Abstract
Human MxA protein inhibits LaCrosse virus (LAC virus; family Bunyaviridae) replication in vertebrate cells and MxA-transgenic mice. LAC virus is transmitted to humans by Aedes triseriatus mosquitoes. In this report, we have shown that transfected mosquito cells expressing the human MxA cDNA are resistant to LAC virus but permissive for Sindbis virus (family Togaviridae) infection.
Expression of the human MxA gene is induced by alpha and beta interferons, often in response to viral infection. Interferons induce an antiviral state in surrounding cells. The binding of alpha and beta interferons to specific receptors activates the JAK/STAT signaling pathway, which activates ≥50 genes, including the Mx gene (15). Mx proteins belong to the dynamin superfamily of large GTPases found in yeasts, plants, and animals (4). Some Mx proteins exhibit broad-spectrum antiviral activity. For example, human MxA has been shown to inhibit replication of viruses from the families Orthomyxoviridae, Rhabdoviridae, Bunyaviridae, Paramyxoviridae, and Togaviridae (2, 4, 8).
The human MxA protein inhibits LaCrosse virus (LAC virus) in cell culture and in transgenic mice (3, 5). Alpha/beta interferon receptor knockout mice do not respond to interferons, do not express Mx protein, and are highly susceptible to viral infections despite an otherwise intact immune system. These mice permit evaluation of MxA-induced virus resistance in vivo without the involvement of other interferon-induced gene products. When these knockout mice transgenically express the human MxA cDNA, they become resistant to previously lethal virus infections (5). This suggested that expression of MxA in mosquito cells, which do not have an interferon response, would also interfere with LAC virus.
LAC virus is transmitted to humans by Aedes triseriatus mosquitoes, which serve as the vector and the reservoir host for LAC virus (9). Thus, the mosquito is a good target for interference strategies to perturb the transmission cycle of LAC virus. In these studies, we demonstrate that mosquito cells expressing the human MxA cDNA are resistant to LAC virus but not Sindbis virus (SIN virus) replication.
Mosquito cells can constitutively express MxA.
pIE1-MxA was derived from pIE1-3 (Novagen), which contains the Autographa californica baculovirus immediate-early (IE1) promoter and hr5 enhancer sequences. pIE1-MxA was constructed by PCR amplification of the ∼2-kb MxA sequence from pHMGMxA (11) using primers (5′-GGATCCGGAAGATGGTTGTTTCCG-3′ and 5′-GGATCCGGACAGAGTGTGGTTAACC-3′) containing BamHI sites flanking the primer sequence. The PCR product was cloned into a TA cloning vector (pCR2.1 TOPO; Invitrogen), excised with BamHI, and cloned into the BamHI site of pIE1-3 (Novagen).
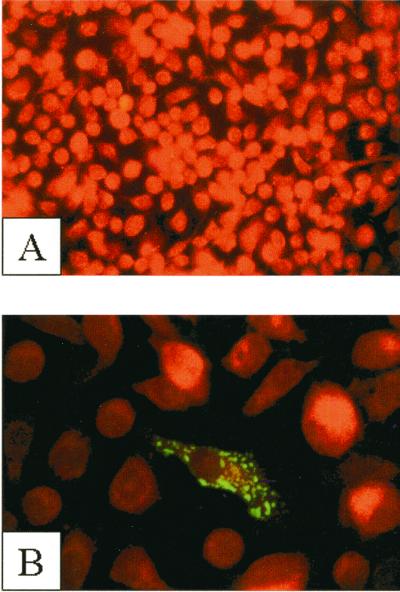
C6/36 (Aedes albopictus) cells were transfected with plasmid pIE1-MxA using Effectene reagent (Qiagen) according to the manufacturer's suggestions. Forty-eight hours posttransfection, the cells were fixed onto glass coverslips using 4% paraformaldehyde. MxA expression was analyzed by immunofluorescence assay (IFA). A mouse monoclonal antibody to MxA, 2C12 (13), was the primary antibody, and a fluorescein isothiocyanate-linked anti-mouse antibody (Kirkegaard & Perry Laboratories, Inc.) was the secondary antibody. The cells were counterstained with Evans blue. Fluorescence was detected in approximately 25% of cells. MxA-specific fluorescence was localized in the cytoplasm of transfected cells (Fig. 1B) in a punctate pattern similar to that seen in vertebrate cell lines. No MxA-specific fluorescence was seen in nontransfected C6/36 cells (Fig. 1A).
FIG. 1.
Expression of human MxA protein in mosquito cell line C6/36. pIE1-MxA-transfected cells were analyzed by IFA. (A) Untransfected C6/36 cells (magnification, ×200); (B) C6/36 cells transfected with pIE1-MxA (magnification, ×1,000).
MxA expression inhibits LAC virus replication in mosquito cells.
C6/36 cells were transfected with pIE1-MxA as described above. Twenty-four hours posttransfection, the cells were challenged with LAC virus (multiplicity of infection, 0.01). At 24 and 48 h postinfection, cells were fixed and analyzed by IFA. LAC virus antigen was detected using rabbit polyclonal hyperimmune serum, and a secondary tetramethyl rhodamine isothiocyanate (TRITC)-linked anti-rabbit antibody (Kirkegaard & Perry Laboratories, Inc.). An Olympus BH-2 fluorescent microscope with a fluorescein isothiocyanate-TRITC filter cube (Chroma Technology Corp.) was used for IFA for MxA and viral antigens. MxA-positive and MxA-negative cells in random, nonoverlapping microscope fields were analyzed for LAC virus-specific antigen. Results were statistically analyzed using two-by-two contingency tables with chi-square analysis.
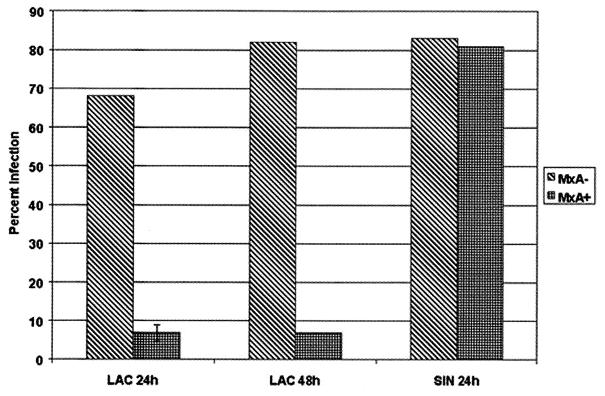
LAC virus antigen was not detected in the majority of MxA-positive cells (Fig. 2B and D). However, dual fluorescence was detected in a small number of cells (Fig. 2C). These cells appeared to have less MxA-specific fluorescence than did cells with no detectable LAC virus antigen, suggesting an Mx dose effect. No LAC virus antigen was detected in uninfected cells (Fig. 2A). Numbers of MxA-positive and MxA-negative cells with or without LAC virus antigen were analyzed (Table 1). LAC virus infection of MxA-positive cells was significantly lower than that of MxA-negative cells at 24- and 48-h time points (P < 0.0001). The mean infection rate (Fig. 3) in MxA-negative cells was 67.6%, and the mean infection rate in MxA-positive cells was 7.4%, at 24 h postinfection. These rates were 81.4 and 6.8%, respectively, at 48 h postinfection. Means were determined from three replicates for each group. Infection rates differed statistically by chi-square analysis (P < 0.0001).
FIG. 2.
Analysis of viral infection in MxA-expressing C6/36 cells. Cells that were transformed with pIE1-MxA and challenged with LAC virus or a recombinant SIN virus were analyzed by IFA. (A) MxA-negative, LAC virus-negative cells at 24 h (magnification, ×200); (B) MxA-positive, LAC virus-positive cells at 24 h (magnification, ×200); (C) MxA-positive, LAC virus-positive cells at 24 h (magnification, ×1,000); (D) MxA-positive, LAC virus-positive cells at 48 h (magnification, ×400); (E) MxA-positive, SIN virus-positive cells at 24 h (magnification, ×1,000).
TABLE 1.
Comparison of LAC virus- or SIN virus-infected cell numbers in MxA-positive and MxA-negative cell populations
| Cell population | No. of cells negative or positive for virus at time
|
|||||
|---|---|---|---|---|---|---|
| LAC virus (24 h)
|
LAC virus (48 h)
|
SIN virus (24 h)
|
||||
| − | + | − | + | − | + | |
| MxA− | 207 | 433 | 56 | 245 | 54 | 258 |
| MxA+ | 358 | 30 | 221 | 16 | 35 | 146 |
FIG. 3.
Comparison of LAC virus and SIN virus infection rates in MxA-negative and MxA-positive cell populations at 24 and 48 h postinfection. LAC virus infection rates differed statistically by chi-square analysis (P < 0.0001). SIN virus infection rates did not differ statistically by chi-square analysis.
MxA-expressing mosquito cells are susceptible to infection by SIN virus.
C6/36 cells transfected with pIE1-MxA were challenged with a recombinant Sindbis virus (SIN virus) which expressed the capsid proteins of Aedes densovirus (AeDNV; family Parvoviridae) (1). SIN virus replication was assayed using a rabbit polyclonal antibody to AeDNV and a TRITC-linked anti-rabbit secondary antibody (Kirkegaard & Perry Laboratories, Inc.). Analysis of MxA expression and dual IFA were conducted as described above. AeDNV-specific antigen was detected in a majority of cells expressing MxA. In cells expressing both MxA and AeDNV antigens, MxA-specific fluorescence was seen in the cytoplasm, while AeDNV-specific fluorescence was seen in the nucleus (Fig. 2E). There was no significant difference in the number of cells with or without AeDNV antigen in MxA-positive and MxA-negative cell populations (Table 1; P = 0.5723). The mean infection rate in MxA-negative cells was 82.7% and in MxA-positive cells was 80.7% at 24 h postinfection (Fig. 3).
MxA protein inhibited LAC virus in mosquito cells, which do not have the alpha/beta interferon pathways. There was a significant reduction in LAC virus replication in mosquito cells expressing MxA (Table 1 and Fig. 3). Similarly, the MxA-positive, alpha/beta interferon receptor knockout mice were also protected from a lethal challenge with LAC virus (5).
Most of the viruses susceptible to MxA inhibition have negative-sense RNA genomes (4); however, Semliki Forest virus (family Togaviridae), which has a positive-sense RNA genome, is inhibited by MxA (8). However, MxA-expressing mosquito cells were susceptible to challenge (Fig. 2 and 3 and Table 1) with SIN virus (family Togaviridae). Different susceptibilities to MxA have been seen with similar viruses and with the same virus in different cell types. For example, MxA inhibits measles virus in human but not in mouse cells (4). The spectrum of antiviral activity of MxA in mosquitoes needs to be determined. If it is broad, MxA expression in mosquitoes may be an effective means to combat a number of mosquito-borne viruses.
The mechanism of MxA-specific interference with LAC virus replication is currently unknown, but there is evidence that MxA interferes with transcription and replication of LAC virus RNA (3). MxA also inhibits vesicular stomatitis virus transcription (12, 14), and mouse Mx1 inhibits influenza virus transcription (7, 10). Alternatively, MxA may inhibit LAC virus replication by binding to ribonucleoprotein complexes, thereby preventing their transport and budding through Golgi membranes. MxA has been shown to bind Thogoto virus, thereby preventing its transport into the nucleus and inhibiting subsequent replication (6). It will be interesting to determine the molecular basis for the antiviral activity of MxA protein in mosquito cells.
Acknowledgments
We thank Peter Staeheli for providing the plasmid pHMG-MxA and Otto Haller for providing the 2C12 monoclonal antibody.
This work was supported by grant AI 46753 from the National Institutes of Health.
REFERENCES
- 1.Allen-Miura T M, Afanasiev B N, Olson K E, Beaty B J, Carlson J O. Packaging of AeDNV-GFP transducing virus by expression of densovirus structural proteins from a Sindbis virus expression system. Virology. 1999;257:54–61. doi: 10.1006/viro.1999.9622. [DOI] [PubMed] [Google Scholar]
- 2.Arnheiter H, Frese M, Kambadur R, Meier E, Haller O. Mx transgenic mice—animal models of health. Curr Top Microbiol Immunol. 1995;206:119–147. doi: 10.1007/978-3-642-85208-4_8. [DOI] [PubMed] [Google Scholar]
- 3.Frese M, Kochs G, Feldmann H, Hertkorn C, Haller O. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J Virol. 1996;70:915–923. doi: 10.1128/jvi.70.2.915-923.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haller O, Frese M, Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev Sci Tech Off Int Epizoot. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 5.Hefti H P, Frese M, Landis H, Paolo C D, Aguzzi A, Haller O, Pavlovic J. Human MxA protein protects mice lacking a functional alpha/beta interferon system against LaCrosse virus and other lethal viral infections. J Virol. 1999;73:6984–6991. doi: 10.1128/jvi.73.8.6984-6991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochs G, Haller O. Interferon-induced human MxA GTPase blocks import of Thogoto virus nucleocapsids. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krug R M, Shaw M, Broni B, Shapiro G, Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985;56:201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis H, Simon-Jodicke A, Kloti A, Paolo C D, Schnorr J-J, Schneider-Schaulies S, Hefti H P, Pavlovic J. Human MxA protein confers resistance to Semliki Forest virus and inhibits the amplification of a Semliki Forest virus-based replicon in the absence of viral structural proteins. J Virol. 1998;72:1516–1522. doi: 10.1128/jvi.72.2.1516-1522.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGaw M, Chandler L, Wasieloski L, Blair C, Beaty B. Effect of LaCrosse virus overwintering on Aedes triseriatus. Am J Trop Med Hyg. 1998;58:168–175. doi: 10.4269/ajtmh.1998.58.168. [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlovic J, Zurcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwemmle M, Weining K C, Richter M F, Schumacher B, Staeheli P. Vesicular stomatitis virus transcription inhibited by purified MxA protein. Virology. 1995;206:545–554. doi: 10.1016/s0042-6822(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 13.Staeheli P, Haller O. Interferon-induced human protein with homology to protein Mx of influenza virus-resistant mice. Mol Cell Biol. 1985;5:2150–2153. doi: 10.1128/mcb.5.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staeheli P, Pavlovic J. Inhibition of vesicular stomatitis virus mRNA synthesis by human MxA protein. J Virol. 1991;65:4498–4501. doi: 10.1128/jvi.65.8.4498-4501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark G R, Kerr I M, Williams B R G, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]