Abstract
PPHN is a common cause of neonatal respiratory failure and is still a serious condition that is associated with high mortality. Objectives: To analyze the clinical characteristics and outcomes of SARS-CoV-2 infection in neonates with PPHN to identify neonatal cases at risk to develop severe illness. Methods: For this systematic review, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and searched Medline, Embase, CINAHL, and PubMed for studies on the development of COVID-19 in neonates with PPHN, published from 1 December 2019 to 29 February 2024, with an English language restriction. Results: Of the 2406 papers that were identified, 21 articles were included in the systematic review. Studies involving thirty-six neonates with PPHN and infected with SARS-CoV-2 were analyzed (twenty-nine survived, six died, and one is still hospitalized). The main causes of PPHN in neonates who had COVID-19 were neonatal respiratory distress syndrome (NRDS) (41.7%), meconium-stained amniotic fluid (MSAF) (16.7%), preterm premature rupture of membranes (PPROM) (11.1%), hypoxic ischemic encephalopathy (HIE) (5.5%), pneumonia (5.5%), and idiopathic (2.8%). Most of those neonates were male (33.3%), belonged to Indian ethnicity (50%), and were delivered via caesarean section (44.4%). COVID-19 in cases with PPHN commonly occurred in neonates born with a pregnancy range from 32 to <37 weeks (moderate to late preterm) (36.1%). The maternal severity of COVID-19 was reported to be severe in three cases only (8.3%); however, SARS-CoV-2 infection in neonates with PPHN was either severe (44.4%) or critical (22.2%). Most of these neonates experienced acute respiratory distress syndrome (ARDS) (58.3%). Early and late multisystem inflammatory syndrome in neonates (MIS-N) were reported in 50% and 11.1%, respectively. A high proportion of neonates were admitted to the intensive care unit (ICU) (58.3%) or needed mechanical ventilation (MV) (47.2%). Neonates with concurrent PPHN and SARS-CoV-2 infection who died had worse severity of COVID-19 [i.e., severity of COVID-19 was critical in 10% (neonates with PPHN who survived group) vs. 83.3% (neonates with PPHN who died group); p = 0.026]. Neonates with PPHN and COVID-19 had a higher relative risk of death if they received more antibiotics (RR 4.14, 95% CI 0.64–6.88) and if their COVID-19 was defined as critical (RR 2.84, 95% CI 0.86–9.39). Male neonates with PPHN and COVID-19 (RR 2.60, 95% CI 0.30–1.17) and those requiring prolonged invasive positive pressure ventilation (RR 2.22, 95% CI 0.64–7.73) also showed an increased relative risk for death. Conclusions: COVID-19 in neonates with PPHN is challenging and may be associated with increased mortality, severity, ICU admission, ARDS, MIS-N, and MV usage. The results should be interpreted with caution owing to the small number of studies and substantial heterogeneity and indicate a need for future research in this area. Due to its benefits, testing for SARS-CoV-2 should be encouraged for newborns with symptoms consistent with COVID-19, especially in neonates with a history of SARS-CoV-2 exposure. Effective protection measures should be implemented during delivery and post-delivery care as necessary.
Keywords: COVID-19, persistent fetal circulation, patent ductus arteriosus, patent foramen ovale, persistent pulmonary hypertension, PPHN, risk, SARS-CoV-2, severity
1. Introduction
Despite advances in neonatal cardiorespiratory care, persistent pulmonary hypertension of the newborn (PPHN) remains a serious condition and is associated with a high mortality rate, ranging between 7% and 35.7% [1,2,3,4]. The most commonly involved systems in PPHN are cardiovascular and respiratory systems with echocardiographic abnormalities and raised cardiac biomarkers; therefore, neonates with PPHN of all ages are assumed to be at high risk for adverse health outcomes from coronavirus disease 2019 (COVID-19). PPHN can cause acute respiratory failure and in many COVID-19 cases, patients have been reported to experience acute respiratory distress syndrome (ARDS) [5]; thus, the co-existence of both conditions can be deleterious. COVID-19 can impact pulmonary blood flow through various mechanisms, some of which overlap with the disease process of PPHN. These mechanisms include the following: (1) inflammation, swelling, and damage of the endothelial cells and blood vessels; (2) small blood vessel clots; (3) blood clots in the veins; and (4) changes in blood flow dynamics due to ARDS and the use of mechanical ventilation.
It is uncertain which PPHN neonates are at high risk for a worse COVID-19 clinical course, although there is an increasing number of studies reporting on real-world patients who suffered PPHN and COVID-19 concurrently [6,7,8]. Recent data indicate that neonatal cases with PPHN may not be at greater risk than others [8,9]; however, some early studies in neonates with PPHN suggested that the risk of severe COVID-19 is higher in this population [10,11,12]. There is limited evidence on the final treatment outcome in neonates who had PPHN and were infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as findings were built on very few cases or small sample populations and many of these studies reported conflicting results [13,14,15]. Due to the lack of comprehensive and updated systematic reviews focusing on COVID-19 in neonates with PPHN, we aimed to analyze the clinical characteristics and outcomes of SARS-CoV-2 infection in neonates with PPHN to identify neonatal cases at risk to develop severe illness, to decrease the mortality rate among those patients, and improve their final healthcare outcomes.
2. Methods
2.1. Design
We followed the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement to perform this systematic review [16]. The systematic review protocol was entered into the International Prospective Register of Systematic Reviews (PROSPERO) under CRD42024596354. Published articles from 1 December 2019 to 29 February 2024, with an English language restriction, were selected for review from four electronic databases (Medline, Embase, CINAHL, and PubMed). The search phrases included Boolean terms ‘AND’ and ‘OR’ with the following keywords in various possible combinations: “pulmonary hypertension”, “patent ductus arteriosus”, “patent foramen ovale”, “PPHN”, “neonate”, “neonates”, “newborn”, “newborns”, “neonatal”, “COVID-19”, “SARS-CoV-2”, “severe acute respiratory syndrome coronavirus 2”, “coronavirus disease 2019”, and “2019 novel coronavirus” (see Supplementary Table S1 for database-specific search syntax). Articles discussing and reporting the development of COVID-19 in neonates with PPHN were selected based on the title and abstract.
The PRISMA 2020 checklist was used (see Supplementary Materials) [16].
2.2. Inclusion–Exclusion Criteria
We included observational studies [case reports, case-series and cohort studies] that reported the real-world development of COVID-19 in neonates with PPHN. We excluded editorials, commentaries, expert opinions, discussion papers, reviews, meta-analyses, or studies available in languages other than English.
2.3. Definitions of COVID-19 Severity, Mother-to-Neonate Transmission of SARS-CoV-2, and Multisystem Inflammatory Syndrome in Neonates (MIS-N)
The severity of COVID-19 was defined as per the Clinical Spectrum of SARS-CoV-2 Infection issued by the National Institutes of Health (i.e., asymptomatic, mild, moderate, severe, and critical) [17].
We applied the World Health Organization (WHO) definition to assess for the mother-to-neonate transmission in neonates with a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) or immunoglobulin M (IgM) test [18]. Cases were categorized as confirmed/possible/unlikely/indeterminate for intrauterine, intra- partum, or early postpartum infection. Stillborn babies were not included in our analysis [18].
MIS-N was defined according to the current United States Centers for Disease Control and Prevention case definition in an individual aged < 28 days [19,20]. The neonates who fulfilled all the criteria for the definition were termed as “most likely MIS-N”. The neonates who presented with a high suspicion for MIS-N but could not fulfil all the criteria were termed as “possible MIS-N” if no alternative diagnosis was suggested, or “unlikely MIS-N” if an alternative diagnosis was available. The neonates who presented within the first 3 days of life were termed as early, and those who presented beyond 3 days until 28 days of life were termed as late MIS-N [21].
2.4. Data Extraction
Six reviewers—Saad Alhumaid, Muneera Alabdulqader, Zainab Al Alawi, Mohammed A Alabdulmuhsin, Hassan I Al Hassar, and Hussain Ahmed Alsouaib—independently screened the papers by reviewing titles and abstracts based on the selection criteria. If there was disagreement and agreement could not be reached after discussion between the two investigators, a third investigator adjudicated.
A standardized data collection form was used to collate information from the studies that we selected and facilitate study quality assessment and data analysis (see Supplementary Tables S2 and S3 for summary of the characteristics of the included studies, main outcome measures, and clinical features in neonates with PPHN and COVID-19 (n = 21 studies), 2020–2023).
2.5. Quality Assessment
Two tools were used appropriately to assess the quality of the studies included in this review: (1) Modified Newcastle–Ottawa Scale (NOS) to evaluate case report and case-series studies [22]; and (2) NOS to evaluate cohort studies [23]. The quality assessment was carried out by six co-authors—Hussain Ali Alhassan, Hassan Al-Helal, Sameer Ahmed Almoraihel, Mohammed Jaber Alomran, Hassan Redha AL-Tarfi, and Abbas Radi Al-Makinah—who independently evaluated potential bias using these two tools.
2.6. Data Analysis
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between the COVID-19-infected PPHN cases who survived and COVID-19-infected PPHN cases who died were analyzed using the Chi-square (χ2) tests (or Fisher’s exact tests for expected cell count <5 in more than 20% of the cells). Relative risks (RRs) and 95% confidence intervals (CIs) of the association of each demographic parameter and clinical variable with the treatment outcomes (i.e., survived or died) of PPHN cases with SARS-CoV-2 infection were calculated. All p-values were based on two-sided tests and significance was set at a p-value less than 0.05. Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics software, version 26.0 (IBM Corp., Armonk, NY, USA) were used for all data wrangling and statistical analyses. The graphical abstract was created with BioRender.com (accessed on 20 October 2024) (agreement no. VG27G3WWB6).
3. Results
3.1. Study Characteristics and Quality
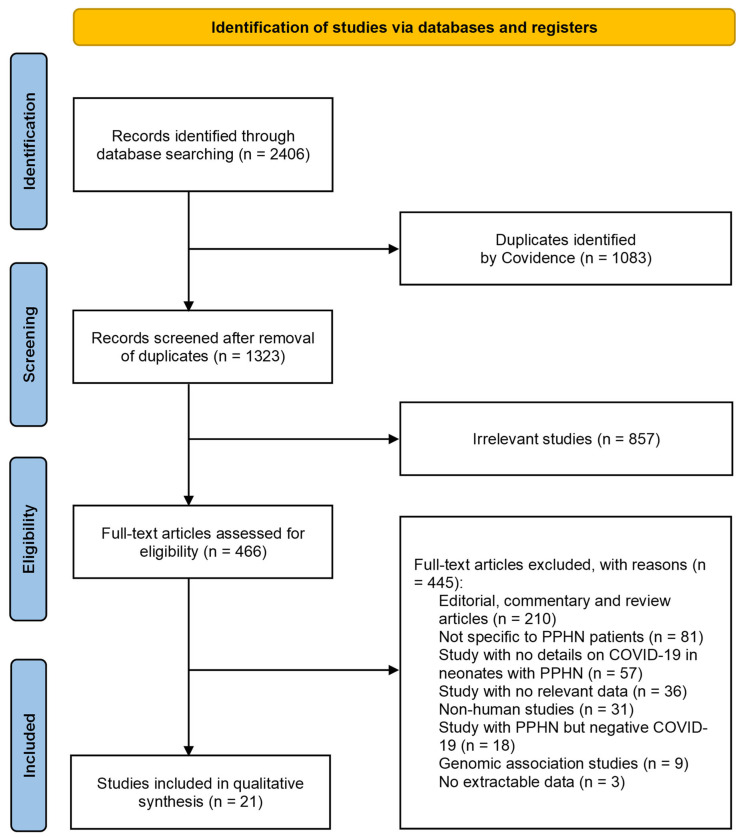
A total of 2406 publications were identified (Figure 1). After the exclusion of duplicates and articles that did not fulfil the study inclusion criteria, twenty-one articles were included in the qualitative synthesis [5,6,7,8,9,10,11,12,13,14,15,24,25,26,27,28,29,30,31,32,33]. The detailed characteristics of the included studies for neonates who were infected with SARS-CoV-2 and had PPHN are shown in Supplementary Tables S2 and S3. There were fourteen case reports [5,9,10,11,12,14,24,25,27,28,29,30,32,33], six case-series studies [6,7,13,15,26,31], and one cohort study [8]. All studies included in this review were retrospective in design except for two studies, which were prospective (n = 2) [6,8]. These studies were conducted in India (n = 7) [6,8,13,14,15,24,31], United States (n = 4) [5,25,26,29], Saudi Arabia (n = 2) [10,33], Iran (n = 2) [9,27], Turkey (n = 1) [11], Brazil (n = 1) [12], Israel (n = 1) [7], Bangladesh (n = 1) [28], the Netherlands (n = 1) [32], and Canada (n = 1) [30]. All were single-center studies, except for three multi-center studies [6,13,15]. All case reports and case-series studies were assessed for bias using the modified NOS. Thirteen studies were deemed to have high methodological quality [9,10,11,12,15,24,27,28,29,30,31,32,33] and seven were deemed to have moderate methodological quality [5,6,7,13,14,25,26]. The only study that utilized a cohort design had a moderate risk of bias based on NOS [8].
Figure 1.
Flow diagram of studies included in the systematic review.
3.2. Demographic Features and Clinical Characteristics of SARS-CoV-2 Infection in Neonates with PPHN
The included studies had a total of 36 neonatal cases with PPHN who were infected with SARS-CoV-2, as detailed in Supplementary Tables S2 and S3. The median interquartile range (IQR) age of hospitalized neonates was 90 min (1 to 1440), with an increased male predominance in neonates diagnosed with PPHN and COVID-19 in most of the studies (12/36 = 33.3%) [5,11,12,14,15,24,26,27,29,30,31]. A majority of the neonates belonged to Indian ethnicity (n = 18/36, 50%) [6,8,13,14,15,24,31], and seven studies reported the following neonatal ethnicities: Persian (n = 2) [9,27], White (Caucasian) (n = 1) [11], Black (n = 1) [5], Hispanic (n = 1) [12], Asian (n = 1) [33], and Arab (n = 1) [10]. Only two neonates were born via the normal spontaneous vaginal delivery (NSVD) (n = 2/36, 5.5%) [27,29]; however, a higher proportion of these neonates were born via the caesarean delivery mode (C-section) (n = 16/36, 44.4%) [5,6,9,10,11,12,24,25,28,30,32]. One neonate was born by induction of labor [33]. Around ten (n = 10/36, 27.8%) of the neonates were born with a normal birthweight (≥2500 g) [6,15,24,26,27,30,33], but a high proportion of neonates were delivered with low birthweight (≥1500 g to <2500 g) (n = 9/36, 25%) [5,6,9,10,11,12,28,31,32]. Two cases were born with extremely low birthweight (<1000 g) [6,25] and one neonate was born with a very low birthweight (<1500 g) [6]. Some neonates were born with a normal gestational age (term: ≥37 weeks) (n = 9/36, 25%) [6,9,14,24,26,27], but most neonates were born with a pregnancy range from 32 to <37 weeks (moderate to late preterm) (n = 13/36, 36.1%) [5,6,10,11,12,15,28,29,30,31,32,33]. Three cases were very preterm (28 to <32 weeks) (n = 3/36, 8.3%) [6,25]. The Apgar score for these neonates was low (range of 0 to 3) (n = 7/36, 19.4%) [5,6,25,30,32], moderately abnormal (range of 4 to 6) (n = 11/36, 30.5%) [5,6,11,27,30,32], and reassuring (range of 7 to 10) (n = 17/36, 47.2%) [6,9,10,11,12,24,27,28,33].
The most frequent medical comorbidities in neonates with PPHN who had moth-er-to-neonate SARS-CoV-2 transmission were arrhythmia (n = 6) [6], atrial thrombi (n = 6) [6], seizures (n = 5) [12,13], hypotension (n = 4) [5,11,15,25], intracranial hemorrhage (ICH) (n = 4) [5,10,25,32], necrotizing enterocolitis (NEC) (n = 3) [6,9,24], sepsis (n = 3) [5,11,12], metabolic acidosis (n = 3) [10,29,33], decreased fetal movements (n = 3) [5,6], lack of fetal movements (n = 3) [6,32], chorioamnionitis (n = 3) [5,12,25], food intolerance (n = 3) [9,24,31], respiratory acidosis (n = 2) [5,27], rash (n = 2) [9,24], decreased fetal heart rate (n = 2) [9,24], vomiting (n = 2) [9,24], catecholamine-resistant vasodilatory shock (CRVS) (n = 2) [6], shock (n = 2) [15], cardiorespiratory failure (n = 2) [10,12], hydrocephalus (n = 2) [5,9], and hypoxic ischemic encephalopathy (HIE) (n = 2) [26,30]. The causes of PPHN were neonatal respiratory distress syndrome (NRDS) (n = 15/36, 41.7%) [5,6,9,10,11,15,25,26,28,29,30,32,33]; meconium-stained amniotic fluid (MSAF) (n = 6/36, 16.7%) [5,6,25,27]; preterm premature rupture of membranes (PPROM) (n = 4/36, 11.1%) [5,6,12,27]; hypoxic ischemic encephalopathy (HIE) (n = 2/36, 5.5%) [26,30]; pneumonia (n = 2/36, 5.5%); and idiopathic (n = 1/36, 2.8%) [24]. The individual oxygen index (OI) for PPHN patients infected with COVID-19 was reported by two studies only and the patient’s OI was moderate (≥15 and <25) (1/36, 2.8%) [10] or severe (≥25 and <40) (1/36, 2.8%) [11]. One study reported on the mean OI for the entire study population (n = 10) [26]. As expected, most common therapies initiated to treat PPHN were invasive positive pressure ventilation (IPPV) (n = 18/36, 50%) [5,6,7,10,13,14,15,24,27,29,32,33], continuous positive airway pressure (CPAP) (n = 17/36, 47.2%) [5,6,7,9,10,11,12,13,24,27,28,29,30,33], inhaled nitric oxide (iNO) (n = 14/36, 38.9%) [5,6,7,10,11,14,25,26,29,30,32,33], high frequency ventilation (HFV) (n = 10/36, 27.8%) [5,6,10,11,13,24,25,33], surfactant (n = 9/36, 25%) [5,9,10,11,12,25,27,29,32], sildenafil (n = 9/36, 25%) [9,11,12,14,15,24,27,28,30], noninvasive positive pressure ventilation (NPPV) (n = 8/36, 22.2%) [12,13,27,28,31], dopamine (n = 7/36, 19.4%) [5,6,9,10,11,24,27], hydrocortisone (n = 7/36, 19.4%) [5,6,10,12,25,32,33], packed red blood cells (n = 6/36, 16.7%) [5,9,10,25,28,29], milrinone (n = 5/36, 13.9%) [6,9,15,27,30], inotropes (n = 5/36, 13.9%) [9,14,26,31,32], fresh frozen plasma (n = 4/36, 11.1%) [9,25,28,33], diuretics (n = 4/36, 11.1%) [9,14,24,28], epinephrine (n = 4/36, 11.1%) [6,10,15,25], dobutamine (n = 4/36, 11.1) [10,11,27,33], antihypertensives (n = 4/36, 11.1%) [9,12,14,27], magnesium sulfate (n = 3/36, 8.3%) [6,25,27], vasopressors (n = 2/36, 5.5%) [12,14], sedation (n = 2/36, 5.5%) [5,30], cardiopulmonary resuscitation (n = 2/36, 5.5%) [5,10], platelets (n = 2/36, 5.5%) [10,29], endothelin receptor antagonists (ERAs) (n = 2/36, 5.5%) [12,14], opioids (n = 2/36, 5.5%) [5,27], vasodilators (n = 2/36, 5.5%) [14,28], and therapeutic hypothermia (n = 2/36, 5.5%) [26,27]. Only one patient was placed on extracorporeal membrane oxygenation (ECMO) with the initial ECMO venoarterial mode [26].
3.3. Demographic Features and Clinical Characteristics of SARS-CoV-2 Infection in Mothers Who Delivered Neonates with PPHN and a History of Mother-to-Neonate SARS-CoV-2 Transmission
The most common medical comorbidities in pregnant mothers who had SARS-CoV-2 infection and delivered neonates with PPHN were gestational diabetes mellitus (n = 4) [5,10,24,28], pregnancy-induced hypertension (n = 3) [10,28,29], preeclampsia (n = 3) [25,29,33], coinfections (n = 3) [including Group B Streptococcus (n = 1), Cytomegalovirus (n = 1) and Parvovirus B19 (n = 1)] [5,32], hypothyroidism (n = 2) [27,28], and placenta previa (n = 2) [6,25]. However, three mother had no medical comorbidities and were previously healthy (3/36, 8.3%) [9,12,30]. Only three studies reported on the vaccination status against SARS-CoV-2 for mothers who delivered PPHN patients infected with COVID-19, and none of these mothers were vaccinated against SARS-CoV-2 before or during pregnancy or at the time of delivery [5,6,31]. Studies reported a different maternal onset of symptoms for SARS-CoV-2 infection: 3 days or 5 days before hospital admission [12,28,32]; 1 day after hospital admission [29]; 1 day, 12 days, 19 days, or 21 days before birth [9,10,24,33]; and 1 day after birth [10]. Only a few studies reported maternal SARS-CoV-2 infection in pregnant women with a history of close contact with a COVID-19 patient (three cases) [6,31,32] or who had abnormal chest x-rays (including patchy consolidation, or multifocal pneumonia and airspace opacities) (three cases) [10,25,28].
Maternal RT-PCR tests for SARS-CoV-2 were positive in eighteen mothers (18/36, 50%) [5,6,7,9,10,11,12,15,25,27,28,29,30,32,33]. Of the total number of mothers who had positive serologic SARS-CoV-2 antibodies (immunoglobulin G only) perinatally from blood specimens (6/36, 16.7%) [6,24,31,32], all were tested by RT-PCR-SARS-CoV-2 except one [31], and only one mother tested positive for SARS-CoV-2 [32]. The diagnosis of maternal SARS-CoV-2 infection was made by RT-PCR testing of the nasopharyngeal swab samples (n = 9) [9,10,11,12,25,28,29,32,33], oropharyngeal swab samples (n = 2) [28,32], vaginal secretion sample (n = 1) [32], blood sample (n = 1) [32], and urine sample (n = 1) [32]. Only one study demonstrated the presence of SARS-CoV-2 in the cells of placenta by utilizing an immunohistochemical investigation for SARS-CoV-2 antigen expression in combination with SARS-CoV-2 RNA in situ hybridization [32]. Maternal SARS-CoV-2 positivity was confirmed in another 9 cases using RT-PCR, but the location of samples was not stated [5,6,7,15,27]. One study reported a positive SARS-CoV-2 test in a mother at hospital admission but the method of COVID-19 testing used and location of sample were not mentioned [30]. Two studies used whole-genome sequencing of SARS-CoV-2 and identified similar variants in mothers and their neonates (Gamma and Delta variants) [12,30].
Some mothers tested positive for COVID-19 within 1 day at the hospital admission (n = 6) [5,12,28,29,30,32]. However, few mothers tested positive for SARS-CoV-2 after birth by 2 days (n = 2) [9,32], 3 days (n = 3) [10,27,32], 5 days (n = 1) [32], 10 days (n = 1) [33], or 19 days (n = 1) [24]. Overall, COVID-19 in mothers resulted in no or low severity of disease in more than fifteen (15/36, 41%) of all included cases. The maternal COVID-19 severity of cases was as follows: asymptomatic = 9, mild = 5, or moderate = 1 [6,9,10,12,15,24,27,31,32,33]. The maternal severity of COVID-19 was reported to be severe in three cases only (3/36, 8.3%) [5,25,28]. Five studies reported to have followed measures to prevent mother-to-neonate SARS-CoV-2 transmission by immediate separation, strict isolation, no skin-to-skin contact, or breastfeeding [5,12,25,28,33], and one study reported full isolation of newborn during delivery and intensive care unit (ICU) admission [10]. Based on timing of mother-to-neonate SARS-CoV-2 transmission according to the WHO classification system, 5/36 (13.9%) were classified as confirmed intrauterine transmissions [5,9,11,12,33] and 4/36 (11.1%) were classified as possible intrauterine transmissions [14,27,30]. There were two confirmed intrapartum infections (2/36, 5.5%) [10,25], two confirmed early postpartum infections (2/36, 5.5%) [24,32], one possible intrapartum infection (1/36, 2.8%) [31], one unlikely intrapartum infection (1/36, 2.8%) [28], and one unlikely intrauterine infection (1/36, 2.8%) [29].
3.4. Diagnosis and Severity of SARS-CoV-2 Infection in Neonates with PPHN
Neonatal RT-PCR tests for SARS-CoV-2 were positive in six neonates only (6/36, 16.7%) [5,9,10,11,12,27], whereas a positive serology yielded neonatal SARS-CoV-2 antibodies (immunoglobulin G only) from blood specimens in eighteen cases (18/36, 50%) [6,11,13,14,15,24,31,33]. The diagnosis of neonatal SARS-CoV-2 infection was made using RT-PCR tests of the nasopharyngeal swab samples (n = 4) [9,10,11,12] and tracheal aspirate (n = 2) [11,12]. One study utilized rapid antigen tests to confirm neonatal SARS-CoV-2 positivity [5]. Only one study revealed neonatal positive results for immunoglobulin M antibodies against SARS-CoV-2 [11]. Neonatal SARS-CoV-2 positivity was confirmed in two cases using RT-PCR; however, the location of samples was not stated [5,27]. One study reported a positive SARS-CoV-2 test in a neonate on the second day of life but the method of COVID-19 testing used and the location of the sample were not mentioned [30]. Some neonates showed positive RT-PCR tests or immunoglobulin antibodies against SARS-CoV-2 at different ages: 1 day (n = 5) [5,9,11,12,33], 2 days (n = 2) [27,30], 3 days (n = 1) [11], 4 days (n = 1) [5], 5 days (n = 2) [10,11], 6 days (n = 3) [9,14,31], 7 days (n = 1) [11], 8 days (n = 1) [11], 11 days (n = 1) [11], 12 days (n = 1) [5], 13 days (n = 1) [12], 15 days (n = 1) [5], 17 days (n = 1) [11], and 18 days (n = 2) [5,24].
The most common neonatal COVID-19 symptoms were respiratory distress (12/36, 33.3%) [5,6,9,12,15,27,28,30,32,33], fever (9/36, 25%) [9,11,13,15,24,29], low O2 saturation (6/36, 16.7%) [5,10,25,27,29,33], shortness of breath (5/36, 13.9%) [5,13], cyanosis (5/36, 13.9%) [24,25,27,28,33], rash (5/36, 13.9%) [13,32], grunting (4/36, 11.1%) [9,10,27,28], tachypnoea (4/36, 11.1%) [9,11,27,28], retractions (3/36, 8.3%) [5,10,27], respiratory failure (3/36, 8.3%) [5,25,29], and tachycardia (3/36, 8.3%) [6,10,28]. The most common neonatal COVID-19 abnormal laboratory findings were high D-dimer (16/36, 44.4%) [6,11,13,14,15,24,28,31,32], high C-reactive protein (15/36, 41.7%) [6,9,11,14,24,27,28,29,31,33], thrombocytopenia (14/36, 38.9%) [6,10,11,24,27,28,29,32,33], high lactate dehydrogenase (13/36, 36.1%) [6,13,14,24,27,31,33], high N-terminal pro b-type natriuretic peptide (NT-proBNP) (10/36, 27.8%) [6,12,14,15,24,33], high liver function tests (10/36, 27.8%) [6,11,27,32,33], high troponin-I (8/36, 22.2%) [6,12,14,24], high ferritin (7/36, 19.4%) [9,12,14,15,24,32], high procalcitonin (6/36, 16.7%) [6,15,24,25,33], leukocytosis (6/36, 16.7%) [6,29], high interleukin-6 (5/36, 13.9%) [6,14,28], high blood urea nitrogen (5/36, 13.9%) [6,10,27], high white blood cells (4/36, 11.1%) [10,24,27,29], high prothrombin time (3/36, 8.3%) [10,25,28], lymphopenia (3/36, 8.3%) [11,28,33], and neutrophilia (3/36, 8.3%) [24,27,28]. Echocardiographic evaluation suggested PPHN in 31/36 (86.1%) [5,6,7,9,10,11,12,13,14,15,24,28,29,30,31,32,33], tricuspid regurgitation in 10/36 (27.8%) [6,9,10,31,32], dilated coronaries in 4/36 (11.1%) [13,31], coronary aneurysm 4/36 (11.1%) [6], and cardiomegaly in 2/36 (5.5%) [9,12] neonates. Chest x-rays shown cardiomegaly (19.4%) [6,31] and ground glass opacity (11.1%) [10,11,24,30]. Ultrasonography shown abnormal nonstress test (13.9%) [6].
SARS-CoV-2 infection in neonates with PPHN was severe (n = 16/36, 44.4%) [6,9,14,15,24,28,29,30,31,32,33] or critical (n = 8/36, 22.2%) [5,6,10,11,12,14,25,27], and most patients experienced ARDS (n = 21/36, 58.3%) [5,6,9,10,11,12,15,24,25,27,28,29,30,32,33]. Early multisystem inflammatory Syndrome in neonates (MIS-N) was reported in eighteen cases (18/36, 50%) [5,6,9,10,11,12,15,25,27,28,30,32,33] and late MIS-N in four cases (4/36, 11.1%) [6,24,29,31]. Based on the definition, a diagnosis of “most likely MIS-N” was considered in 21/36 (58.3%) cases [6,9,11,12,13,15,24,25,27,28,30,32,33], “possible MIS-N” in 8/36 (22.2%) cases [8,10,14,15,29,31], and “unlikely MIS-N” in 1/36 cases (2.8%) [5].
3.5. Management, Treatment Outcomes, and Relative Risk Associated with Mortality in PPHN Cases Infected with SARS-CoV-2
As expected, most prescribed pharmacotherapy agents in these PPHN cases to treat COVID-19 were antibiotics (20/36, 55.5%) [5,6,9,10,11,12,14,15,24,25,28,29,32,33], intravenous immunoglobulin (15/36, 41.7%) [9,11,12,13,14,15,27,28,31,32,33], steroids (14/36, 38.9%) [5,9,12,13,14,15,24,27,30,31], aspirin (7/36, 19.4%) [6,14,31,32], anticoagulants (3/36, 8.3%) [12,14,31], and remdesivir (2/36, 5.5%) [25,27]. Two studies reported use of prone position (n = 2/36, 5.5%) [25,30]. The median neonatal need of supplemental oxygen was 10 (IQR, 6 to 16), mechanical ventilation (MV) was 10.5 (IQR, 4.2 to 16.7), and inhaled nitric oxide (iNO) was 8 (IQR, 5.2 to 11.7), while the median neonatal length of stay in hospital was 20 days (IQR, 14 to 23.5). Final treatment outcomes of the neonates who had PPHN with a history of mother-to-neonate SARS-CoV-2 transmission with mortality were documented in six cases (n = 6/36, 16.7%) [6,10,11,12,13,14], while twenty-nine (n = 29/36, 80.5%) cases recovered [5,6,7,8,9,13,14,15,24,25,26,27,28,29,31,32,33], and one case was still in the ICU (n = 1/36, 2.8%) [30], as shown in Table 1.
Table 1.
Characteristics and clinical presentation of COVID-19-infected PPHN cases (n = 21 studies), 2020–2023.
| Variable | All (n = 36) a | Variable | All (n = 36) a |
|---|---|---|---|
| Age (hours) | Maternal COVID-19 severity | ||
| <1 | 10 (27.8) | Asymptomatic | 9 (25) |
| 1–3 | 2 (5.5) | Mild | 5 (13.9) |
| >3 | 10 (27.8) | Moderate | 1 (2.8) |
| Gender | Severe | 3 (8.3) | |
| Female | 7 (19.4) | Mother-to-neonate transmission of SARS-CoV-2 | |
| Male | 12 (33.3) | Confirmed intrauterine | 5 (13.9) |
| Ethnicity | Possible intrauterine | 4 (11.1) | |
| Indian | 18 (50) | Confirmed intrapartum | 2 (5.5) |
| Persian | 2 (5.5) | Confirmed early postpartum | 2 (5.5) |
| Bengali | 1 (2.8) | Possible intrapartum | 1 (2.8) |
| White (Caucasian) | 1 (2.8) | Unlikely intrapartum | 1 (2.8) |
| Black b | 1 (2.8) | Unlikely intrauterine | 1 (2.8) |
| Hispanic | 1 (2.8) | Neonatal COVID-19 symptoms | |
| Asian | 1 (2.8) | Respiratory distress | 12 (33.3) |
| Arab | 1 (2.8) | Fever | 9 (25) |
| Delivery mode | Low O2 sat | 6 (16.7) | |
| Caesarean | 16 (44.4) | SOB | 5 (13.9) |
| NSVD | 2 (5.5) | Cyanosis | 5 (13.9) |
| Induced labor | 1 (2.8) | Rash | 5 (13.9) |
| Weight (grams) | Grunting | 4 (11.1) | |
| Normal: ≥2500 | 10 (27.8) | Tachypnoea | 4 (11.1) |
| Low: ≥1500–2499 | 9 (25) | Retractions | 3 (8.3) |
| Very low: <1500 | 1 (2.8) | Respiratory failure | 3 (8.3) |
| Extremely low: <1000 | 2 (5.5) | Tachycardia | 3 (8.3) |
| Gestational age (weeks) | Neonatal abnormal laboratory findings | ||
| Term (≥37 weeks) | 9 (25) | High D-dimer | 16 (44.4) |
| Moderate to late preterm (32 to <37 weeks) | 13 (36.1) | High CRP | 15 (41.7) |
| Very preterm (28 to <32 weeks) | 3 (8.3) | Thrombocytopenia | 14 (38.9) |
| Apgar score | High LDH | 13 (36.1) | |
| Low: 0–3 | 7 (19.4) | High NT-proBNP | 10 (27.8) |
| Moderately abnormal: 4–6 | 11 (30.5) | High LFTs | 10 (27.8) |
| Reassuring: 7–10 | 17 (47.2) | High troponin-I | 8 (22.2) |
| Neonatal comorbidities | High ferritin | 7 (19.4) | |
| Arrhythmia | 6 (16.7) | High procalcitonin | 6 (16.7) |
| Atrial thrombi | 6 (16.7) | Leukocytosis | 6 (16.7) |
| Seizures | 5 (13.9) | High IL-6 | 5 (13.9) |
| Hypotension | 4 (11.1) | High BUN | 5 (13.9) |
| ICH | 4 (11.1) | High WBCs | 4 (11.1) |
| NEC | 3 (8.3) | High PTT | 3 (8.3) |
| Sepsis | 3 (8.3) | Lymphopenia | 3 (8.3) |
| Metabolic acidosis | 3 (8.3) | Neutrophilia | 3 (8.3) |
| Decreased fetal movements | 3 (8.3) | Neonatal abnormal radiological findings | |
| Lack of fetal movements | 3 (8.3) | Echo: PPHN | 31 (86.1) |
| Chorioamnionitis | 3 (8.3) | Echo: TR | 10 (27.8) |
| Food intolerance | 3 (8.3) | CXR: Cardiomegaly | 7 (19.4) |
| Respiratory acidosis | 2 (5.5) | USG: Abnormal NST | 5 (13.9) |
| Rash | 2 (5.5) | CXR: GGO | 4 (11.1) |
| Decreased fetal HR | 2 (5.5) | Echo: Dilated coronaries | 4 (11.1) |
| Vomiting | 2 (5.5) | Echo: Coronary aneurysm | 4 (11.1) |
| CRVS | 2 (5.5) | Echo: Cardiomegaly | 2 (5.5) |
| Shock | 2 (5.5) | Neonatal COVID-19 severity | |
| Cardiorespiratory failure | 2 (5.5) | Severe | 16 (44.4) |
| Hydrocephalus | 2 (5.5) | Critical | 8 (22.2) |
| HIE | 2 (5.5) | If neonate suffered ARDS | |
| Cardiogenic shock | 1 (2.8) | Yes | 21 (58.3) |
| Septic shock | 1 (2.8) | No | 1 (2.8) |
| MOD | 1 (2.8) | If experienced MIS-N | |
| Coinfection with Klebsiella oxytoca | 1 (2.8) | Most likely | 21 (58.3) |
| MI | 1 (2.8) | Possible | 8 (22.2) |
| Myocarditis | 1 (2.8) | Unlikely | 1 (2.8) |
| CLD | 1 (2.8) | Type of MIS-N | |
| PVD | 1 (2.8) | Early | 18 (50) |
| Bronchiectasis | 1 (2.8) | Late | 4 (11.1) |
| PPHN etiology | Neonatal treatment of SARS-CoV-2 infection | ||
| NRDS | 15 (41.7) | Antibiotics | 20 (55.5) |
| MSAF | 6 (16.7) | IVIG | 15 (41.7) |
| PPROM | 4 (11.1) | Steroids | 14 (38.9) |
| HIE | 2 (5.5) | Aspirin | 7 (19.4) |
| Pneumonia | 2 (5.5) | Anticoagulants | 3 (8.3) |
| Idiopathic | 1 (2.8) | Remdesivir | 2 (5.5) |
| Initiated PPHN treatments | Prone positioning | 2 (5.5) | |
| IPPV | 18 (50) | Duration on supplemental oxygen (days) | |
| CPAP | 17 (47.2) | <7 | 4 (11.1) |
| iNO | 14 (38.9) | 7 to <14 | 9 (25) |
| HFV | 10 (27.8) | ≥14 | 3 (8.3) |
| Surfactant | 9 (25) | Duration of MV (days) | |
| Sildenafil | 9 (25) | <7 | 5 (13.9) |
| NPPV | 8 (22.2) | 7 to <14 | 8 (22.2) |
| Dopamine | 7 (19.4) | ≥14 | 4 (11.1) |
| Hydrocortisone | 7 (19.4) | Duration on iNO use (days) | |
| Packed RBCs | 6 (16.7) | <7 | 4 (11.1) |
| Milrinone | 5 (13.9) | 7 to <14 | 3 (8.3) |
| Inotropes | 5 (13.9) | ≥14 | 1 (2.8) |
| FFP | 4 (11.1) | Duration of hospital stay (days) | |
| Diuretics | 4 (11.1) | <7 | 0 |
| Epinephrine | 4 (11.1) | 7 to <14 | 3 (8.3) |
| Dobutamine | 4 (11.1) | ≥14 | 14 (38.9) |
| Antihypertensives | 4 (11.1) | Final treatment outcome | |
| Magnesium sulfate | 3 (8.3) | Survived | 29 (80.5) |
| Vasopressors | 2 (5.5) | Died | 6 (16.7) |
| Sedation | 2 (5.5) | Still hospitalized | 1 (2.8) |
| CPR | 2 (5.5) | ||
| Platelets | 2 (5.5) | ||
| ERAs | 2 (5.5) | ||
| Opioids | 2 (5.5) | ||
| Vasodilators | 2 (5.5) | ||
| Therapeutic hypothermia | 2 (5.5) | ||
| Maternal comorbidities | |||
| Gestational DM | 4 (11.1) | ||
| PIH | 3 (8.3) | ||
| Preeclampsia | 3 (8.3) | ||
| No comorbidities | 3 (8.3) | ||
| Hypothyroidism | 2 (5.5) | ||
| Placenta previa | 2 (5.5) | ||
| Coinfection with Group B Streptococcus | 1 (2.8) | ||
| Coinfection with CMV | 1 (2.8) | ||
| Coinfection with Parvovirus B19 | 1 (2.8) |
Abbreviations: ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; CLD, chronic lung disease; CMV, Cytomegalovirus; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CPR, cardiopulmonary resuscitation; CRP, C-reactive protein; CRVS, catecholamine-resistant vasodilatory shock; CXR, chest X-rays; DM, diabetes mellitus; ERAs, endothelin receptor antagonists; Echo, echocardiogram; FFP, fresh frozen plasma; GGO, ground-glass opacity; HFV, high frequency ventilation; HIE, hypoxic ischemic encephalopathy; HR, heart rate; ICH, intracranial hemorrhage; IL-6, interleukin 6; iNO, inhaled nitric oxide; IPPV, invasive positive pressure ventilation; LDH, lactic acid dehydrogenase; LFTs, liver function tests; MI, myocardial infarction; MIS-N, multisystem inflammatory syndrome in neonates; MOD, multiple organ dysfunction; MSAF, meconium-stained amniotic fluid; MV, mechanical ventilation; NEC, necrotizing enterocolitis; NPPV, noninvasive positive pressure ventilation; NRDS, neonatal respiratory distress syndrome; NST, nonstress test; NT-proBNP, N-terminal pro b-type natriuretic peptide; O2sat, oxygen saturation; PIH, pregnancy-induced hypertension; PPHN, persistent pulmonary hypertension of the newborn; PPROM, preterm premature rupture of membranes; PTT, partial thromboplastin time; PVD, pulmonary vascular disease; RBCs, red blood cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOB, shortness of breath; TR, tricuspid regurgitation; USG, ultrasonography; WBCs, white blood cells. a Data are presented as number (%). Data were calculated on patients for whom the information was available. Percentages do not total 100% owing to missing data. b Patients with black ethnicity include African-American, Black African, African, and Afro-Caribbean patients.
Patients were stratified based on treatment outcome (survival or death). A summary of the characteristics and clinical presentation of COVID-19-infected PPHN cases that were found to be statistically significant with regards to final treatment outcome in 36 neonates who had either survived (n = 30) or died (n = 6) is shown in Table 2. Neonates in the PPHN group who had COVID-19 and died had higher rates of cardiorespiratory failure (0 vs. 33.3%) and pneumonia as a cause of PPHN (0 vs. 33.3%) compared to the neonates in the PPHN group who had COVID-19 and survived (p values = 0.047). Neonates with PPHN who had COVID-19 and survived received a significantly higher proportion of sildenafil (16.7% vs. 66.7%; p = 0.037), epinephrine (6.7% vs. 33.3%; p = 0.03), vasopressors (3.3 vs. 33.3%; p = 0.047), endothelin receptor antagonists (0 vs. 33.3%; p = 0.047), and antibiotics (50% vs. 83.3%; p = 0.048). Neonates with PPHN who had COVID-19 and died had a longer use of inhaled nitric oxide [need of iNO for 7 to <14 days: 3.3% vs. 33.3% and need of iNO for ≥14 days: 0% vs. 16.7%; p = 0.034]. Neonates with PPHN who had COVID-19 and died had a significantly higher percentage of SARS-CoV-2 infection with critical severity (10% vs. 83.3%) but a lower percentage of COVID-19 cases were found to be severe (50% vs. 16.7%); p = 0.026.
Table 2.
Characteristics and clinical presentation of COVID-19-infected PPHN cases, stratified by treatment outcome (n = 21 studies), 2020–2023.
| Variable | Findings | ||
|---|---|---|---|
| Survived (n = 30) a | Died (n = 6) a | p-Value b | |
| Neonatal comorbidities | |||
| Cardiorespiratory failure | 0 | 2 (33.3) | 0.047 * |
| PPHN etiology | |||
| Pneumonia | 0 | 2 (33.3) | 0.047 * |
| Initiated PPHN treatments | |||
| Sildenafil | 5 (16.7) | 4 (66.7) | 0.037 * |
| Epinephrine | 2 (6.7) | 2 (33.3) | 0.03 * |
| Vasopressors | 1 (3.3) | 2 (33.3) | 0.047 * |
| ERAs | 0 | 2 (33.3) | 0.047 * |
| Neonatal need of iNO (days) | |||
| <7 | 4 (13.3) | 0 | 0.034 * |
| 7 to <14 | 1 (3.3) | 2 (33.3) | |
| ≥14 | 0 | 1 (16.7) | |
| Neonatal COVID-19 severity | |||
| Severe | 15 (50) | 1 (16.7) | 0.026 * |
| Critical | 3 (10) | 5 (83.3) | |
| Neonatal treatment of SARS-CoV-2 infection | |||
| Antibiotics | 15 (50) | 5 (83.3) | 0.048 * |
Abbreviations: COVID-19, coronavirus disease 2019; ERAs, endothelin receptor antagonists; iNO, inhaled nitric oxide; PPHN, persistent pulmonary hypertension of the newborn. a Data are presented as a number (%). b Chi-square (χ2) test was used to compare between the survival and death groups. Percentages do not total 100% owing to missing data. * Represents significant differences.
Potential determining variables associated in survival and death groups were analyzed through relative risk analysis, as shown in Table 3. The neonates who received a higher proportion of antibiotics (RR 4.14, 95% CI 0.64–6.88), neonates who had a worse severity of COVID-19 defined as critical (RR 2.84, 95% CI 0.86–9.39), neonates of the male gender (RR 2.60, 95% CI 0.30–1.17), or neonates who had a longer duration of invasive positive pressure ventilation use (RR 2.22, 95% CI 0.64–7.73) were associated with an increased relative risk for death. The relative risk of death was also high in PPHN patients infected with SARS-CoV-2 who had steroids (RR 1.87, 95% CI 0.74–4.74), NRDS as a cause of PPHN (RR 1.78, 95% CI 0.69–4.52), sildenafil to treat PPHN (RR 1.70, 95% 0.83–3.46), IVIG to treat COVID-19 (RR 1.67, 95% 0.65–4.29), and high NT-proBNP (RR 1.63, 95% CI 0.79–3.33).
Table 3.
Relative risk for mortality among of COVID-19-infected PPHN cases (n = 21 studies), 2020–2023.
| Variable | Survived (n = 30) | Died (n = 6) | Relative Risk | 95% CIs |
|---|---|---|---|---|
| Gender (male) | 9 (30) | 3 (50) | 2.60 | 0.30–1.17 |
| Neonatal comorbidities (hypotension) | 3 (10) | 1 (16.7) | 1.23 | 0.82–1.86 |
| Neonatal comorbidities (sepsis) | 1 (3.3) | 2 (33.3) | 1.28 | 0.85–1.93 |
| PPHN etiology (NRDS) | 13 (43.3) | 2 (33.3) | 1.78 | 0.69–4.52 |
| PPHN treatments (sildenafil) | 3 (10) | 3 (50) | 1.70 | 0.83–3.46 |
| PPHN treatments (epinephrine) | 3 (10) | 1 (16.7) | 1.54 | 0.89–2.64 |
| PPHN treatments (HFV) | 7 (23.3) | 3 (50) | 1.55 | 0.75–3.20 |
| PPHN treatments (IPPV) | 14 (46.7) | 4 (66.7) | 2.22 | 0.64–7.73 |
| PPHN treatments (dobutamine) | 2 (6.7) | 2 (33.3) | 1.23 | 0.81–1.87 |
| PPHN treatments (surfactant) | 6 (20) | 3 (50) | 1.24 | 0.70–2.20 |
| Neonatal COVID-19 severity (critical) | 3 (10) | 5 (83.3) | 2.84 | 0.86–9.39 |
| Neonatal COVID-19 symptoms (fever) | 7 (23.3) | 2 (33.3) | 1.24 | 0.70–2.20 |
| Neonatal abnormal laboratory findings (high NT-proBNP) | 7 (23.3) | 3 (50) | 1.63 | 0.79–3.33 |
| Neonatal abnormal laboratory findings (high troponin-I) | 5 (16.7) | 3 (50) | 1.30 | 0.74–2.29 |
| Neonatal abnormal laboratory findings (high ferritin) | 5 (16.7) | 2 (33.3) | 1.36 | 0.77–2.38 |
| Neonatal abnormal radiological findings (CXR GGO) | 2 (6.7) | 2 (33.3) | 1.28 | 0.85–1.92 |
| Neonatal treatment of SARS-CoV-2 infection (IVIG) | 11 (36.7) | 4 (66.7) | 1.67 | 0.65–4.29 |
| Neonatal treatment of SARS-CoV-2 infection (steroids) | 12 (40) | 3 (50) | 1.87 | 0.74–4.74 |
| Neonatal treatment of SARS-CoV-2 infection (antibiotics) | 15 (50) | 5 (83.3) | 4.14 | 0.64–6.88 |
Abbreviation: CIs, confidence intervals; CXR, chest X-rays; GGO, ground glass opacity; HFV, high frequency ventilation; IPPV, invasive positive pressure ventilation; NRDS, neonatal respiratory distress syndrome; NT-proBNP, N-terminal pro b-type natriuretic peptide; PPHN, persistent pulmonary hypertension of the newborn.
4. Discussion
This review analyzed 36 neonates with PPHN caused by various risk factors and etiologies who also developed COVID-19. It utilized data from 21 observational studies to offer insights into the clinical progression and management outcomes. To the best of our knowledge, this is the largest review to report on the development of SARS-CoV-2 infection in neonates with PPHN. We found the main causes of PPHN in neonates who had COVID-19 were NRDS (41.7%), MSAF (16.7%), PPROM (11.1%), and HIE (5.5%). Most of the neonates diagnosed with PPHN who developed COVID-19 were ≤1-day-old (18/36, 50%), male (12/36, 33.3%), Indian ethnicity (18/36, 50%), and delivered via caesarean section (16/36, 44.4%).
COVID-19 in neonates with PPHN commonly occurred in moderate to late preterm (gestational age 32 to <37 weeks) (36.1%) or term newborns (gestational age ≥37 weeks) (25%) and neonates delivered with a normal birthweight (≥2500 g) (27.8%) or low birthweight (≥1500–2499) (25%). These findings are consistent with previous observations that shown PPHN is rare in very low birthweight neonates (birthweight < 1500 g) [34] and that the male sex is associated with an increased risk of PPHN [35,36], and usually occurs in a significant number of moderate to late preterm (gestational age 32 to <37 weeks) [37] or term (gestational age ≥ 37 weeks) neonates [35,38] or after a caesarean-section delivery [35,36]. Our results extend the findings that PPHN is infrequent in very preterm newborns (<32 weeks) [39,40] or those delivered with a low birthweight (<1500 g) [34,40]. On the contrary, PPHN was reported to be common and highly diagnosed in very preterm or low birthweight neonates [41,42,43]. The observed low rates of PPHN in very preterm newborns may be explained by the frequent use of iNO, sildenafil, and steroids [44,45,46], in addition to the low rate of refractory pulmonary hypertension to supportive cardiorespiratory care and conventional therapies [47,48,49]. A gender difference is identified in our study and more male neonates developed PPHN with COVID-19. This may be explained by the sexual dimorphism that favors structural and functional lung maturation in female neonates [50], or higher prevalence of pulmonary diseases [51,52], and increased risk for respiratory complications in male neonates [53]. However, our findings are inconsistent with previous studies that found that the prevalence of PPHN among White (Caucasian) and Black neonates was higher than other ethnicities such as Asians or Indians [54,55]. Differences in the incidence of PPHN in neonates infected with SARS-CoV-2 by ethnicity could be attributed mainly to the differences in the echocardiographic criteria used to diagnose PPHN, study design, setting, and population characteristics, or partly be explained by low socioeconomic status [56], difference in access to health care, lack of accessibility to medical treatment [57], difficulties in social distancing or being part of multigenerational or multi-family households [58], or a lack of awareness among parents when they present with symptoms of COVID-19 leading them to seek medical attention [59].
Our findings represent the most recent evidence on the co-existence of PPHN and COVID-19 along with their association with various clinical outcomes in one study. The results of our systematic review showed that PPHN in neonates who had COVID-19 was associated with increased mortality (16.7%), severity [SARS-CoV-2 infection was severe (44.4%) or critical (22.2%)], ARDS (58.3%), MIS-N (58.3%), ICU admission (58.3%), and MV usage (47.2%). It is necessary to point out that although most cases of COVID-19 in the neonatal population were asymptomatic or mild and had good prognosis [60], the overall rate of ICU admission and mortality rate we report in neonates who had PPHN and COVID-19 suggests that the risk of severe disease and mortality from SARS-CoV-2 infection is very much higher in neonates with PPHN compared to neonates without PPHN. For example, COVID-19-related ICU admission among neonates aged 11 days (IQR, 1 to 22) without PPHN who had various medical comorbidities was low (61/918, 6.6%) and the COVID-19-related death rate was reported in one neonate only from all cases with presentation suggestive of MIS-N (1/918, 0.1%) [61]. Nevertheless, our findings should be interpreted cautiously due to the small number of studies and substantial heterogeneity; thus, further research in this area is warranted.
Our findings align with some studies that found that mother-to-neonate transmission of SARS-CoV-2 is of clinical significance [62,63] and pregnant women infected with COVID-19 are at increased risk of adverse obstetrical outcomes, compared with the general population [64,65]. Among the COVID-19 positive mothers, there were cases of preeclampsia, PPROM, abortion, and death [66,67,68]. In neonates with PPHN, the SARS-CoV-2 infection outcome was associated with a relatively higher rate of ICU admission (58.3%), antibiotics use (55.5%), need of ventilatory support (47.2%), C-section delivery (44.4%), preterm birth (44.4%), neonatal respiratory distress (41.7%), and perinatal death (16.7%). Therefore, regular screening for COVID-19, early identification of infection in asymptomatic pregnant women, and safe practices to avoid acquiring an infection during early gestational ages, may be associated with more favorable pregnancy outcomes [69,70]. Mothers and their neonates should be isolated to prevent neonatal transmission, with effective protection measures during and after delivery to prevent SARS-CoV-2 transmission [71,72]. Testing for viral pathogens, including SARS-CoV-2, may be necessary for newborns showing COVID-19 symptoms, especially those with a history of exposure. Gathering illness histories from pregnant mothers, household members, and contacts is crucial to identify newborns at risk for SARS-CoV-2 infection.
In our review, neonates diagnosed with PPHN who had COVID-19 showed a positive serology for IgG SARS-CoV-2 antibodies in 50% of cases and IgM in 2.8% cases. In terms of maternal serology, some women were positive for IgG (16.7%). With the upscaling of COVID-19 vaccination in pregnancy, the possibility of maternal IgG tests being positive following vaccination needs to be considered. Only three studies reported on the vaccination status against SARS-CoV-2 for mothers who delivered PPHN patients infected with COVID-19, of which none of the mothers had received a vaccination. Therefore, it is important to document the maternal vaccination history to prevent a misdiagnosis of MIS-N [21]. Most published PPHN cases with SARS-CoV-2 reported early MIS-N diagnoses in the neonatal period. These cases are likely secondary to maternal infection during pregnancy, causing a fetal inflammatory response from transferred maternal antibodies or transplacental virus transfer [31], leading to an altered endogenous immune response [13]. Another possibility is immune dysregulation from postnatal infection [73]. To manage MIS-N caused by COVID-19 in PPHN neonates, a high proportion of patients were treated with IVIG (41.7%) and steroids (38.9%), as they have a basis in the immune-mediated hyperinflammatory pathogenesis [74,75]. A significant proportion of neonates with MIS-N received aspirin (19.4%) or anticoagulants (8.3%). Aspirin is recommended in all mild MIS-N cases to improve platelet count and prevent thrombosis, particularly with coronary artery aneurysms. Heparin is advised for moderate to severe MIS-N cases with elevated D-dimers and a low ejection fraction to prevent thrombosis [76].
We found that the mortality rate in PPHN neonates infected with COVID-19 was significantly high in patients who received sildenafil or antibiotics or neonates who had a critical severity of COVID-19. Mortality due to sildenafil in PPHN neonates infected with SARS-CoV-2 is unlikely and may be attributable to the underlying pulmonary vascular disease instead of sildenafil use [77]. Because of the overlapping clinical signs and symptoms between COVID-19 and sepsis, a high percentage of the PPHN neonates was treated with large-spectrum antibiotics (55.5%). Taking into consideration the high need for ICU admission in neonates with MIS-N (58.3%) and given that an antibiotic treatment is often guaranteed in younger infants, the length of antibiotic therapy should be judiciously evaluated in order to avoid the spread of multi-drug resistant organisms [78]. It should be noted that the difference in mortality due to PPHN based on the severity of COVID-19 might be attributed mainly to the differences in the severity of the PPHN illness and/or inclusion criteria, or the level of health care infrastructure and general care-seeking practices in low- and middle-income countries. Mortality in COVID-19 cases with PPHN included in our review might have been complicated by multi-inflammatory systemic infection in children (i.e., cytokine storm), and the patients died due to the fact of subsequent multiple organ failure induced by the viral invasion [79]. Nonetheless, PPHN is not common and has been estimated to occur in approximately two cases per one-thousand live births; however, a serious disorder of postnatal transition accounts for a significant proportion of admissions to tertiary neonatal ICUs [37]. Neonates with PPHN often require advanced cardiorespiratory support and are at significant risk of mortality and morbidities [80]. Timely diagnosis and meticulous management lead to reduced morbidities and improved clinical outcomes [81].
Strengths and Limitations
To the best of our knowledge, this is the first systematic review conducted to review the available literature on clinical characteristics and outcomes of SARS-CoV-2 infection in neonates with PPHN. Six authors independently conducted screening, data extraction, and quality assessments to reduce selection and reviewer biases.
We recognize that our study had some limitations. First, our review is limited to published case reports of SARS-CoV-2 infection in neonates with PPHN, with most studies being retrospective. This design may introduce reporting bias due to reliance on clinical records. Second, more severe cases with worse outcomes are more likely to be published, while asymptomatic or mildly ill neonates with PPHN and COVID-19 that did not require hospitalization are underrepresented in the literature. Consequently, treatment outcomes like hospitalization, ICU admission, oxygen requirement, and death are likely overestimated. Third, a direct comparison between studies was limited, and a meta-analysis was not performed due to clinical and methodological heterogeneity, such as patients’ varying medical comorbidities and causes of PPHN. Last, we only focused on the studies that were in the English language.
5. Conclusions
COVID-19 in neonates with PPHN is challenging and maybe associated with increased mortality, severity, ICU admission, ARDS, MIS-N, and MV usage. The results should be interpreted with caution owing to the small number of studies and substantial heterogeneity and indicate a need for future research in this area. Due to its benefits, testing for SARS-CoV-2 should be encouraged for newborns with symptoms consistent with COVID-19, especially in neonates with a history of SARS-CoV-2 exposure. Effective protection measures should be implemented during delivery and post-delivery care as necessary.
Abbreviations
hypoxic ischemic encephalopathy; MIS-N, multisystem inflammatory syndrome in neonates; MSAF, meconium-stained amniotic fluid; NEC, necrotizing enterocolitis; NRDS, neonatal respiratory distress syndrome; PPHN, persistent pulmonary hypertension of the newborn; PPROM, preterm premature rupture of membranes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children11111305/s1, Table S1: Database-specific search syntax; Table S2: Characteristics of the included studies and main outcome measures in neonates with persistent pulmonary hypertension of the newborn (PPHN) and coronavirus disease 2019 (COVID-19) (n = 21 studies), 2020–2023; Table S3: Clinical features in neonates with documented persistent pulmonary hypertension of the newborn (PPHN) and coronavirus disease 2019 (COVID-19) (n = 21 studies), 2020–2023; and Table S4: The PRISMA 2020 checklist.
Author Contributions
S.A., A.A.A., M.A. (Muneera Alabdulqader), Z.A.A. and M.A.A.G. (Mohammed A. Al Ghamdi) contributed equally to the systematic review. S.A., A.A.A., M.A. (Muneera Alabdulqader) and Z.A.A. were the core team leading the systematic review. S.A., M.A. (Muneera Alabdulqader), Z.A.A., M.A.A. (Mohammed A Alabdulmuhsin), H.I.A.H. and H.A.A. (Hussain Ahmed Alsouaib) identified and selected the studies. H.A.A. (Hussain Ali Alhassan), H.A.-H., S.A.A., M.J.A., H.R.A.-T. and A.R.A.-M. performed the quality assessment of the studies. S.A., A.A.A., M.A. (Muneera Alabdulqader), Z.A.A., T.T.A., M.A.A. (Mohammad Abdullah Alkhwaitem), M.A. (Murtadha Alsuliman), A.N.B. and K.K.A. collected the data. S.A., A.A.A., M.A. (Muneera Alabdulqader), Z.A.A., M.A.A.G. (Mohammed A. Al Ghamdi), A.S.A.M., M.A.A. (Mariam Ali Almajhad) and A.H.A. drafted the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Steurer M.A., Baer R.J., Oltman S., Ryckman K.K., Feuer S.K., Rogers E., Keller R.L., Jelliffe-Pawlowski L.L. Morbidity of persistent pulmonary hypertension of the newborn in the first year of life. J. Pediatr. 2019;213:58–65.e4. doi: 10.1016/j.jpeds.2019.06.053. [DOI] [PubMed] [Google Scholar]
- 2.Roofthooft M., Elema A., Bergman K., Berger R. Patient characteristics in persistent pulmonary hypertension of the newborn. Pulm. Med. 2011;2011:858154. doi: 10.1155/2011/858154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakwan N., Jain S., Kumar K., Hosono S., Hammoud M., Elsayed Y.Y., Ariff S., Hasan B., Khowaja W., Poon W.B. An Asian multicenter retrospective study on persistent pulmonary hypertension of the newborn: Incidence, etiology, diagnosis, treatment and outcome. J. Matern.-Fetal Neonatal Med. 2020;33:2032–2037. doi: 10.1080/14767058.2018.1536740. [DOI] [PubMed] [Google Scholar]
- 4.Alhumaid S., Alnaim A.A., Al Ghamdi M.A., Alahmari A.A., Alabdulqader M., Al HajjiMohammed S.M., Alalwan Q.M., Al Dossary N., Alghazal H.A., Al Hassan M.H. International treatment outcomes of neonates on extracorporeal membrane oxygenation (ECMO) with persistent pulmonary hypertension of the newborn (PPHN): A systematic review. J. Cardiothorac. Surg. 2024;19:493. doi: 10.1186/s13019-024-03011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer M.L. A neonate with vertical transmission of COVID-19 and acute respiratory failure: A case report. Adv. Neonatal Care. 2021;21:482–492. doi: 10.1097/ANC.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri M., Tomar M., Gaonkar S., Rastogi A., Shenoi A. Pilot study analyzing combination of point-of-care echocardiography and clinical correlation in unveiling cryptic multi-inflammatory syndrome in neonates during coronavirus disease 2019 pandemic. J. Indian Acad. Echocardiogr. Cardiovasc. Imaging. 2022;6:89–99. doi: 10.4103/jiae.jiae_64_21. [DOI] [Google Scholar]
- 7.Ishqeir A., Nir A., Aptowitzer I., Godfrey M., Pediatric Cardiology Unit, Shaare Zedek Medical Center, Jerusalem, Israel Increased incidence of Persistent Pulmonary Hypertension of the Newborn following third trimester maternal COVID-19 infection. Eur. Heart J. 2021;42:ehab724.1843. doi: 10.1093/eurheartj/ehab724.1843. [DOI] [Google Scholar]
- 8.Tomar M., Chaudhuri M., Goel T., Agarwal V., Bidhan S., Jain A., Rastogi A., Saxena V., Tomar H.S. Profile of cardiac involvement in children after exposure to COVID-19. Indian Pediatr. 2023;60:385–388. doi: 10.1007/s13312-023-2886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamali Z., Sinaei R., Razi L. Multisystem inflammatory syndrome in a newborn (MIS-N): Clinical evidence and neurodevelopmental outcome. Curr. Pediatr. Rev. 2023;19:210–212. doi: 10.2174/1573396318666220806143047. [DOI] [PubMed] [Google Scholar]
- 10.Algadeeb K.B., AlMousa H.H., AlKadhem S.M., Alduhilan M.O., II, Almatawah Y. A novel case of severe respiratory symptoms and persistent pulmonary hypertension in a Saudi neonate with SARS-CoV-2 infection. Cureus. 2020;12:e10472. doi: 10.7759/cureus.10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ergon E.Y., Akbay S., Aytemiz G., Çelik E.C.A., Çalıskan Polat A., Umit Z., Paytoncu S. A novel case of neonatal acute respiratory distress syndrome with SARS-CoV-2 infection: Potential perinatal transmission. Arch. Argent. De Pediatr. 2021;119:e531–e535. doi: 10.5546/aap.2021.eng.e531. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves-Ferri W.A., Carvalheiro C.G., Mussi-Pinhata M.M., Cavasin B.P.D., Fonseca B.A.L.d. Gamma variant vertically transmitted from a mild symptomatic pregnant woman associated with fatal neonatal COVID. Braz. J. Infect. Dis. 2022;26:102385. doi: 10.1016/j.bjid.2022.102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balleda L., Pasupula S., Kolla S., Thimmapuram C.R. Clinical profile, laboratory parameters, management and outcomes of newborns with multisystem inflammatory syndrome (mis-n) due to transplacental transfer of SARS-CoV 2 antibodies: A study from a tertiary care institute. J. Clin. Neonatol. 2022;11:65–70. doi: 10.4103/jcn.jcn_1_22. [DOI] [Google Scholar]
- 14.Gupta P., Tamatam P.R., Dhulipudi B., Vardhelli V., Deshabhotla S., Oleti T.P. Neonatal multisystem inflammatory syndrome (MIS-N) associated with maternal SARS-CoV-2 exposure. Indian J. Pediatr. 2022;89:827–828. doi: 10.1007/s12098-022-04167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.More K., Aiyer S., Goti A., Parikh M., Sheikh S., Patel G., Kallem V., Soni R., Kumar P. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: A case series. Eur. J. Pediatr. 2022;181:1883–1898. doi: 10.1007/s00431-022-04377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health COVID-19 Treatment Guidelines. Clinical Spectrum of SARS-CoV-2 Infection 2024. [(accessed on 30 January 2024)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf.
- 18.World Health Organization Definition and Categorization of the Timing of Mother-to-Child Transmission of SARS-CoV-2. Scientific Brief 2021. [(accessed on 30 January 2024)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1.
- 19.United States Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome (MIS). Case Definitions and Reporting. MIS-C Case Definition. [(accessed on 30 January 2024)]; Available online: https://www.cdc.gov/mis/hcp/case-definition-reporting/index.html.
- 20.Molloy E.J., Nakra N., Gale C., Dimitriades V.R., Lakshminrusimha S. Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: Optimizing definition and management. Pediatr. Res. 2023;93:1499–1508. doi: 10.1038/s41390-022-02263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascarenhas D., Goyal M., Haribalakrishna A., Nanavati R., Ish P., Kunal S. Multisystem inflammatory syndrome in neonates (MIS-N): A systematic review. Eur. J. Pediatr. 2023;182:2283–2298. doi: 10.1007/s00431-023-04906-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazerbachi F., Sawas T., Vargas E.J., Prokop L.J., Chari S.T., Gleeson F.C., Levy M.J., Martin J., Petersen B.T., Pearson R.K. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: A systematic review and meta-analysis. Gastrointest. Endosc. 2018;87:30–42.e15. doi: 10.1016/j.gie.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott. Ott. Hosp. Res. Inst. 2011;2:1–12. [Google Scholar]
- 24.Borkotoky R.K., Barua P.B., Paul S.P., Heaton P.A. COVID-19-related potential multisystem inflammatory syndrome in childhood in a neonate presenting as persistent pulmonary hypertension of the newborn. Pediatr. Infect. Dis. J. 2021;40:e162–e164. doi: 10.1097/INF.0000000000003054. [DOI] [PubMed] [Google Scholar]
- 25.Easterlin M.C., De Beritto T., Yeh A.M., Wertheimer F.B., Ramanathan R. Extremely preterm infant born to a mother with severe COVID-19 pneumonia. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620946621. doi: 10.1177/2324709620946621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi S., Quinones Cardona V., Menkiti O.R. Use of vasopressin in persistent pulmonary hypertension of the newborn: A case series. SAGE Open Med. Case Rep. 2022;10:2050313X221102289. doi: 10.1177/2050313X221102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalani-Moghaddam F., Pouralizadeh N., Pourdowlat G., Sarfarazi-Moghaddam S., Gharib M.H., Pakdel M. The youngest surviving COVID-19 patient: A case report. Int. J. Surg. Case Rep. 2022;94:107065. doi: 10.1016/j.ijscr.2022.107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malek A., Khadga M., Zahid M.N., Mojib S., Debnath R., Khan S., Haque M., Godman B., Islam S., Khan S. Multisystem inflammatory syndrome of a neonate from a COVID-19-infected mother: A case report. Cureus. 2022;14:e23046. doi: 10.7759/cureus.23046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarty K.L., Tucker M., Lee G., Pandey V. Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection. Pediatrics. 2021;147:e2020010132. doi: 10.1542/peds.2020-010132. [DOI] [PubMed] [Google Scholar]
- 30.Moore S.S., Altit G. Detection of Pulmonary Hypertension in an Infant with Covid Related Chronic Lung Disease. [(accessed on 30 January 2024)]. Available online: https://neonatalhemodynamics.com/wp-content/uploads/2022/03/MooreCaseNHRC_MAR2022.pdf.
- 31.Pawar R., Gavade V., Patil N., Mali V., Girwalkar A., Tarkasband V., Loya S., Chavan A., Nanivadekar N., Shinde R. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: A case series. Children. 2021;8:572. doi: 10.3390/children8070572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoenmakers S., Snijder P., Verdijk R.M., Kuiken T., Kamphuis S.S., Koopman L.P., Krasemann T.B., Rousian M., Broekhuizen M., Steegers E.A. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J. Pediatr. Infect. Dis. Soc. 2021;10:556–561. doi: 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaiba L.A., Hadid A., Altirkawi K.A., Bakheet H.M., Alherz A.M., Hussain S.A., Sobaih B.H., Alnemri A.M., Almaghrabi R., Ahmed M. Case report: Neonatal multi-system inflammatory syndrome associated with SARS-CoV-2 exposure in two cases from Saudi Arabia. Front. Pediatr. 2021;9:652857. doi: 10.3389/fped.2021.652857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aikio O., Metsola J., Vuolteenaho R., Perhomaa M., Hallman M. Transient defect in nitric oxide generation after rupture of fetal membranes and responsiveness to inhaled nitric oxide in very preterm infants with hypoxic respiratory failure. J. Pediatr. 2012;161:397–403.e1. doi: 10.1016/j.jpeds.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Steinhorn R.H. Advances in neonatal pulmonary hypertension. Neonatology. 2016;109:334–344. doi: 10.1159/000444895. [DOI] [PubMed] [Google Scholar]
- 36.Razzaq A., Quddusi A.I., Nizami N. Risk factors and mortality among newborns with persistent pulmonary hypertension. Pak. J. Med. Sci. 2013;29:1099. doi: 10.12669/pjms.295.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh-Sukys M.C., Tyson J.E., Wright L.L., Bauer C.R., Korones S.B., Stevenson D.K., Verter J., Stoll B.J., Lemons J.A., Papile L.-A. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 38.Teng R.-J., Wu T.-J. Persistent pulmonary hypertension of the newborn. J. Formos. Med. Assoc. 2013;112:177–184. doi: 10.1016/j.jfma.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirza H., Ziegler J., Ford S., Padbury J., Tucker R., Laptook A. Pulmonary hypertension in preterm infants: Prevalence and association with bronchopulmonary dysplasia. J. Pediatr. 2014;165:909–914.e1. doi: 10.1016/j.jpeds.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Buffoni I., Buratti S., Mallamaci M.F., Pezzato S., Lampugnani E., Buffelli F., Fulcheri E., Moscatelli A. Sudden Onset of Severe Pulmonary Hypertension in a Preterm Infant: A Case Report on the Role of Maternal Use of Serotonin Re-Uptake Inhibitors During Pregnancy and Concurrent Risk Factors. Front. Pediatr. 2022;10:855419. doi: 10.3389/fped.2022.855419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mourani P.M., Sontag M.K., Younoszai A., Miller J.I., Kinsella J.P., Baker C.D., Poindexter B.B., Ingram D.A., Abman S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seth S.A., Soraisham A.S., Harabor A. Risk factors and outcomes of early pulmonary hypertension in preterm infants. J. Matern.-Fetal Neonatal Med. 2018;31:3147–3152. doi: 10.1080/14767058.2017.1365129. [DOI] [PubMed] [Google Scholar]
- 43.Berenz A., Vergales J.E., Swanson J.R., Sinkin R.A. Evidence of early pulmonary hypertension is associated with increased mortality in very low birth weight infants. Am. J. Perinatol. 2017;34:801–807. doi: 10.1055/s-0037-1598246. [DOI] [PubMed] [Google Scholar]
- 44.Soraisham A.S., Harabor A., Shivananda S., Alvaro R., Xiang Y.Y., Lee S.K., Shah P.S., Network C.N. Trends and variations in the use of inhaled nitric oxide in preterm infants in Canadian neonatal intensive care units. Am. J. Perinatol. 2016;33:715–722. doi: 10.1055/s-0036-1571329. [DOI] [PubMed] [Google Scholar]
- 45.Koenders V., Appels A., van Straaten H., Dutman A., Hemels M. Postnatal corticosteroid response in neonates <32 weeks and relation with placental pathology. Eur. J. Pediatr. 2023;182:265–274. doi: 10.1007/s00431-022-04672-9. [DOI] [PubMed] [Google Scholar]
- 46.Baczynski M., Ginty S., Weisz D.E., McNamara P.J., Kelly E., Shah P., Jain A. Short-term and long-term outcomes of preterm neonates with acute severe pulmonary hypertension following rescue treatment with inhaled nitric oxide. Arch. Dis. Child.-Fetal Neonatal Ed. 2017;102:F508–F514. doi: 10.1136/archdischild-2016-312409. [DOI] [PubMed] [Google Scholar]
- 47.Steiner M., Salzer U., Baumgartner S., Waldhoer T., Klebermass-Schrehof K., Wald M., Langgartner M., Berger A. Intravenous sildenafil i. v. as rescue treatment for refractory pulmonary hypertension in extremely preterm infants. Klin. Pädiatrie. 2014;226:211–215. doi: 10.1055/s-0034-1375697. [DOI] [PubMed] [Google Scholar]
- 48.Kahveci H., Yilmaz O., Avsar U.Z., Ciftel M., Kilic O., Laloglu F., Ozturk K. Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn. Pediatr. Pulmonol. 2014;49:1205–1213. doi: 10.1002/ppul.22985. [DOI] [PubMed] [Google Scholar]
- 49.Lakshminrusimha S., Konduri G., Steinhorn R. Considerations in the management of hypoxemic respiratory failure and persistent pulmonary hypertension in term and late preterm neonates. J. Perinatol. 2016;36:S12–S19. doi: 10.1038/jp.2016.44. [DOI] [PubMed] [Google Scholar]
- 50.Gortner L., Shen J., Tutdibi E. Sexual dimorphism of neonatal lung development. Klin. Pädiatrie. 2013;225:64–69. doi: 10.1055/s-0033-1333758. [DOI] [PubMed] [Google Scholar]
- 51.Yarci E., Canpolat F.E. Evaluation of morbidities and complications of neonatal intensive care unit patients with respiratory disorders at different gestational ages. Am. J. Perinatol. 2022;29:1533–1540. doi: 10.1055/s-0041-1722942. [DOI] [PubMed] [Google Scholar]
- 52.Hillman N.H., Lam H.S. Kendig’s Disorders of the Respiratory Tract in Children. Elsevier; Amsterdam, The Netherlands: 2019. Respiratory disorders in the newborn; pp. 338–366.e6. [Google Scholar]
- 53.Su Z., Lin L., Fan X., Jia C., Huang X., Wei J., Wu F. Increased risk for respiratory complications in male extremely preterm infants: A propensity score matching study. Front. Endocrinol. 2022;13:823707. doi: 10.3389/fendo.2022.823707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janevic T., Zeitlin J., Auger N., Egorova N.N., Hebert P., Balbierz A., Howell E.A. Association of race/ethnicity with very preterm neonatal morbidities. JAMA Pediatr. 2018;172:1061–1069. doi: 10.1001/jamapediatrics.2018.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ong M.-S., Abman S., Austin E.D., Feinstein J.A., Hopper R.K., Krishnan U.S., Mullen M.P., Natter M.D., Raj J.U., Rosenzweig E.B. Racial and ethnic differences in pediatric pulmonary hypertension: An analysis of the pediatric pulmonary hypertension network registry. J. Pediatr. 2019;211:63–71.e6. doi: 10.1016/j.jpeds.2019.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gopalan H.S., Misra A. COVID-19 pandemic and challenges for socio-economic issues, healthcare and National Health Programs in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:757–759. doi: 10.1016/j.dsx.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raman R., Rajalakshmi R., Surya J., Ramakrishnan R., Sivaprasad S., Conroy D., Thethi J.P., Mohan V., Netuveli G. Impact on health and provision of healthcare services during the COVID-19 lockdown in India: A multicentre cross-sectional study. BMJ Open. 2021;11:e043590. doi: 10.1136/bmjopen-2020-043590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das M., Bhattacharyya A. Social distanciation through COVID-19: A narrative analysis of Indian Peri-Urban Elderly. Soc. Sci. Humanit. Open. 2021;4:100139. doi: 10.1016/j.ssaho.2021.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roy D., Tripathy S., Kar S.K., Sharma N., Verma S.K., Kaushal V. Study of knowledge, attitude, anxiety & perceived mental healthcare need in Indian population during COVID-19 pandemic. Asian J. Psychiatry. 2020;51:102083. doi: 10.1016/j.ajp.2020.102083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevisanuto D., Cavallin F., Cavicchiolo M.E., Borellini M., Calgaro S., Baraldi E. Coronavirus infection in neonates: A systematic review. Arch. Dis. Child.-Fetal Neonatal Ed. 2021;106:330–335. doi: 10.1136/archdischild-2020-319837. [DOI] [PubMed] [Google Scholar]
- 61.Devin J., Marano R., Mikhael M., Feaster W., Sanger T., Ehwerhemuepha L. Epidemiology of neonatal COVID-19 in the United States. Pediatrics. 2022;150:e2022056297. doi: 10.1542/peds.2022-056297. [DOI] [PubMed] [Google Scholar]
- 62.Kyle M.H., Hussain M., Saltz V., Mollicone I., Bence M., Dumitriu D. Vertical transmission and neonatal outcomes following maternal SARS-CoV-2 infection during pregnancy. Clin. Obstet. Gynecol. 2022;65:195–202. doi: 10.1097/GRF.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeganathan K., Paul A.B. Vertical transmission of SARS-CoV-2: A systematic review. Obstet. Med. 2022;15:91–98. doi: 10.1177/1753495X211038157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubey P., Reddy S.Y., Manuel S., Dwivedi A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: An updated systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:490–501. doi: 10.1016/j.ejogrb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal–fetal transmission: A systematic review and meta-analysis. Eur. J. Med. Res. 2020;25:39. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A., Ferdosian F., Bahrami R. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: A review. Fetal Pediatr. Pathol. 2020;39:246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agolli A., Agolli O., Velazco D.F.S., Ahammed M.R., Patel M., Cardona-Guzman J., Garimella R., Rummaneethorn N., Bista S., Abreu R. Fetal complications in COVID-19 infected pregnant woman: A systematic review and meta-analysis. Avicenna J. Med. 2021;11:200–209. doi: 10.1055/s-0041-1736540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lei D., Wang C., Li C., Fang C., Yang W., Chen B., Wei M., Xu X., Yang H., Wang S. Clinical characteristics of COVID-19 in pregnancy: Analysis of nine cases. Chin. J. Perinat. Med. 2020;12:159–165. [Google Scholar]
- 69.Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Roggero P., Prefumo F., Do Vale M.S., Cardona-Perez J.A. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allotey J., Fernandez S., Bonet M., Stallings E., Yap M., Kew T., Zhou D., Coomar D., Sheikh J., Lawson H. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shahbazi Sighaldeh S., Ebrahimi Kalan M. Care of newborns born to mothers with COVID-19 infection; a review of existing evidence. J. Matern.-Fetal Neonatal Med. 2022;35:2203–2215. doi: 10.1080/14767058.2020.1777969. [DOI] [PubMed] [Google Scholar]
- 72.Yeo K.T., Oei J.L., De Luca D., Schmölzer G.M., Guaran R., Palasanthiran P., Kumar K., Buonocore G., Cheong J., Owen L.S. Review of guidelines and recommendations from 17 countries highlights the challenges that clinicians face caring for neonates born to mothers with COVID-19. Acta Paediatr. 2020;109:2192–2207. doi: 10.1111/apa.15495. [DOI] [PubMed] [Google Scholar]
- 73.De Rose D.U., Pugnaloni F., Calì M., Ronci S., Caoci S., Maddaloni C., Martini L., Santisi A., Dotta A., Auriti C. Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): A systematic review. Viruses. 2022;14:750. doi: 10.3390/v14040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rauniyar R., Mishra A., Kharel S., Giri S., Rauniyar R., Yadav S., Chaudhary G. IVIG plus glucocorticoids versus IVIG alone in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: A systematic review and meta-analysis. Can. J. Infect. Dis. Med. Microbiol. 2022;2022:9458653. doi: 10.1155/2022/9458653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tagarro A., Domínguez-Rodríguez S., Mesa J.M., Epalza C., Grasa C., Iglesias-Bouzas M.I., Fernández-Cooke E., Calvo C., Villaverde S., Torres-Fernández D. Treatments for multi-system inflammatory syndrome in children—Discharge, fever, and second-line therapies. Eur. J. Pediatr. 2023;182:461–466. doi: 10.1007/s00431-022-04649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henderson L.A., Canna S.W., Friedman K.G., Gorelik M., Lapidus S.K., Bassiri H., Behrens E.M., Kernan K.F., Schulert G.S., Seo P. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022;74:e1–e20. doi: 10.1002/art.42062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He Z., Zhu S., Zhou K., Jin Y., He L., Xu W., Lao C., Liu G., Han S. Sildenafil for pulmonary hypertension in neonates: An updated systematic review and meta-analysis. Pediatr. Pulmonol. 2021;56:2399–2412. doi: 10.1002/ppul.25444. [DOI] [PubMed] [Google Scholar]
- 78.Pasero D., Cossu A.P., Terragni P. Multi-drug resistance bacterial infections in critically ill patients admitted with COVID-19. Microorganisms. 2021;9:1773. doi: 10.3390/microorganisms9081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sher E.K., Ćosović A., Džidić-Krivić A., Farhat E.K., Pinjić E., Sher F. COVID-19 a triggering factor of autoimmune and multi-inflammatory diseases. Life Sci. 2023;319:121531. doi: 10.1016/j.lfs.2023.121531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barrington K.J., Finer N., Pennaforte T., Altit G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2017;1:CD000399. doi: 10.1002/14651858.CD000399.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma V., Berkelhamer S., Lakshminrusimha S. Persistent pulmonary hypertension of the newborn. Matern. Health Neonatol. Perinatol. 2015;1:14. doi: 10.1186/s40748-015-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.