Abstract
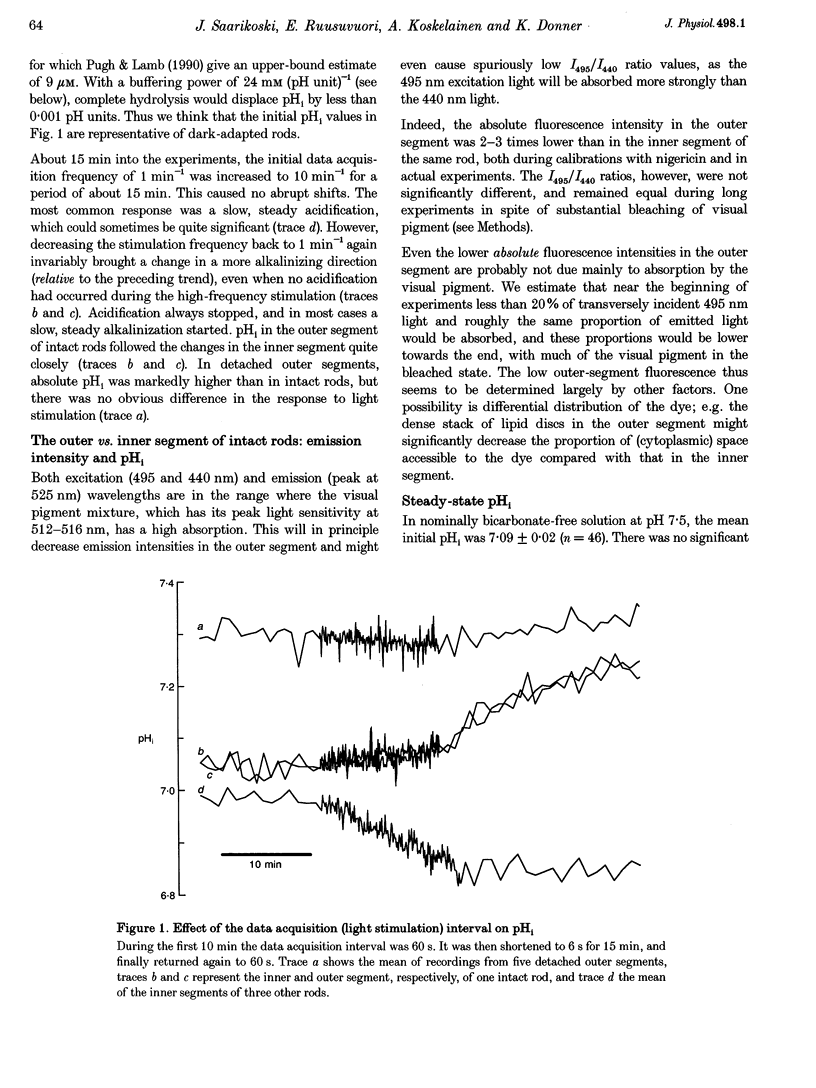
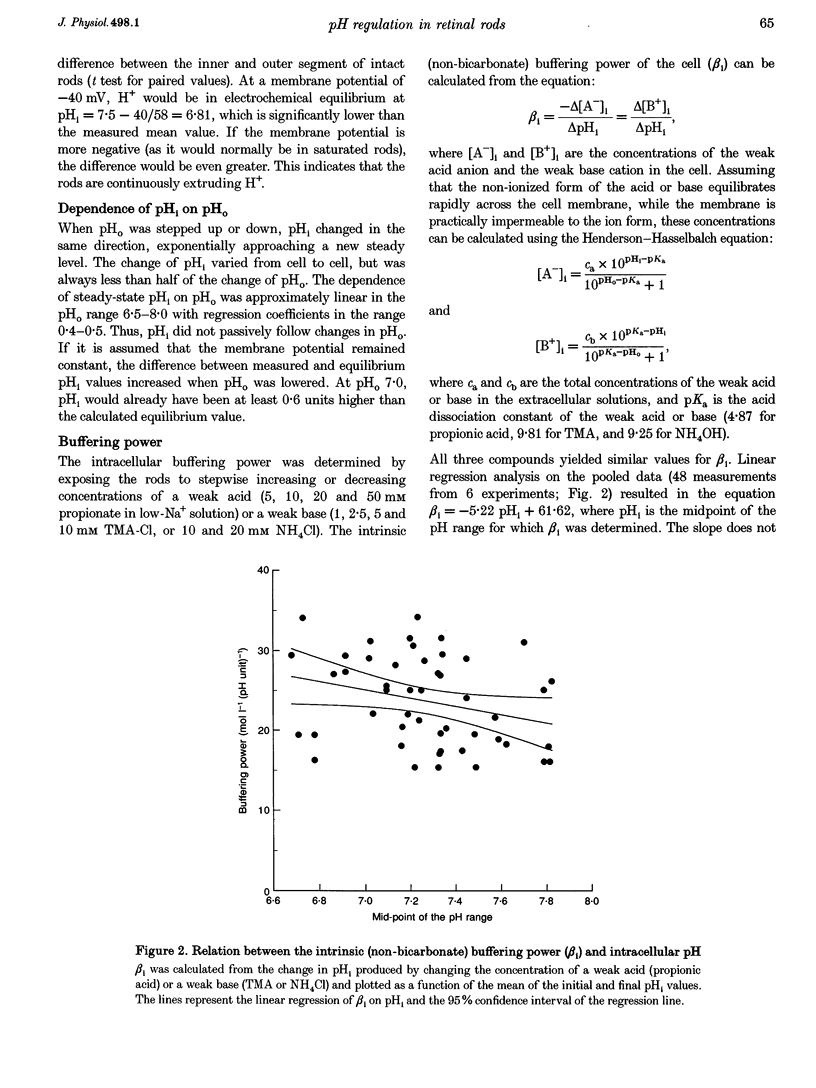
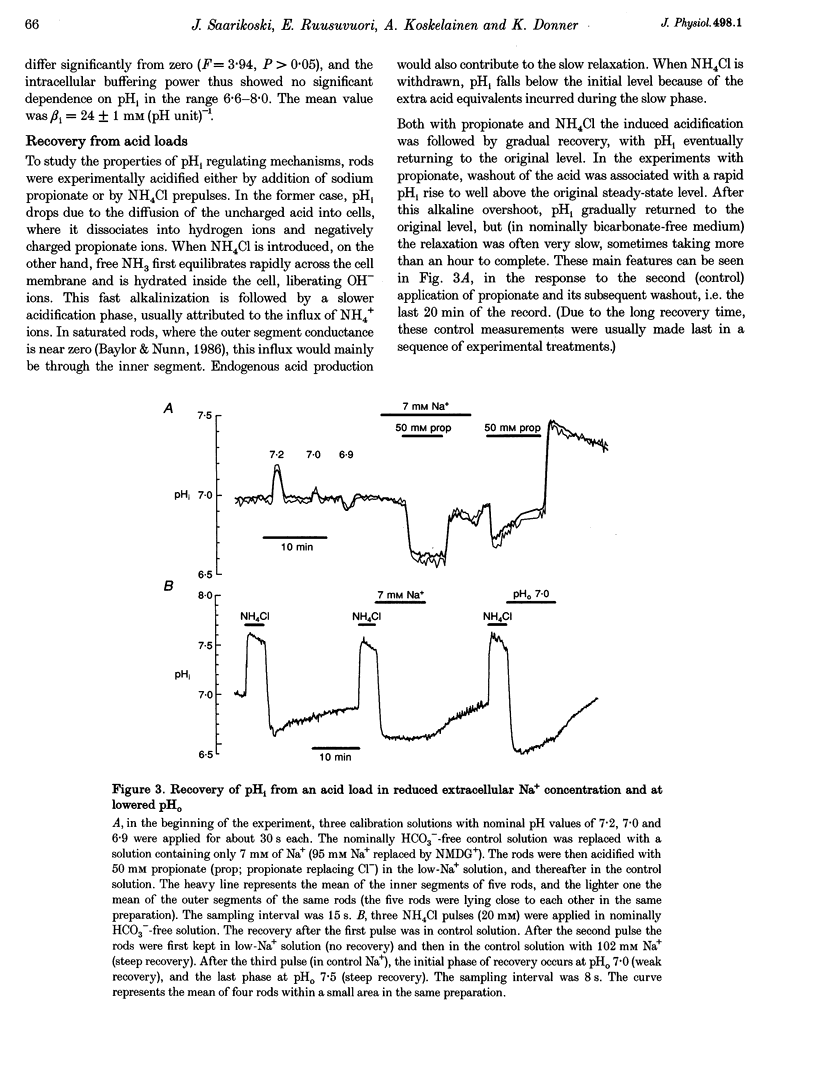
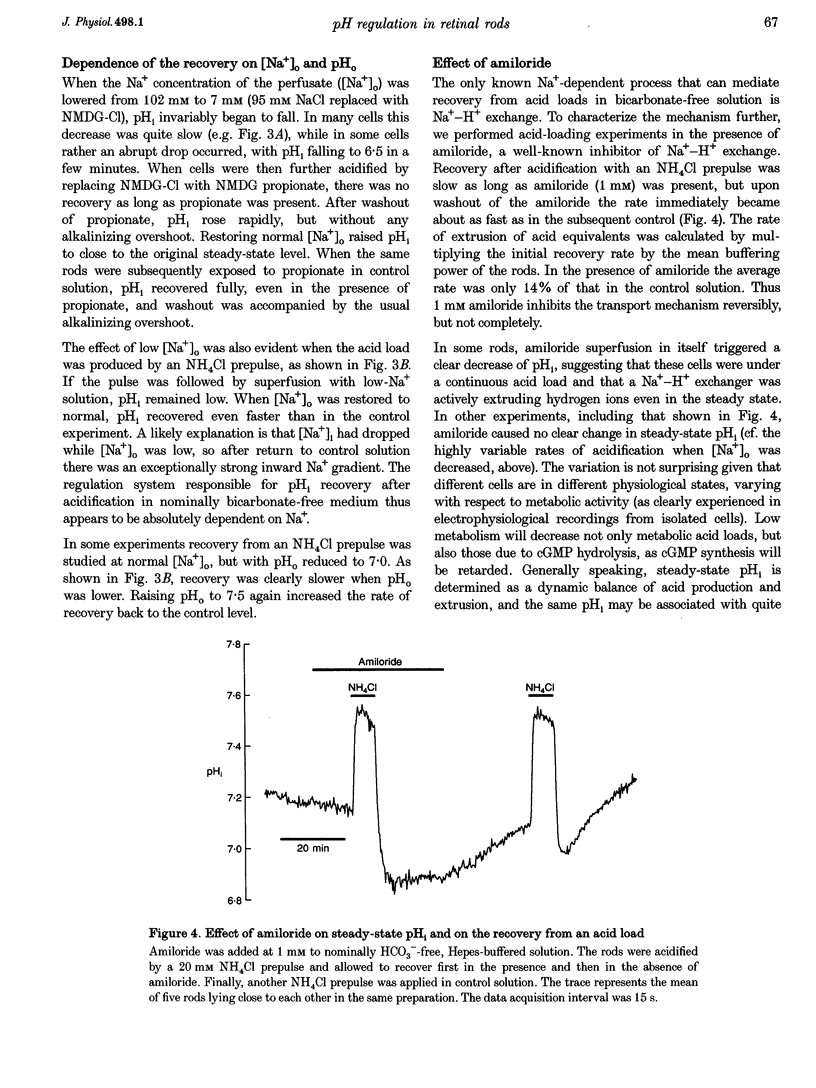
1. We measured intracellular pH (pHi) in rods isolated from the retina of the axolotl salamander, Ambystoma mexicanum, using the fluorescent indicator 2',7'-bis(carboxyethyl)-5(and -6)-carboxyfluorescein (BCECF). 2. The light exposures associated with data acquisition had no marked effect on pHi. There was no sharp change between the value obtained from the first exposure of dark-adapted rods and subsequent readings. Increasing the acquisition frequency from 1 to 10 min-1 either had no effect, or brought about a slow acidification, which was stopped or reversed when the low frequency was restored. 3. In nominally HCO3(-)-free solution at pH 7.5, the rods had a steady-state pHi of 7.09 +/- 0.02 (n = 46) and a buffering power (beta i) of 24 +/- 1 mM (pH unit)-1 (n = 48). The buffering power was virtually constant in the pH range 6.6-8.0. In the same range, pHi dependent linearly on perfusion pH (pHo) with regression coefficients of 0.4-0.5. 4. There were no significant differences between the inner and outer segment of intact rods as regards steady-state pHi or responses to experimental treatments. 5. Recovery from an intracellular acid load imposed by sodium propionate or an NH4Cl prepulse in nominally bicarbonate-free perfusate was completely blocked by decreasing the extracellular Na+ concentration to 7 mM, and slowed by 86% by applying 1 mM amiloride. 6. Introduction of 2% CO2-13 mM HCO3- caused an alkalinization that was often preceded by a transient acidification. Steady-state pHi was on average 0.1 pH units higher than in nominally bicarbonate-free solution. The mean acid extrusion rate, calculated on the assumption that CO2-HCO3- behaves as an open system, was 19% higher (31 +/- 2 mM h-1) than in a solution buffered only by Hepes (26 +/- 2 mM h-1). 7. In the presence of CO2-HCO3-, 100 microM 4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid (DIDS) decreased the acid extrusion rate by 20% on average. Lowering the extracellular Cl-concentration to 7 mM raised pHi, but did not significantly affect the acid extrusion rate. 8. We conclude that retinal rods regulate pHi by both Na(+)-H+ exchange and mechanism(s) involving HCO3(-)-Cl- exchange. In the present conditions, the Na(+)-H+ exchanger appears as the dominant mechanism for acid extrusion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgula G. A., Karwoski C. J., Steinberg R. H. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29(9):1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Dawis S. M., Graeff R. M., Heyman R. A., Walseth T. F., Goldberg N. D. Regulation of cyclic GMP metabolism in toad photoreceptors. Definition of the metabolic events subserving photoexcited and attenuated states. J Biol Chem. 1988 Jun 25;263(18):8771–8785. [PubMed] [Google Scholar]
- Donner K., Copenhagen D. R., Reuter T. Weber and noise adaptation in the retina of the toad Bufo marinus. J Gen Physiol. 1990 Apr;95(4):733–753. doi: 10.1085/jgp.95.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K., Hemilä S., Kalamkarov G., Koskelainen A., Shevchenko T. Rod phototransduction modulated by bicarbonate in the frog retina: roles of carbonic anhydrase and bicarbonate exchange. J Physiol. 1990 Jul;426:297–316. doi: 10.1113/jphysiol.1990.sp018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K., Koskelainen A., Djupsund K., Hemilä S. Changes in retinal time scale under background light: observations on rods and ganglion cells in the frog retina. Vision Res. 1995 Aug;35(16):2255–2266. doi: 10.1016/0042-6989(94)00319-h. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Kenning N. A., O'Neill S. C., Pocock G., Richards C. D., Valdeolmillos M. A novel method for absolute calibration of intracellular pH indicators. Pflugers Arch. 1989 Mar;413(5):553–558. doi: 10.1007/BF00594188. [DOI] [PubMed] [Google Scholar]
- Gaillard S., Dupont J. L. Ionic control of intracellular pH in rat cerebellar Purkinje cells maintained in culture. J Physiol. 1990 Jun;425:71–83. doi: 10.1113/jphysiol.1990.sp018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh-Scheidt L. M., Griff E. R., Linsenmeier R. A. Light-evoked oxygen responses in the isolated toad retina. Exp Eye Res. 1995 Jul;61(1):73–81. doi: 10.1016/s0014-4835(95)80060-3. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. C., Molday R. S. Glucose metabolism in photoreceptor outer segments. Its role in phototransduction and in NADPH-requiring reactions. J Biol Chem. 1994 Jul 8;269(27):17954–17959. [PubMed] [Google Scholar]
- Katsura K., Mellergård P., Theander S., Ouyang Y. B., Siesjö B. K. Buffer capacity of rat cortical tissue as well as of cultured neurons and astrocytes. Brain Res. 1993 Aug 6;618(2):283–294. doi: 10.1016/0006-8993(93)91277-y. [DOI] [PubMed] [Google Scholar]
- Katz B. J., Oakley B., 2nd Evidence for Na+/H+ exchange in vertebrate rod photoreceptors. Exp Eye Res. 1990 Aug;51(2):199–207. doi: 10.1016/0014-4835(90)90073-4. [DOI] [PubMed] [Google Scholar]
- Koskelainen A., Donner K., Kalamkarov G., Hemilä S. Changes in the light-sensitive current of salamander rods upon manipulation of putative pH-regulating mechanisms in the inner and outer segment. Vision Res. 1994 Apr;34(8):983–994. doi: 10.1016/0042-6989(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. Effects of temperature changes on toad rod photocurrents. J Physiol. 1984 Jan;346:557–578. doi: 10.1113/jphysiol.1984.sp015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyertholen E. P., Wilson M. J., Ostroy S. E. The effects of hepes, bicarbonate and calcium on the cGMP content of vertebrate rod photoreceptors and the isolated electrophysiological effects of cGMP and calcium. Vision Res. 1986;26(4):521–533. doi: 10.1016/0042-6989(86)90001-5. [DOI] [PubMed] [Google Scholar]
- Miki N., Baraban J. M., Keirns J. J., Boyce J. J., Bitensky M. W. Purification and properties of the light-activated cyclic nucleotide phosphodiesterase of rod outer segments. J Biol Chem. 1975 Aug 25;250(16):6320–6327. [PubMed] [Google Scholar]
- Musser G. L., Rosen S. Localization of carbonic anhydrase activity in the vertebrate retina. Exp Eye Res. 1973 Jan 1;15(1):105–119. doi: 10.1016/0014-4835(73)90195-4. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol. 1989 Dec;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-yang Y., Mellergård P., Siesjö B. K. Regulation of intracellular pH in single rat cortical neurons in vitro: a microspectrofluorometric study. J Cereb Blood Flow Metab. 1993 Sep;13(5):827–840. doi: 10.1038/jcbfm.1993.105. [DOI] [PubMed] [Google Scholar]
- Pocock G., Richards C. D. Hydrogen Ion Regulation in Rat Cerebellar Granule Cells Studied by Single-Cell Fluorescence Microscopy. Eur J Neurosci. 1992;4(2):136–143. doi: 10.1111/j.1460-9568.1992.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 1990;30(12):1923–1948. doi: 10.1016/0042-6989(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Raley-Susman K. M., Sapolsky R. M., Kopito R. R. Cl-/HCO3- exchange function differs in adult and fetal rat hippocampal neurons. Brain Res. 1993 Jun 18;614(1-2):308–314. doi: 10.1016/0006-8993(93)91049-x. [DOI] [PubMed] [Google Scholar]
- Saarikoski J., Kaila K. Simultaneous measurement of intracellular and extracellular carbonic anhydrase activity in intact muscle fibres. Pflugers Arch. 1992 Jul;421(4):357–363. doi: 10.1007/BF00374224. [DOI] [PubMed] [Google Scholar]
- Schwiening C. J., Boron W. F. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(-)-HCO3- exchange. J Physiol. 1994 Feb 15;475(1):59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Owen W. G., Fernandez H. R. The generation of the late receptor potential: an excitation-inhibition phenomenon. Vision Res. 1972 Sep;12(9):1519–1531. doi: 10.1016/0042-6989(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Winkler B. S. Buffer dependence of retinal glycolysis and ERG potentials. Exp Eye Res. 1986 Jun;42(6):585–593. doi: 10.1016/0014-4835(86)90048-5. [DOI] [PubMed] [Google Scholar]
- Winkler B. S. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981 Jun;77(6):667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F., Borgula G. A., Steinberg R. H. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992 May;54(5):685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]


