Abstract
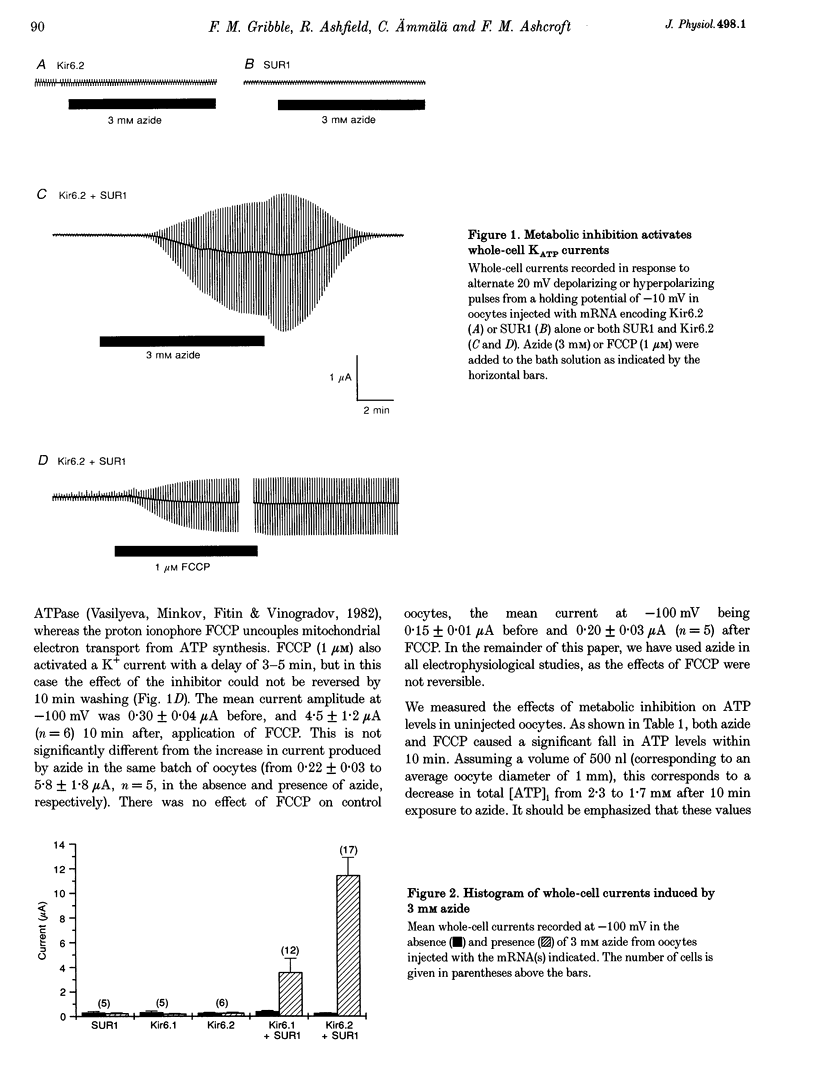
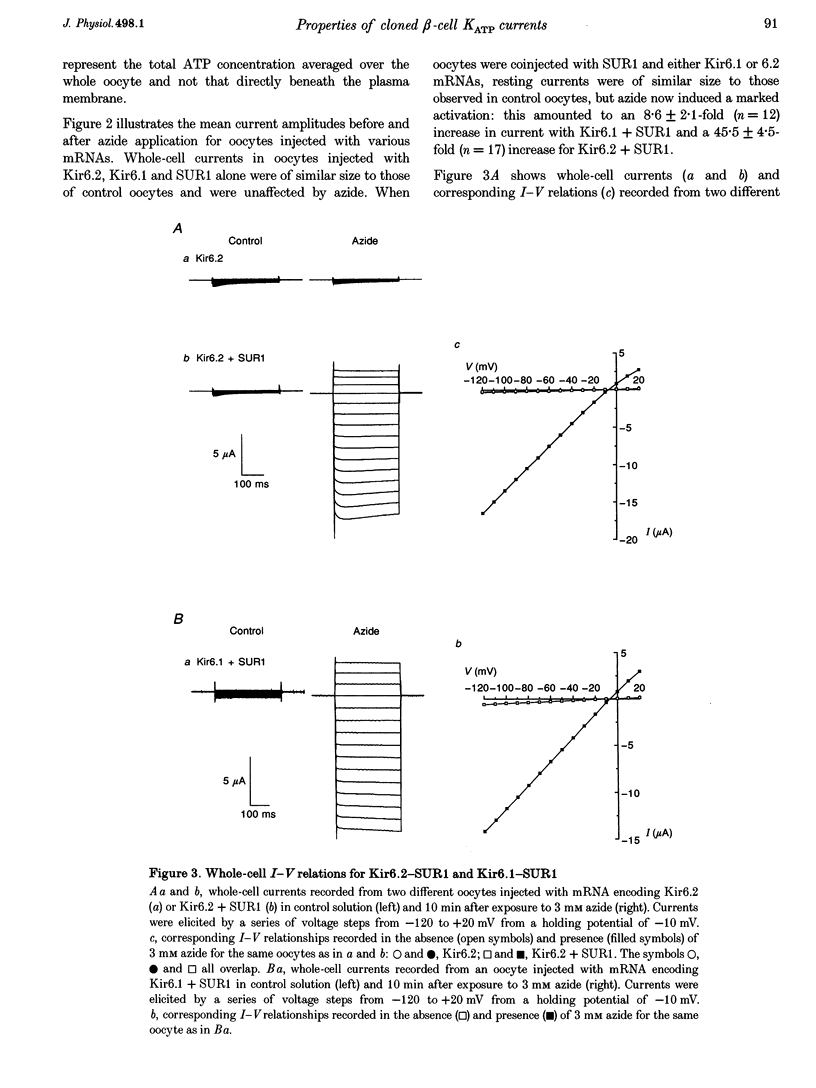
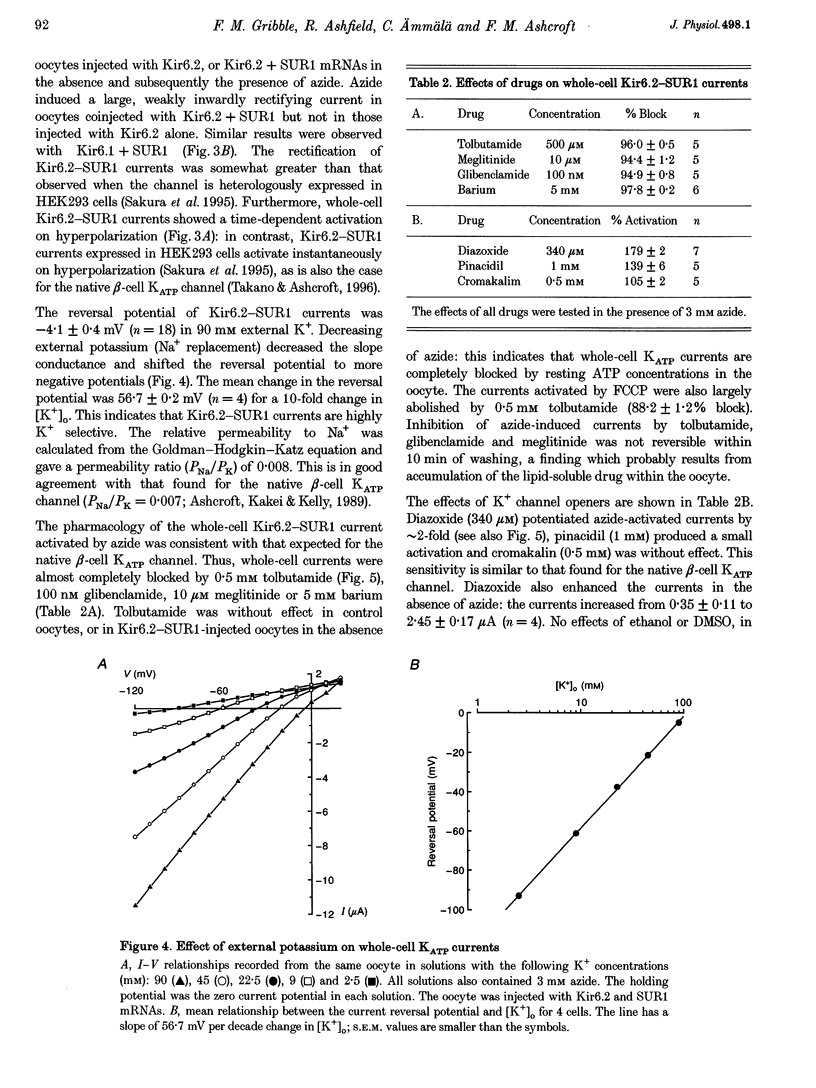
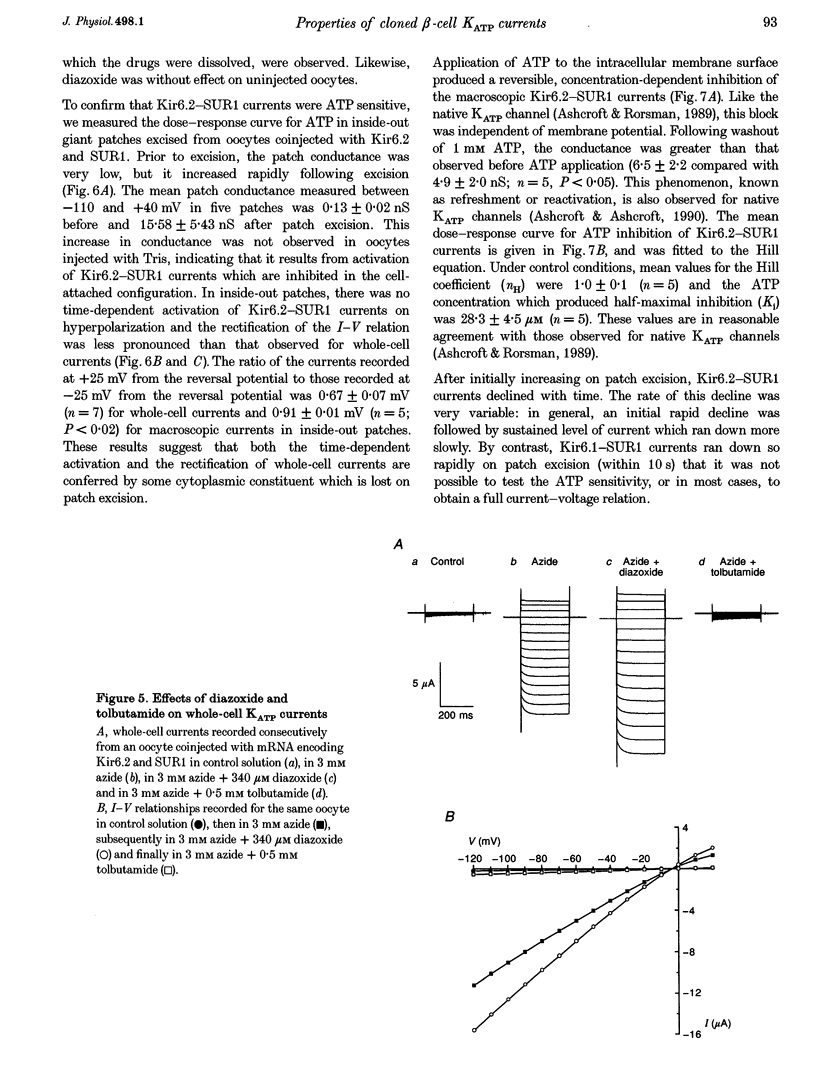
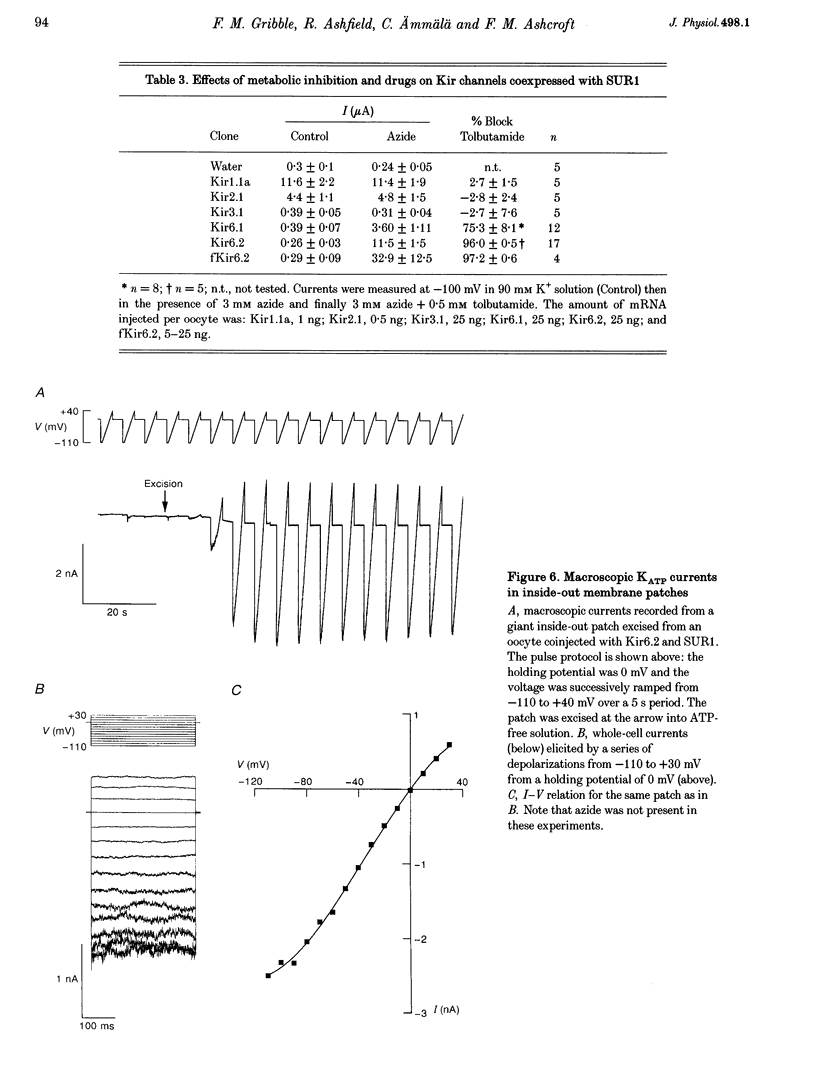
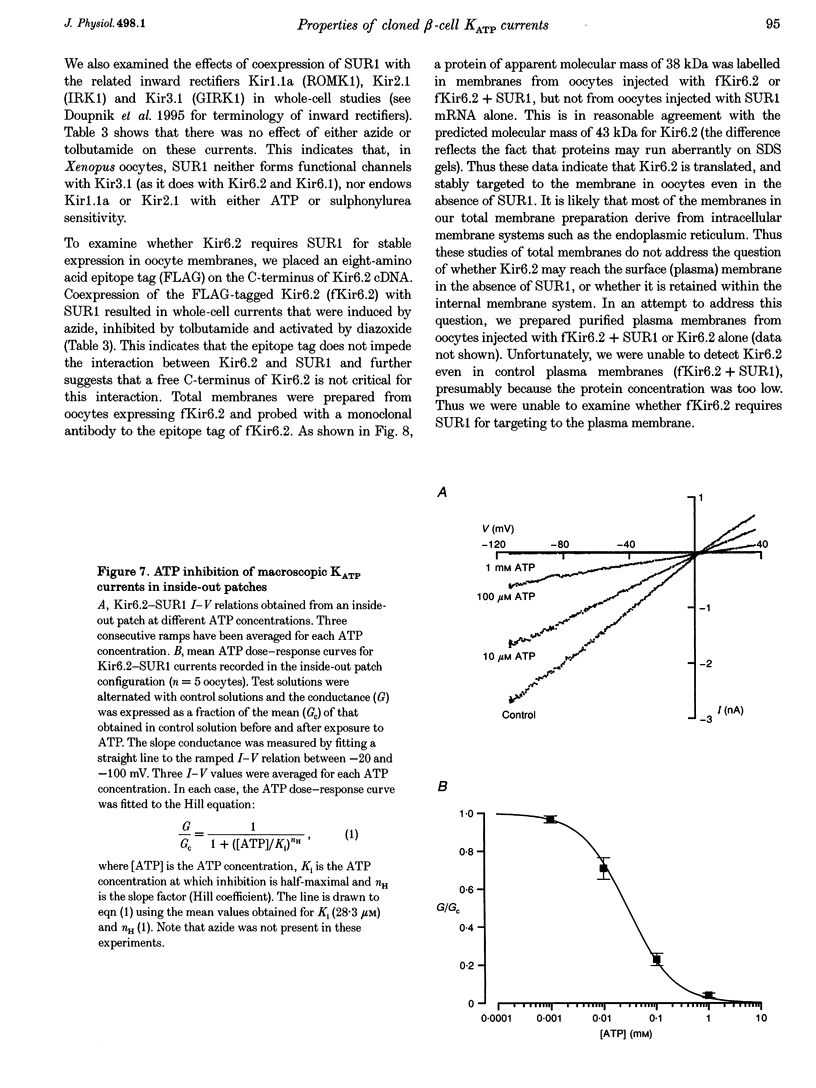
1. We have studied the electrophysiological properties of cloned ATP-sensitive K+ channels (KATP channels) heterologously expressed in Xenopus oocytes. This channel comprises a sulphonylurea receptor subunit (SUR) and an inwardly rectifying K+ channel subunit (Kir). 2. Oocytes injected with SUR1 and either Kir6.2 or Kir6.1 exhibited large inwardly rectifying K+ currents when cytosolic ATP levels were lowered by the metabolic inhibitors azide or FCCP. No currents were observed in response to azide in oocytes injected with Kir6.2, Kir6.1 or SUR1 alone, indicating that both the sulphonylurea receptor (SUR1) and an inward rectifier (Kir6.1 or Kir6.2) are needed for functional channel activity. 3. The pharmacological properties of Kir6.2-SUR1 currents resembled those of native beta-cell ATP-sensitive K+ channel currents (KATP currents): the currents were > 90% blocked by tolbutamide (500 microM), meglitinide (10 microM) or glibenclamide (100 nM), and activated 1.8-fold by diazoxide (340 microM), 1.4-fold by pinacidil (1 mM) and unaffected by cromakalim (0.5 mM). 4. Macroscopic Kir6.2-SUR1 currents in inside-out patches were inhibited by ATP with a Ki of 28 microM. Kir6.1-SUR1 currents ran down within seconds of patch excision preventing analysis of ATP sensitivity. 5. No sensitivity to tolbutamide or metabolic inhibition was observed when SUR1 was coexpressed with either Kir1.1a or Kir2.1, suggesting that these proteins do not couple in Xenopus ocytes. 6. Our data demonstrate that the Xenopus oocyte constitutes a good expression system for cloned KATP channels and that expression may be assayed by azide-induced metabolic inhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995 Apr 21;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ammälä C., Moorhouse A., Ashcroft F. M. The sulphonylurea receptor confers diazoxide sensitivity on the inwardly rectifying K+ channel Kir6.1 expressed in human embryonic kidney cells. J Physiol. 1996 Aug 1;494(Pt 3):709–714. doi: 10.1113/jphysiol.1996.sp021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Moorhouse A., Gribble F., Ashfield R., Proks P., Smith P. A., Sakura H., Coles B., Ashcroft S. J., Ashcroft F. M. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996 Feb 8;379(6565):545–548. doi: 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Kakei M., Kelly R. P. Rubidium and sodium permeability of the ATP-sensitive K+ channel in single rat pancreatic beta-cells. J Physiol. 1989 Jan;408:413–429. doi: 10.1113/jphysiol.1989.sp017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. The sulfonylurea receptor. Biochim Biophys Acta. 1992 Dec 15;1175(1):45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. The ABC of channel regulation. Cell. 1995 Sep 8;82(5):693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Nicoll D. A., Philipson K. D. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature. 1991 Aug 22;352(6337):715–718. doi: 10.1038/352715a0. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995 Nov 17;270(5239):1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J. P., Wang C. Z., Aguilar-Bryan L., Bryan J., Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996 May;16(5):1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Tsuura Y., Namba N., Masuda K., Gonoi T., Horie M., Seino Y., Mizuta M., Seino S. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995 Mar 17;270(11):5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Ko Y. H., Pedersen P. L. The first nucleotide binding fold of the cystic fibrosis transmembrane conductance regulator can function as an active ATPase. J Biol Chem. 1995 Sep 22;270(38):22093–22096. doi: 10.1074/jbc.270.38.22093. [DOI] [PubMed] [Google Scholar]
- Miller D. S., Horowitz S. B. Intracellular compartmentalization of adenosine triphosphate. J Biol Chem. 1986 Oct 25;261(30):13911–13915. [PubMed] [Google Scholar]
- Niki I., Ashcroft F. M., Ashcroft S. J. The dependence on intracellular ATP concentration of ATP-sensitive K-channels and of Na,K-ATPase in intact HIT-T15 beta-cells. FEBS Lett. 1989 Nov 6;257(2):361–364. doi: 10.1016/0014-5793(89)81572-8. [DOI] [PubMed] [Google Scholar]
- Sakura H., Ammälä C., Smith P. A., Gribble F. M., Ashcroft F. M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995 Dec 27;377(3):338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Takano M., Ashcroft F. M. The Ba2+ block of the ATP-sensitive K+ current of mouse pancreatic beta-cells. Pflugers Arch. 1996 Feb;431(4):625–631. doi: 10.1007/BF02191912. [DOI] [PubMed] [Google Scholar]
- Trube G., Rorsman P., Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta-cells. Pflugers Arch. 1986 Nov;407(5):493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Tsubaki M. Fourier-transform infrared study of azide binding to the Fea3-CuB binuclear site of bovine heart cytochrome c oxidase: new evidence for a redox-linked conformational change at the binuclear site. Biochemistry. 1993 Jan 12;32(1):174–182. doi: 10.1021/bi00052a023. [DOI] [PubMed] [Google Scholar]
- Tucker S. J., Bond C. T., Herson P., Pessia M., Adelman J. P. Inhibitory interactions between two inward rectifier K+ channel subunits mediated by the transmembrane domains. J Biol Chem. 1996 Mar 8;271(10):5866–5870. doi: 10.1074/jbc.271.10.5866. [DOI] [PubMed] [Google Scholar]
- Vasilyeva E. A., Minkov I. B., Fitin A. F., Vinogradov A. D. Kinetic mechanism of mitochondrial adenosine triphosphatase. Inhibition by azide and activation by sulphite. Biochem J. 1982 Jan 15;202(1):15–23. doi: 10.1042/bj2020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Patel S. Isolation of plasma membrane complexes from Xenopus oocytes. J Membr Biol. 1989 Feb;107(2):189–201. doi: 10.1007/BF01871724. [DOI] [PubMed] [Google Scholar]