| Ames 23635 | 35.00 B | 3.57 SV | 5.07 a | 1.92 EFG | 0.36 TU | 54.47 |
| Ames 23634 | 19.23 I | 3.39 VYZ | 12.91 B | 6.90 A | 0.71 P | 32.61 |
| Ames 20048 | 14.55 L | 0.05 d | 7.81 JK | 4.43 A–F | 0.62 Q | 35.65 |
| Ames 19089 | 9.20 R | 6.59 E | 6.2 TU | 3.65 B–F | 0.83 O | 31.93 |
| Ames 18595 | 7.29 TU | 5.04 JK | 6.60 S | 4.65 A–E | 1.28 KL | 27.17 |
| Ames 18591 | 6.08 Z–c | 5.08 IJK | 7.51 M | 4.90 A–D | 1.55 GH | 28.31 |
| Ames 18590 | 5.56 c | 3.72 RST | 6.88 P | 5.29 A–D | 1.86 D | 18.94 |
| Ames 18587 | 21.40 G | 3.63 R–U | 5.57 Y | 3.07 C–F | 0.10 Z | 38.76 |
| Ames 18585 | 33.87 C | 7.15 C | 9.48 D | 3.77 B–F | 0.42 S | 51.70 |
| Ames 18581 | 7.87 S | 3.71 RST | 6.1 UV | 4.48 A–E | 1.15 M | 24.75 |
| Ames 18577 | 3.13 d | 0.74 b | 6.74 QR | 5.51 A–D | 1.79 E | 19.48 |
| Ames 18575 | 9.06 R | 3.37 YZ | 6.85 PQ | 4.58 A–E | 1.41 J | 29.81 |
| Ames 18573 | 5.73 bc | 5.14 HIJ | 5.15 a | 3.31 C–F | 1.47 I | 21.97 |
| Ames 18570 | 12.77 NO | 5.24 HI | 7.26 N | 5.81 ABC | 0.60 Q | 36.26 |
| Ames 18569 | 6.29 Y–b | 3.97 PQ | 5.65 Y | 3.80 B–F | 0.83 O | 26.51 |
| Ames 18568 | 20.68 H | 6.06 G | 9.52 D | 5.54 ABC | 0.39 ST | 38.23 |
| Ames 18566 | 9.09 R | 3.49 U–Z | 7.44 M | 4.92 A–D | 1.50 HI | 24.97 |
| Ames 18559 | 6.85 UVY | 3.34 Z | 5.37 Z | 3.62 B–F | 0.84 O | 29.34 |
| Ames 13900 | 7.06 UV | 5.23 HIJ | 7.76 KL | 5.59 ABC | 1.32 K | 25.34 |
| Ames 13899 | 9.62 R | 4.14 OP | 8.07 I | 4.91 A–D | 1.61 F | 31.23 |
| Ames 10235 | 12.35 OP | 3.55 T–Y | 4.28 c | 2.98 C–F | 2.61 A | 26.63 |
| Ames 10234 | 16.69 J | 0.37 c | 6.67 RS | 3.83 B–F | 0.19 Y | 31.14 |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, EOCom: essential oil composition, RT: retention time.
In this study, other minor essential oil compositions (38) i.e., a-pinene, camphene, sabiene, di-limonene, and 1-borneol were found lower than 10%v/v contents (Table S4). These minor essential oil compositions showed high variability among the coriander genotypes.
Many previous studies reported that the main essential oil compositions of the coriander fruits were linalool, p-cymene, and limonene. However, the essential oil compositions may show variation based on the different soil and climatic conditions, altitude, seasonal factors, another environmental effect, and different chemical variations or chemotypes can be revealed in some cases [50].
Mandal and Mandal [51] reported that linalool is the major and greatest (40–70%v/v) essential oil composition of coriander fruits. This component is found in many plants as a monoterpene alcohol and shows an antimicrobial effect on the different bacteria. The result in this study showed that linalool content was found to be partly similar with the Mandal and Mandal [51].
The main essential oil compositions may vary depending on the genotype, geographical conditions, agricultural applications, climatic, and soil properties. Weisany et al. [52] reported that coriander apiole and carvone were the first two main essential oil compositions in coriander/soybean intercropping and mycorrhizae application. Another study reported that n-decanal, 2E-dodecanal, 2E-decanal, 2E-tridecen-1-al, and n-nonane were the main among the 24 essential oil compositions of coriander leaves grown under salinity and foliar-applied silicon [53]. Similarly, El-Zaeddi et al. [54] reported that the main compositions were noted as E-2-dodecenal, dodecanal, and octane at different harvest times of coriander in Spain ecological conditions.
The differences in essential oil contents and compositions can be explained by some factors such as growing conditions (irrigation, used fertilizer species and doses), environmental conditions (light, nutrient availability, temperature, and day length), maturation process, or different genotypes [7,8,55,56,57,58]. Sangwan et al. [59] reported that the linalool contents of coriander fruits were affected by climatic factors such as cloudy days, lower temperatures during maturation, and high rainfall amounts during the vegetation periods.
3.6. Fixed Oil Content and Fatty Acid Profiles
The results showed that statistically significant differences were found for the fruit-fixed oil of the coriander genotypes (Table 8). The fixed oil content varied between 10.22 and 34.03%v/w. When genotypes were compared with cultivars (average 28.24%v/w), it was observed that 13 genotypes had high values and 22 genotypes had low values. The genotypes with the highest fixed oil contents were Ames 10234 (34.03%v/w), Ames 23634 (33.63%v/w), Ames 18559 (33.19%v/w), Ames 13900 (32.21%v/w), and Ames 33640 (31.85%v/w), while the genotypes with the lowest genotypes were Ames 10235 (10.22%v/w), Ames 18568 (10.35%v/w), Ames 18587 (12.52%v/w), Ames 18585 (13.56%v/w), and Ames 18575 (13.72%v/w).
Table 8.
Fixed oil contents of the high-yielding different origin coriander genotypes.
| G/C | FOC (%v/w) | G/C | FOC (%v/w) | G/C | FOC (%v/w) |
|---|---|---|---|---|---|
| PI 664512 | 25.70 S | Ames 24921 | 29.49 N | Ames 18577 | 16.31 h |
| PI 633685 | 24.98 U | Ames 24909 | 30.91 J | Ames 18575 | 13.72 k |
| PI 483232 | 25.35 T | Ames 24907 | 30.60 K | Ames 18573 | 28.86 O |
| PI 478378 | 22.11 b | Ames 23640 | 31.85 G | Ames 18570 | 31.39 I |
| PI 269472 | 25.89 R | Ames 23635 | 24.06 Z | Ames 18569 | 16.27 ı |
| PI 193493 | 20.35 c | Ames 23634 | 33.63 C | Ames 18568 | 10.35 n |
| PI 174129 | 20.21 d | Ames 20048 | 24.50 Y | Ames 18566 | 30.18 L |
| PI 171592 | 19.35 f | Ames 19089 | 28.56 P | Ames 18559 | 33.19 E |
| Pelmus | 22.43 a | Ames 18595 | 30.04 M | Ames 13900 | 32.21 F |
| Gürbüz | 33.93 B | Ames 18591 | 27.90 Q | Ames 13899 | 14.39 j |
| Gamze | 33.27 D | Ames 18590 | 24.73 V | Ames 10235 | 10.22 o |
| Erbaa | 20.20 d | Ames 18587 | 12.52 m | Ames 10234 | 34.03 A |
| Arslan | 31.4 H | Ames 18585 | 13.56 l | ||
| Ames 29174 | 19.50 e | Ames 18581 | 17.60 g |
Differences between means indicated with the same letter are not significant. G/C: genotypes/cultivars, FOC: fixed oil content.
Previous studies reported that fixed oil of coriander changed between 5.8 and 22.9%v/w [59], 1.63–24.26%v/w [29], 15.01–17%v/w [60] and 4.6–25.1%v/w [61]. The obtained results from this study on fixed oil were found to be similar with the previous studies.
In the fruits of different coriander genotypes and cultivars, eighteen fixed oil acids were identified (85.10–100.00%v/v of total oil samples), with petroselinic, palmitic, elaidic, behenic, and arachidic acids being the most abundant compositions that totally constituted around 62.62–99.48%v/v of the investigated fixed acids (Table 9 and Table S5). Petroselinic asit (C18:1n12) was found in the highest quantity (24.47–87.70%v/v), followed by elaidic acid (C18:1n9t) (1.55–47.44%v/v), palmitic acid (C16:0) (7.13–23.04%v/v), behenic acid (C22:0) (3.17–12.56%v/v), and arachidic acid (C20:0) (1.08–13.33%v/v).
Table 9.
Major fixed oil acids of the high-yielding coriander genotypes.
| G/C-FOA | Petroselinic (C18:1n12) | Palmitic (C16:0) | Elaidic (C18:1n9t) | Behenic (C22:0) | Arachidic (C20:0) | Total |
|---|---|---|---|---|---|---|
| RT | 20.94 | 17.73 | 21.82 | 28.02 | 24.42 | |
| PI 664512 | 63.12 O | 10.83 d | 7.11 Y | 7.11 N | 3.48 K | 91.65 |
| PI 633685 | 61.17 Q | 12.19 O | 10.60 N | 9.08 F | 2.54 R | 95.58 |
| PI 483232 | 66.00 K | 10.37 g | 8.20 S | 7.51 K | 2.15 V | 94.23 |
| PI 478378 | 41.26 ı | 10.97 c | 27.27 B | 7.49 K | 2.20 U | 89.19 |
| PI 269472 | 46.56 f | 14.97 D | 18.23 G | 5.13 V | 2.99 M | 87.88 |
| PI 193493 | 58.08 S | 12.80 K | 6.94 Z | 7.06 O | 5.65 E | 90.53 |
| PI 174129 | 39.92 j | 13.26 I | 26.00 C | 5.92 S | 2.20 U | 87.30 |
| PI 171592 | 55.90 U | 13.52 H | 12.73 J | 6.86 P | 2.98 N | 91.99 |
| Pelmus | 58.10 S | 12.03 R | 9.72 P | 7.68 I | 5.07 H | 92.60 |
| Gürbüz | 71.65 G | 14.86 E | 4.65 e | nd | nd | 91.16 |
| Gamze | 24.47 n | 23.04 A | 47.44 A | nd | nd | 94.95 |
| Erbaa | 46.16 g | 12.33 M | 15.84 I | 5.04 Z | 9.86 C | 89.23 |
| Arslan | 65.52 L | 11.08 Z | 11.05 L | 6.03 R | 1.23 f | 94.91 |
| Ames 29174 | 62.69 P | 12.48 L | 7.93 T | 3.89 f | 3.92 J | 90.91 |
| Ames 24921 | 49.66 d | 12.15 P | 23.49 D | 7.02 O | 2.51 S | 94.83 |
| Ames 24909 | 53.95 Z | 14.48 F | 17.32 H | 5.61 T | 1.70 c | 93.06 |
| Ames 24907 | 74.97 D | 10.34 h | 2.14 ı | 9.24 E | nd | 96.69 |
| Ames 23640 | 67.73 I | 11.54 U | 4.43 f | 12.56 A | 1.08 g | 97.34 |
| Ames 23635 | 59.87 R | 11.00 b | 10.38 O | 4.13 e | 1.89 Z | 87.27 |
| Ames 23634 | 51.12 b | 12.30 N | 10.73 M | 10.19 D | 10.84 B | 95.18 |
| Ames 20048 | 51.36 a | 10.21 ı | 7.45 V | 5.26 U | 1.92 Y | 76.20 |
| Ames 19089 | 48.82 e | 11.39 V | 11.42 K | 7.29 M | 5.63 F | 84.55 |
| Ames 18595 | 46.58 f | 10.38 g | 22.32 E | 4.19 d | 5.16 G | 88.63 |
| Ames 18591 | 66.47 J | 12.11 Q | nd | 7.86 H | 4.68 I | 91.12 |
| Ames 18590 | 39.38 k | 7.22 l | 6.13 c | 7.66 I | 2.23 T | 62.62 |
| Ames 18587 | 31.17 m | 11.73 T | 20.15 F | 10.70 B | 1.84 a | 75.59 |
| Ames 18585 | 64.31 N | 10.79 e | 4.08 g | 4.15 e | 1.59 d | 84.92 |
| Ames 18581 | 45.84 h | 17.16 B | 4.81 d | 3.17 g | 1.90 Z | 72.88 |
| Ames 18577 | 54.52 Y | nd | 8.85 R | 4.51 c | 2.67 P | 70.55 |
| Ames 18575 | 50.22 c | 13.28 I | 6.68 a | 4.54 c | 1.83 b | 76.55 |
| Ames 18573 | 56.82 T | 13.18 J | 2.92 h | 5.09 Y | 7.94 D | 85.95 |
| Ames 18570 | 65.45 M | 10.49 f | 7.44 V | 4.84 a | 2.71 O | 90.93 |
| Ames 18569 | 73.23 F | 7.13 m | 1.55 j | 7.58 J | 3.30 L | 92.79 |
| Ames 18568 | 74.88 E | 16.78 C | 7.82 U | nd | nd | 99.48 |
| Ames 18566 | 36.53 l | 11.06 a | 7.12 Y | nd | 13.33 A | 68.04 |
| Ames 18559 | 87.70 A | 10.04 j | nd | nd | nd | 97.74 |
| Ames 13900 | 80.53 B | 11.91 S | nd | nd | nd | 92.44 |
| Ames 13899 | 79.71 C | 9.76 k | nd | 7.42 L | nd | 96.89 |
| Ames 10235 | 68.80 H | 11.33 Y | 6.30 b | 10.31 C | 1.48 e | 98.22 |
| Ames 10234 | 55.06 V | 13.77 G | 9.32 Q | 6.22 Q | 2.62 Q | 86.99 |
Differences between means indicated with the same letter are not significant. G/C: genotype/cultivar, FOA: fixed oil acid, RT: retention time, nd: not detected.
The variability in the content of petroselinic acid was found to be highly different among the coriander genotypes, and it ranged from 24.47 to 87.70%v/v (Table 9). The highest petroselinic acid content was obtained from the Ames 18559 genotype with 87.70%, followed by the Ames 13900 with 80.53%v/v and Ames 13899 with 79.71%v/v. The lowest petroselinic acid was obtained from Gamze cultivar with 24.47%v/v, and followed by Ames 18587 genotype with 31.17%v/v. Comparing cultivars, it was observed that the cultivars of Gürbüz (71.65%v/v) and Arslan (65.52%v/v) had higher petroselinic acid values than 25 genotypes and other cultivars. The obtained results on the petroselinic acid values of different origin coriander genotypes from this study were found to be similar to those of Sriti et al. [60], Nguyen et al. [61], and Nguyen et al. [62], who reported the petroselinic acid values between 42.20 and 76.37%v/v, 48.80 and 76.40%v/v and 51.80 and 74.00%v/v, respectively.
The elaidic acid values of the coriander genotypes showed wide variation, and these values changed between 1.55 and 47.44%v/v (Table 9). The Gamze cultivar, with PI 478378 and PI 174129 genotypes, had the highest values, and Ames 18569, Ames 24907, and Ames 18573 genotypes had the lowest elaidic values. This study could be the first report on the elaidic acid of coriander because no previous study has been found on the elaidic acid ratios of coriander.
In the study, palmitic acid values of coriander genotypes were found to be statistically significant at the 0.05% level (p < 0.05) (Table 9). As seen in Table 9, palmitic acid values of coriander genotypes varied between 7.13 and 23.04%v/v. The lowest palmitic acid value was detected in the Ames 18569 genotype, and the highest palmitic acid value was detected in the Gamze cultivar, followed by the Ames 18581 genotype. Three genotypes had high values, and 32 genotypes had low values compared to means of coriander cultivars (14.67%v/v). Compared to palmitic acid values of coriander genotypes with the results of previous studies, Ertas et al. [63] and Neffati and Marzouk [64] reported high values from this study. However, Nguyen et al. [61] and Nguyen et al. [62] reported similar findings with this study. It could be thought that the differences from this study reported by Ertas et al. [63] and Neffati and Marzouk [64] were due to climate and growing conditions, environmental factors of the region where the research was conducted, genotype differences, and the large number.
As seen in Table 9, the behenic acid values of coriander genotypes varied between 3.17 and 12.56%v/v. The lowest behenic acid was detected in Ames 18581 genotype, and the highest behenic acid ratio was detected in Ames 33640 genotype. When the genotypes were compared with the cultivars (average 6.25%v/v), it was observed that 18 genotypes had high values, and 17 genotypes had low values in terms of behenic acid values. The genotypes containing the highest behenic acid values were Ames 33640 (12.56%v/v), Ames 18587 (10.70%v/v), Ames 10235 (10.31%v/v), Ames 23634 (10.19%v/v), and Ames 24907 (9.24%v/v), and the lowest genotypes were Ames 18581 (3.17%v/v), Ames 29174 (3.89%v/v), Ames 23635 (4.13%v/v), Ames 18585 (4.15%v/v), and Ames 18595 (4.19%v/v). The behenic acid values of coriander genotypes were compared with the results of previous studies; the findings reported by Nguyen et al. [61] (0.01–1.60%v/v) and Nguyen et al. [62] (0.10–2.30%v/v) were found lower than the obtained from this study. The higher values in this study may be due to genotypic differences, climatic conditions, sample preparation for acids, and applied analytical procedures and crop management practices.
Arachidic acid values ranged from 1.08 to 13.33%v/v in all the genotypes (Table 9). The genotype Ames 18566 showed the maximum arachidic acid, followed by Ames 23634 (10.84%v/v), Ames 18573 (7.94%v/v), PI 193493 (5.65%v/v), and Ames 19089 (5.63%v/v). Ames 33640 had a minimum value for the arachidic acid. Genotypes compared with the cultivars means (5.38%v/v), it was observed that five genotypes had high values and 30 genotypes had low values.
In earlier studies, Nguyen et al. [61] reported that arachidic acid was one of the major acids, and it changed between 0.10 and 0.30%v/v in ecological conditions in France. Similarly, Nguyen et al. [62] noted that it was changed between 0.10 and 5.90%, and Sriti et al. [60] reported between 0.15 and 2.19%v/v. The present results are incompatible with the mentioned results with respect to arachidic acid. Yaldiz and Camlica [7] (2019) and Camlica and Yaldiz [65] reported that the fixed acids vary according to variety, soil structure, and climatic conditions.
The thirteen minor fixed oil acids were found lower than 10%v/v, and values are given in Table S5.
3.7. UPOV Criteria
Coriander cultivars and genotypes were identified in terms of UPOV criteria (Table S6). Anthocyanin coloration in the flower was found to be absent (1) in one cultivar (Pelmus) with 6 genotypes and present (9) in four cultivars (Erbaa, Gamze, Arslan, Gürbüz) with 105 genotypes. The intensity of anthocyanin coloration in the flowers was found to be weak (3) in four cultivars (Gamze, Arslan, Gürbüz, and Pelmus) with 81 genotypes, medium (5) in one cultivar (Erbaa) with 28 genotypes, and strong (7) in 2 genotypes. Anthocyanin coloration of hypocotyl in seedlings is absent or very weak (1) in 9 genotypes, weak (3) in 52 genotypes and one cultivar (Arslan), medium (5) in 32 genotypes and four cultivars (Erbaa, Gamze, Pelmus, Gürbüz), and strong (7) in 18 genotypes. The cotyledon shape was found as narrow elliptic (1) in 36 genotypes, elliptic (2) in 43 genotypes and three cultivars (Erbaa, Arslan, Gürbüz), and broad elliptic (3) in 32 genotypes and two cultivars (Gamze, Pelmus). Density of foliage in plants was found to be sparse (3) in 9 genotypes, medium (5) in 72 genotypes and four cultivars (Erbaa, Gamze, Pelmus, Gürbüz), and dense (7) in 30 genotypes and one cultivar (Arslan). Coloration in foliage was found as yellowish green (1) in four cultivars (Erbaa, Gamze, Pelmus, Gürbüz) with 35 genotypes, green (3) in one cultivar (Arslan) with 46 genotypes, and dark green (5) in 30 genotypes. The number of leaflets in basal leaf was found to be three leaves (1) in four cultivars (Erbaa, Gamze, Arslan, Gürbüz) with 54 genotypes and five leaves (2) in one cultivar (Pelmus) with 57 genotypes. The size of the terminal leaflet in the leaf was found to be small (3) in one cultivar (Arslan) with 35 genotypes, medium (5) in two cultivars (Gamze, Gürbüz) with 42 genotypes, and large (7) in two cultivars (Erbaa, Pelmus) with 34 genotypes. Structure of feathering in basal leaf was found to be fine (1) in three cultivars (Arslan, Pelmus, Gürbüz) with 84 genotypes and medium (2) in two cultivars (Erbaa, Gamze) with 27 genotypes. The leaf serration status was found to be sparse (3) in 39 genotypes, medium (5) in 45 genotypes and three cultivars (Erbaa, Arslan, Gürbüz), and dense (7) in 27 genotypes and two cultivars (Gamze, Pelmus). The fruit shape was found to be rounded (2) in five cultivars with 111 genotypes. The brown color intensity in the fruit was found to be light (3) in one cultivar (Pelmus) with 15 genotypes, medium (5) in one cultivar (Arslan) with 58 genotypes, and dark (7) in three cultivars (Erbaa, Gamze, Gürbüz) with 41 genotypes. Erbaa, Gamze, and Gürbüz cultivars were found similar in terms of anthocyanin density in the flower, anthocyanin density difference in the flower, green color intensity in the plant, green color of the plant, number of leaves, and brown color intensity in the fruit. So, the results of the UPOV criteria values showed large variability among the coriander cultivars and genotypes.
3.8. Cluster Analysis
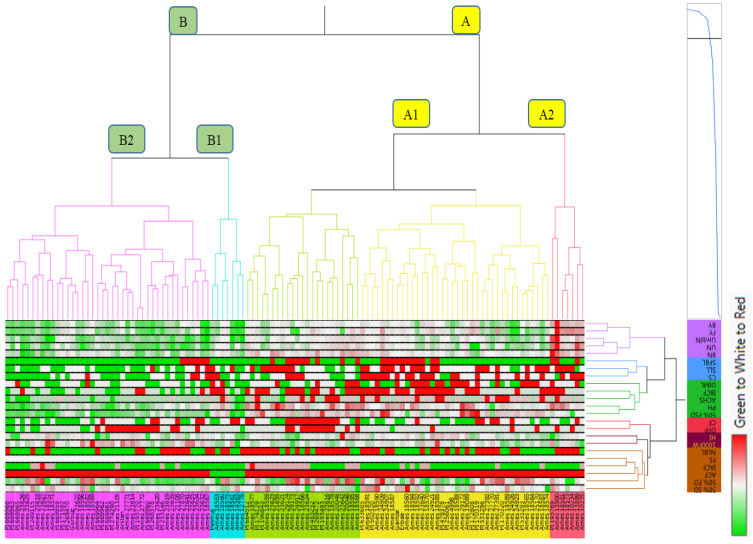
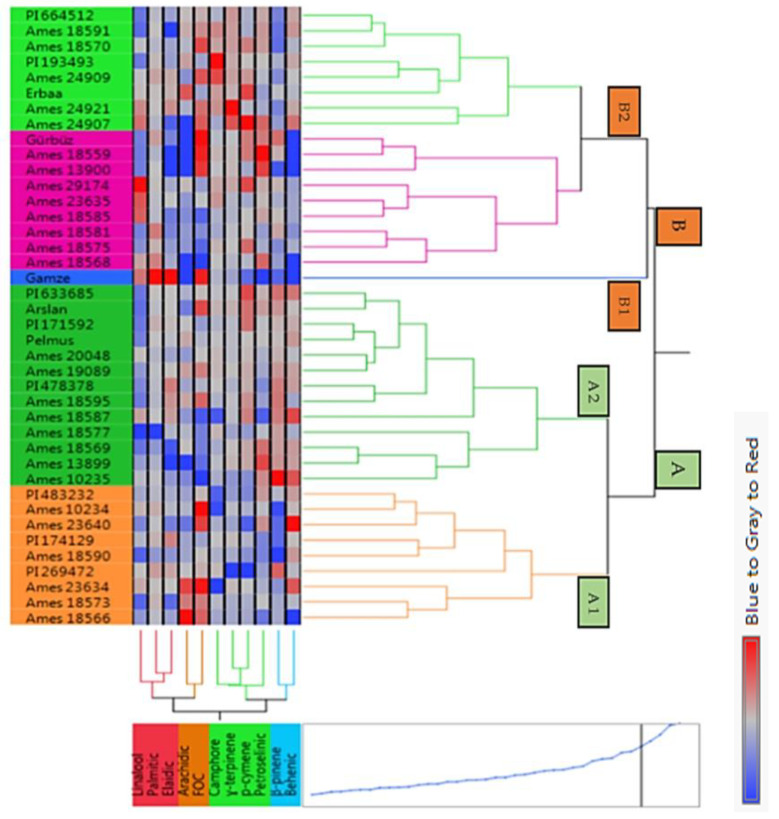
Cluster analysis of the coriander cultivars and genotypes used in the study was performed according to morphological, yield, and some UPOV criteria (Figure 1). Also, the cluster analysis was conducted to determine genetic diversity depending on the fixed oil content, major essential oil compositions, and major fixed acids among the high-yielding selected coriander genotypes and cultivars (Figure 2). As a result of cluster analysis, coriander cultivars and genotypes were divided into two main groups (A and B) (Figure 1).
Figure 1.
Cluster analysis result of the coriander genotypes depending on the examined morphological and yield values. Green to white to red colors show the difference among the examined properties and genotypes. Green color indicates lower data values, white color shows average value, and red represents higher data values.
Figure 2.
Cluster analysis result of the different origin high-yielding coriander genotypes depending on the examined properties. Blue to grey to red colors show the difference among the examined properties and genotypes. Red and light shades of red colors indicate higher data values, and light shades of blue to blue colors represent lower data values.
Sixty-six genotypes and two cultivars (Erbaa and Gamze) took place in group A, and 45 genotypes and three cultivars (Arslan, Pelmus, and Gürbüz) took place in group B. Group A is divided into two subgroups, A1 and A2. There were 59 genotypes and two cultivars (Erbaa and Gamze) in subgroup A1. Among the 59 genotypes in this group, 50 genotypes were determined to be Ames-coded genotypes, and 9 genotypes were determined to be PI-coded genotypes. There were seven genotypes in the A2 subgroup. These nine genotypes consist of genotypes originating from Israel, the Netherlands, Germany, the USA, Tajikistan (2), and Ethiopia.
Group B was divided into two subgroups, B1 and B2. There were six genotypes and one cultivar (Pelmus) in subgroup B1. Six genotypes in this group consist of the Ames-coded genotype, and two genotypes were originating from Russia. There were 39 genotypes and two cultivars (Gürbüz, Arslan) in the B2 subgroup. Among the 39 genotypes in this group, 24 genotypes were determined to be Ames-coded genotypes, and 15 genotypes were determined to be PI-coded genotypes. The Ankara-origin coriander cultivars Arslan and Gürbüz were grouped together within the same subgroup of the main category based on the examined properties.
Figure 2 showed that cluster analysis divided into two A and B main groups for coriander genotypes and cultivars based on the fixed oil, major essential oil compositions, and major fixed acids. While most of the coriander genotypes took place in the A main group, three cultivars (Erbaa, Gürbüz, and Gamze) were found in the B main group. These main groups are also divided into two subgroups: A1, A2, B1, and B2. The subgroup A1 contained nine genotypes, and subgroup A2 contained 11 genotypes and 2 cultivars (Arslan and Pelmus). The subgroup B1 included only the Gamze cultivar, and the subgroup B2 included 15 genotypes and two cultivars (Erbaa and Gürbüz). The B1 subgroup was separated with the values of the fixed oil acids as palmitic, elaidic, and petroselinic. The A1 subgroup was separated from the other groups with β-Pinene essential oil composition and behenic acid.
Gamze and Erbaa with Arslan and Pelmus cultivars were found in the same main group in both Figure 1 and Figure 2. As a result, it can be concluded that these cultivars showed similar genetic variation depending on the examined properties.
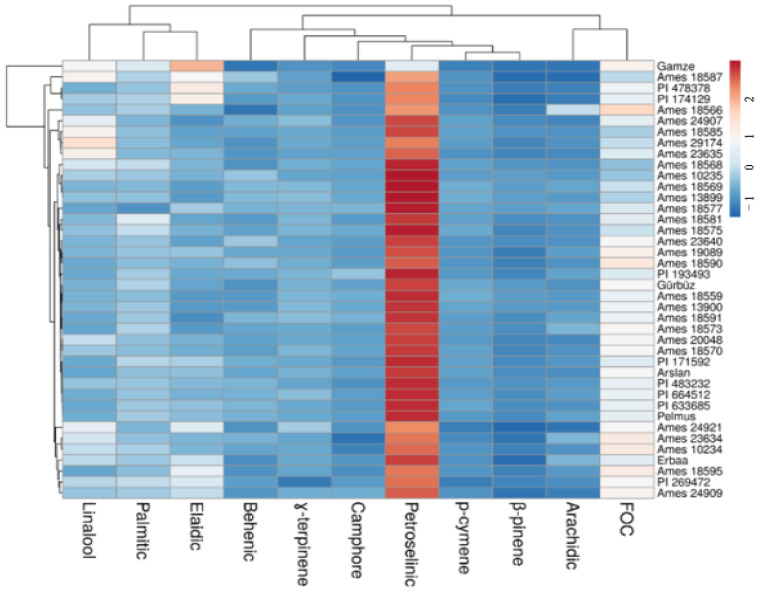
3.9. Heat Map and Principal Coordinate Analyses
The heat map and principal coordinate analyses were conducted to determine the relationship among the coriander genotypes and cultivars with the major essential oil compositions, fixed oil, and major fixed acids (Figure 3 and Figure 4). The heat map analysis revealed that the high-yielding coriander genotypes and cultivars were separated with the fixed oil petroselinic acid values. Especially, the Gamze cultivar took place alone in a group because it included the highest elaidic acid and the lowest petroselinic acid. The Ames 18566 (originating from the Czech Republic) and PI 269472 (originating from Pakistan) showed differences for the arachidic acid and γ-terpinene, respectively.
Figure 3.
Heat map analysis result of the selected coriander genotypes depending on the examined properties. From orange to red (between 1 and 2) colors indicate higher data values, and from light shades of blue (between 0 and 1) to blue (between −1 and 0) colors represent lower data values.
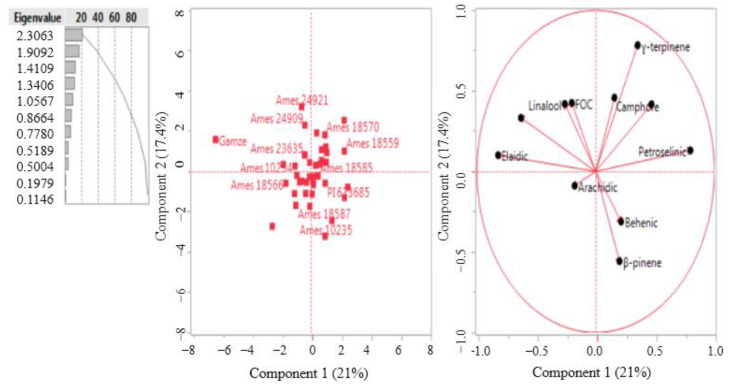
Figure 4.
Principal coordinate analysis result of the examined properties in different selected coriander genotypes.
From five principal components, PC 1 to PC5, extracted from the original data and having an eigenvalue greater than one, accounting for 72.94% of the total variation, suggesting that these principal component scores might be used to summarize the original 11 variables in any further data analysis (Figure 4). Accordingly, the first principal component had high positive coefficients for petroselinic acid. The major contributing character for the diversity in the second principal component (PC2) was linalool, while the third principal component (PC3) was fixed oil content. The fourth principal component contributed diversity for the character of arachidic acid. The fifth principal component was palmitic and behenic acid. On the basis of PCA, most of the important quality properties for the selected genotypes were presented in PC1, PC2, PC3, and PC4. A high PC score for a particular genotype in a particular component showed high values for the variables in that particular genotype.
3.10. Correlation Analysis
The correlation analysis was conducted to determine the relationship among the morphological and yield values. A total of 29 correlations were identified among the examined characteristics, with 22 showing highly significant positive correlations (Table 10). The days to 50% fruit setting had the most correlations, interacting with seven of the characteristics studied. The highest significant positive correlation was found between the number of umbels and the number of umbelletes, with a correlation coefficient of r = 0.902 **. This was followed by the correlation between fruit yield and biological yield, which had a coefficient of r = 0.856 **. It was clearly reported that the highly significant positive correlation was found between the flowering and fruit setting days. Positive correlations were also observed between the days to 50% flowering and branch number, as well as between thousand fruit weight and harvest index. Highly significant negative correlations were noted between thousand fruit weight and days to 50% fruit setting, plant height, umbel number, and umbelletes. Additionally, two positive correlations were found: one between days to 50% flowering and branch number, and another between thousand fruit yield and harvest index. A negative correlation was detected between branch number and thousand fruit yield, with a coefficient of r = −0.207 *.
Table 10.
Correlation analysis results of the morphological and yield values of the different coriander genotypes.
| Characters | 50% FD | 50% FSD | PH | BN | UN | UmblN | 1000 FW | FY | BY | HI |
|---|---|---|---|---|---|---|---|---|---|---|
| 50% SD | 0.014 | −0.135 | −0.173 | −0.089 | −0.011 | −0.02 | 0.076 | −0.009 | 0.022 | −0.068 |
| 50% FD | 0.246 ** | 0.174 | 0.212 * | 0.164 | 0.182 | −0.117 | 0.109 | 0.1 | −0.063 | |
| 50% FSD | 0.66 ** | 0.413 ** | 0.252 ** | 0.347 ** | −0.475 ** | 0.321 ** | 0.246 ** | 0.039 | ||
| PH | 0.67 ** | 0.46 ** | 0.529 ** | −0.348 ** | 0.459 ** | 0.46 ** | 0.038 | |||
| BN | 0.752 ** | 0.752 ** | −0.207 * | 0.53 ** | 0.574 ** | 0.027 | ||||
| UN | 0.902 ** | −0.243 ** | 0.652 ** | 0.724 ** | −0.024 | |||||
| UmblN | −0.267 ** | 0.648 ** | 0.782 ** | −0.042 | ||||||
| 1000 FW | −0.053 | −0.06 | 0.204 * | |||||||
| FY | 0.856 ** | 0.073 | ||||||||
| BY | −0.161 |
*: Significant at 5%, **: Significant at 1%, SD: seedling days, FD: flowering days, FSD: fruit setting days, PH: plant height, BN: branch number, UN: umbel number, UmbltN: umbellet number, 1000 FW: 1000 fruit weight, FY: fruit yield, BY: biological yield, HI: harvest index.
Kumar et al. [66] reported that positive correlations were found between the plant height-branch number, 50% flowering days-branch number, and umbel-umbellet. Similarly, Nagappa et al. [67] noted that the correlation study revealed that plant height, number of primary branches per plant, days to 50% flowering, number of umbels per plant, number of umbelettes per umbel, harvest index, thousand fruit weight, and oil content all exhibited significant positive associations with fruit yield per plant at both the phenotypic and genotypic levels. The obtained results from this study on the correlation analysis showed similar findings with the previous studies.
4. Conclusions
The ANOVA analysis reveals that selectable, wide-promising genotypes from the existing genetic stock have desired properties, such as fruit yield, essential oil content, fixed oil ratio, and their compositions and acids. Also, the results of the study showed that the genotypes, which had high yield and yield components with some chemical properties, can be used for coriander production purposes for future breeding studies. So, Ames 13900 and Ames 18595 genotypes can be recommended for the fruit yield, fixed oil, and essential oil contents. Also, these genotypes can be suggested for the petroselinic acid production. The cluster analysis divided into two main groups, and most of the genotypes took place in the A main group. Similar findings were found from the heat map analysis. The PCA analysis showed over 70% variation, and three fixed oil acids, fixed oil content, and linalool essential oil composition were found as major contributors. The morphological, yield, and quality properties of the different origin plants would be significant to select for future breeding programs. So, this study can contribute to the selection of promising coriander genotypes based on the desired properties for the breeders. In conclusion, the promising coriander genotypes may be used for different programs, increasing their areas of use.
Acknowledgments
This study is part of the MSc thesis of the first author. The authors would like to thank the United States Department of Agriculture (USDA) for supplying coriander genotypes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology13110866/s1, Table S1: 50% seedling, flowering, and fruit setting days of the different coriander genotypes. Table S2: Plant height, branch number, umber number, and umbellate number values of different origin coriander genotypes. Table S3: 1000 fruit weight, fruit yield, biological yield, and harvest index of different coriander genotypes. Table S4: Minor essential oil compositions of the high-yielding coriander genotypes. Table S5: Minor fixed oil acids of the different origin coriander genotypes and cultivars. Table S6: UPOV criteria of different origin coriander genotypes and cultivars.
Author Contributions
Conceptualization, G.Y.; methodology, G.Y. and M.C.; software, A.B. and M.C.; validation, A.B., G.Y. and M.C.; formal analysis, A.B. and M.C.; investigation, G.Y. and M.C.; resources, G.Y. and M.C.; data curation, A.B. and M.C.; writing-original draft preparation, G.Y. and M.C.; writing-review and editing, G.Y. and M.C.; visualization, G.Y. and M.C.; supervision, G.Y.; project administration, G.Y.; funding acquisition, G.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Scientific Research Project Fund, Faculty of Agriculture, Bolu Abant İzzet Baysal University, Türkiye, grant number 2020.10.07.1455.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Turkish Statistical Institute of Ankara, Türkiye Crop Production Statistics. [(accessed on 15 March 2024)]; Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr.
- 2.Food and Agriculture Organization of the United Nations. [(accessed on 15 March 2024)]. Available online: https://www.fao.org/faostat/en/#data/TCL.
- 3.Wangensteen H., Samuelsen A.B., Malterud K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. doi: 10.1016/j.foodchem.2004.01.047. [DOI] [Google Scholar]
- 4.Diederichsen A. Promoting the Conservation and Use of Underutilized and Neglected Crops 3. Institute of Plant Genetics and Crop Plant Research; Gatersleben, Germany: International Plant Genetic Resources Institute; Rome, Italy: 1996. Coriander (Coriandrum sativum L.) [Google Scholar]
- 5.Msaada K., Hosni K., Ben Taarit M., Chahed T., Kchouk M.E., Marzouk B. Changes on essential oil composition of coriander (Coriandrum sativum L.) fruits during three stages of maturity. Food Chem. 2007;102:1131–1134. doi: 10.1016/j.foodchem.2006.06.046. [DOI] [Google Scholar]
- 6.Msaada K., Hosni K., Ben Taarit M., Chahed T., Marzouk B. Variations in the essential oil composition from different parts of Coriandrum sativum L. cultivated in Tunisia. Ital. J. Biochem. 2007;56:47–52. [PubMed] [Google Scholar]
- 7.Yaldiz G., Camlica M. Variation in the fruit phytochemical and mineral composition, and phenolic content and antioxidant activity of the fruit extracts of different fennel (Foeniculum vulgare L.) genotypes. Ind. Crops Prod. 2019;142:111852. doi: 10.1016/j.indcrop.2019.111852. [DOI] [Google Scholar]
- 8.Yaldiz G., Camlica M. Essential oils content, composition and antioxidant activity of selected basil (Ocimum basilicum L.) genotypes. South Afr. J. Bot. 2022;151:675–694. doi: 10.1016/j.sajb.2022.10.045. [DOI] [Google Scholar]
- 9.Msaada K., Hosni K., Taarit M.B., Hammami M., Marzouk B. Effects of Growing Region and Maturity Stages on Oil Yield And Fatty Acid Composition Of Coriander (Coriandrum sativum L.) Fruit. Sci. Hortic. 2009;120:525–531. doi: 10.1016/j.scienta.2008.11.033. [DOI] [Google Scholar]
- 10.Msaada K., Taarit M.B., Hosni K., Hammami M., Marzouk B. Regional and maturational effects on essential oils yields and composition of coriander (Coriandrum sativum L.) fruits. Sci. Hortic. 2009;122:116–124. doi: 10.1016/j.scienta.2009.04.008. [DOI] [Google Scholar]
- 11.Singh D., Jain U.K., Rajput S.S., Khandelwal V., Shiv K.N. Genetic variation for seed yield and its components and their association in coriander (Coriandrum sativum L.) germplasm. J. Spices Aromat. Crops. 2006;15:25–29. [Google Scholar]
- 12.Ebrahimi S.N., Hadian J., Ranjbar H. Essential oil compositions of different accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010;24:1287–1294. doi: 10.1080/14786410903132316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomar Rukam S., Kulkarni G.U., Parakhia M.V., Thakkar J.R., Rathod V.M., Solanki R.K., Golakiya B.A. Genetic diversity analysis in coriander (Coriandrum sativum) genotypes through morphological and molecular characterization. Res. J. Biotechnol. 2014;9:1–11. [Google Scholar]
- 14.Santha S., Jhansirani P., Swaminathan V., Arumugam T. Morphological and seed yield traits of coriander (Coriandrum sativum L.) local land races of Tamil Nadu. Pharma Innov. J. 2022;11:1875–1880. [Google Scholar]
- 15.BMGM . Bolu General Directorate of Meteorology. BMGM; Bolu, Türkiye: 2020. [Google Scholar]
- 16.Association of Official Analytical Chemists . Fatty acids in oil and fats. In: Helrich K., editor. AOAC Official Methods of Analysis. 15th ed. Volume II. Association of Official Analytical Chemists; Arlington, VA, USA: 1990. pp. 963–964. [Google Scholar]
- 17.UPOV . International Union for the Protectıon of New Varıetıes of Plants, Coriander, Guidelines for the Conduct of Tests for Dıstınctness, Unıformıty and Stabılıty. UPOV; Geneve, Switzerland: 2011. [Google Scholar]
- 18.Avci M. Excel’de Araştırma Sonuçlarının Istatistiki Analizi. Ankara, Türkiye. [(accessed on 7 January 2021)]. Available online: https://yeniavciistatistik.blogspot.com/
- 19.Peterson R.G. Agricultural Field Experiments: Design and Analysis. 1st ed. CRC Press; Boca Raton, FL, USA: Marcel Dekker Inc.; Boca Raton, FL, USA: 1994. 428p [Google Scholar]
- 20.Sezek M. Master’s Thesis. Atatürk University Graduate School of Natural and Applied Sciences Department of Field Crops; Erzurum, Turkey: 2014. Effects of Sowing Dates on Yield, Yield Components and Essential Oil Content of Coriander (Coriandrum sativum L.) Cultivars. [Google Scholar]
- 21.Özyazıcı G. Determination of some agricultural characteristics of coriander genotypes in Siirt conditions. KSU J. Nat. Sci. 2017;20:346–350. [Google Scholar]
- 22.Guha S., Sharangi A.B., Debnath S. Phenology and green leaf yield of coriander at different sowing dates and harvesting times. J. Food Agric. Environ. 2014;12:251–254. [Google Scholar]
- 23.Gökduman G.A. Master’s Thesis. Süleyman Demirel University; Isparta, Turkey: 2018. Determination of Yield and Quality Characteristics of Some Coriander (Coriandrum sativum L.) Genotypes in Isparta Conditions. [Google Scholar]
- 24.Moniruzzaman M., Rahman M.M., Hossain M.M., Sirajul Karim A.J.M., Khaliq Q.A. Evaluation of coriander (Coriandrum sativum L.) genotypes for seed yield and yield contributing characters. Bangladesh J. Agric. Res. 2013;38:189–202. doi: 10.3329/bjar.v38i2.15882. [DOI] [Google Scholar]
- 25.Kozlowski T.T., Pallardy S.G. Physiology of Woody Plants. 2nd ed. Academic Press; Cambridge, MA, USA: 1997. Reproductive Growth; pp. 68–86. [Google Scholar]
- 26.Trethowan R.M. Defining a genetic ideotype for crop improvement. In: Fleury D., Whitford R., editors. Crop Breeding: Methods in Molecular Biology. Volume 1145. Humana Press; New York, NY, USA: 2014. pp. 1–20. [DOI] [PubMed] [Google Scholar]
- 27.Augspurger C.K. Reproductive synchrony of a tropical shrub: Experimental studies on effects of pollinators and seed predators in Hybanthus Prunifolius (Violaceae) Ecology. 2014;62:775–788. doi: 10.2307/1937745. [DOI] [Google Scholar]
- 28.Shiwangi P., Hadimani H.P., Satish D., Awati M., Kantharaju V., Biradar I.B. Assessment of genetic variability parameters in coriander (Corıandrum Satıvum L.) genotypes for growth, foliage yield and quality traits. Plant Arch. 2020;20:721–726. [Google Scholar]
- 29.Emiralioglu O., Yaldiz G. Determination of yield and quality properties of coriander (Coriandrum sativum L.) cultivar and populations in Bolu conditions. Acad. J. Agric. 2020;9:307–316. [Google Scholar]
- 30.Özyazıcı G. Effect of different phosphorus doses on yield and some agricultural properties in coriander (Coriandrum sativum L.) Plant. Turk. J. Agric. Res. 2020;7:192–200. [Google Scholar]
- 31.Özbek D.G. Master’s Thesis. Erciyes University Graduate School of Natural and Applied Sciences Department of Field Crops; Kayseri, Turkey: 2020. The Effects of Zinc Application on the Yield and Quality Criteria of Coriander (Coriandrum sativum L.) in Kayseri Conditions. [Google Scholar]
- 32.Hongal A., Basavaraja N., Hongal S., Hegde N.K., Kulkarni S. Evaluation of coriander (Coriandrum sativum L.) genotypes for yield and quality under Hill Zone (Zone-9) of Karnataka, India. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:2494–2502. doi: 10.20546/ijcmas.2018.705.287. [DOI] [Google Scholar]
- 33.Devi A.R., Sharabgi A.B. Morphological Character and Seed Yield Potential of Coriander Genotypes under Gangetic Alluvial Region of West Bengal. Int. J. Curr. Microbiol. Appl. Sci. 2019;8:775–782. doi: 10.20546/ijcmas.2019.804.085. [DOI] [Google Scholar]
- 34.Joble J., Prasad V.M., Bahadur V. Evaluation of Different Varieties of Coriander (Coriandrum sativum L.) Int. J. Plant Soil Sci. 2022;34:1231–1238. doi: 10.9734/ijpss/2022/v34i232537. [DOI] [Google Scholar]
- 35.Bhandari M.M., Gupta A. Variation and association analysis in coriander. Euphytica. 1991;58:1–4. doi: 10.1007/BF00035333. [DOI] [Google Scholar]
- 36.Qureshi S.N., Anwar R., Kashif M., Ghafoor A. Evaluation of winter vegetables for genetic divergence and characterization of genotypes. Pak. J. Bot. 2009;41:1117–1126. [Google Scholar]
- 37.Datta S., Choudhuri P. Evaluation of coriander germplasm under tetra zone of West Bengal. Haryana J. Hortic. Sci. 2006;35:348–349. [Google Scholar]
- 38.Nowak J., Szemplinski W. Influence of sowing date on yield and fruit quality of coriander (Coriandrum sativum L.) Acta Sci. Pol. Hortorum Cultus. 2014;13:83–96. [Google Scholar]
- 39.Bhadkariya S.k., Gupta A., Bobade A., Kasana B.S., Tomar L.S. Effect of different times of sowing on growth, yield and seed quality of coriander (Coriandrum sativum L.) cv Cimpo S-33. Bharatiya Krishi Anusandhan Patrika. 2007;22:229–232. [Google Scholar]
- 40.Moosavi S.G.R., Seghatoleslami M.J., Zareie M.H. The effect of planting date and plant density on morphological traits and essential oil yield of coriander (Coriandrum sativum L.) Int. J. Agric. Crop Sci. 2012;4:496–501. [Google Scholar]
- 41.Carrubba A., Torre R., Saiano F., Alonzo G. Effect of sowing time on Coriander performance in a semi-arid Mediterranean environment. Crop Sci. 2006;46:437–447. doi: 10.2135/cropsci2005.0169. [DOI] [Google Scholar]
- 42.Moniruzzaman M., Rahman M.M., Hossain M.M., Sirajul Karim A.J.M., Khaliq Q.A. Effect of sowing dates and genotypes on the yield of coriander (Coriandrum sativum L.) Bangladesh J. Agric. Res. 2015;40:109–119. doi: 10.3329/bjar.v40i1.23764. [DOI] [Google Scholar]
- 43.Zheljazkova V.D., Pickett K.M., Caldwell C.D., Pincock J.A., Roberts J.C., Mapplebeck L. Cultivar and sowing date effects on seed yield and oil composition of coriander in Atlantic Canada. Ind. Crops Prod. 2008;28:88–94. doi: 10.1016/j.indcrop.2008.01.011. [DOI] [Google Scholar]
- 44.Rashed N.M., Darwesh R.K. A comparative study on the effect of microclimate on planting date and water requirements under different nitrogen sources on coriander (Coriandrum sativum L.) Ann. Agric. Sci. 2015;60:227–243. doi: 10.1016/j.aoas.2015.10.009. [DOI] [Google Scholar]
- 45.Thakur A., Thakur P. Effect of water stress on yield and seed quality of coriander (Coriandrum sativum L.) Int. J. Bio-Resour. Stress Manag. 2018;9:159–163. doi: 10.23910/IJBSM/2018.9.1.3C0581. [DOI] [Google Scholar]
- 46.Nagappa M.K., Babu Rao B., Paramappa M.K., Kranthi Kumar T. Harvest index as influenced by genotype in coriander under Godavari Zone. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:414–421. doi: 10.20546/ijcmas.2017.607.049. [DOI] [Google Scholar]
- 47.Kassu K.T., Dawıt H.H., Wubengeda A.Y., Almaz A.T., Asrat M.T. Yield and yield components of coriander under different sowing dates and seed rates in tropical environment. Adv. Hortic. Sci. 2018;32:193–203. [Google Scholar]
- 48.Borah G., Bora P.K., Mahanta B.P., Saikia S.P., Haldar S. Quality control, ontogenetic variability and sensory profiling of ‘cilantro-mimic’ spiny coriander (Eryngium foetidum L.): A flavour perspective. Food Chem. Adv. 2023;3:100370. doi: 10.1016/j.focha.2023.100370. [DOI] [Google Scholar]
- 49.Fattahi B., Arzani K., Souri M.K., Barzegar M. Morphophysiological and phytochemical responses to cadmium and lead stress in coriander (Coriandrum sativum L.) Ind. Crops Prod. 2021;171:113979. doi: 10.1016/j.indcrop.2021.113979. [DOI] [Google Scholar]
- 50.Heywood V.H. The Conservation of Genetic and Chemical Diversity in Medicinal and Aromatic Plants. In: Şener B., editor. Biodiversity: Biomolecular Aspects of Biodiversity and Innovative Utilization. Springer; Boston, MA, USA: 2002. pp. 13–22. [Google Scholar]
- 51.Mandal S., Mandal M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015;5:421–428. doi: 10.1016/j.apjtb.2015.04.001. [DOI] [Google Scholar]
- 52.Weisany W., Tahir N.A., Schenk P.M. Coriander/soybean intercropping and mycorrhizae application lead to overyielding and changes in essential oil profiles. Eur. J. Agron. 2021;126:126283. doi: 10.1016/j.eja.2021.126283. [DOI] [Google Scholar]
- 53.Amiripour A., Jahromi M.G., Soori M.K., Mohammadi Torkashvand A. Changes in essential oil composition and fatty acid profile of coriander (Coriandrum sativum L.) leaves under salinity and foliar-applied silicon. Ind. Crops Prod. 2021;168:113599. doi: 10.1016/j.indcrop.2021.113599. [DOI] [Google Scholar]
- 54.El-Zaeddi H., Martínez-Tomé J., Calín-Sánchez Á., Burló F., Carbonell-Barrachina Á.A. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean Regions of Spain. Foods. 2016;5:41. doi: 10.3390/foods5020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Circella G., Franz C., Novak J., Resch H. Influence of day length and leaf insertion on the composition of marjoram essential oil. Flavour Fragr. J. 1995;10:371–374. doi: 10.1002/ffj.2730100607. [DOI] [Google Scholar]
- 56.Skoula M., Abbes J.E., Johnson C.B. Genetic variation of volatiles and rosmarinic acid in populations of Salvia fruticosa mill growing in Crete. Biochem. Syst. Ecol. 2000;28:551–561. doi: 10.1016/S0305-1978(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 57.Laribi B., Kouki K., Hamdi M.M., Bettaieba T. Coriander (Coriandrum sativum L.) and Its Bioactive Constituents. Fitoterapia. 2015;103:9–26. doi: 10.1016/j.fitote.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Yaldiz G., Camlica M. Agro-morphological and phenotypic variability of sweet basil genotypes for breeding purposes. Crop Sci. 2021;61:621–642. doi: 10.1002/csc2.20391. [DOI] [Google Scholar]
- 59.Sangwan N.S., Faroqi A.H.A., Shabih F., Sangwan R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001;34:3–21. doi: 10.1023/A:1013386921596. [DOI] [Google Scholar]
- 60.Sriti J., Neffati M., Msaada K., Taloe T., Marzouk B. Biochemical characterization of coriander cakes obtained by extrusion. J. Chem. 2013;2013:871631. doi: 10.1155/2013/871631. [DOI] [Google Scholar]
- 61.Nguyen O.H., Talou T., Cerny M., Evon P., Merah O. Oil and fatty acid accumulation during coriander (Coriandrum sativum L.) fruit ripening under organic cultivation. Crop J. 2015;3:366–369. doi: 10.1016/j.cj.2015.05.002. [DOI] [Google Scholar]
- 62.Nguyen O.H., Talou T., Evon P., Cerny M., Merah O. Fatty acid composition and oil content during coriander fruit development. Food Chem. 2020;326:127034. doi: 10.1016/j.foodchem.2020.127034. [DOI] [PubMed] [Google Scholar]
- 63.Ertas O.N., Güler T., Çiftçi M., Dalkılıç B., Yılmaz O. The effect of a dietary supplement coriander seeds on the fatty acid composition of breastmuscle in Japanese Quail. Rev. De Med. Vet. 2005;156:514–518. [Google Scholar]
- 64.Neffati M., Marzouk B. Roots volatiles and fatty acids of coriander (Coriandrum sativum L.) grown in Saline Medium. Acta Physiol. Plant. 2009;31:455–461. doi: 10.1007/s11738-008-0253-4. [DOI] [Google Scholar]
- 65.Camlica M., Yaldiz G. Analyses and evaluation of the main chemical components in different tobacco (Nicotiana tabacum L.) genotypes. Grasas Y Aceites. 2021;72:e389. doi: 10.3989/gya.0801192. [DOI] [Google Scholar]
- 66.Kumar M.A., Choudhary G., Garhwal O.P., Netwal M. Correlation coefficient and path analysis for yield traits in coriander (Coriandrum sativum L.) genotypes. Electron. J. Plant Breed. 2021;13:253–257. [Google Scholar]
- 67.Nagappa M.K., Giridhar K., Dorajeerao A.V.D. Correlation between yield and its attributes in coriander. Plant Arch. 2017;17:941–947. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article.