Abstract
Background: Vibration-Controlled Transient Elastography (VCTE) with FibroScan is a non-invasive, reliable diagnostic tool for Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD), enabling early detection and management to prevent severe liver diseases. VCTE’s ease and portability suit primary care, streamlining referrals, promoting lifestyle changes, reducing costs, and benefiting underserved communities. Methods: Studies on point-of-care VCTE were systematically reviewed, followed by meta-analysis using a random-effects model. Pooled proportions with 95% confidence intervals were reported, and heterogeneity was assessed using I2%. Results: A total of twenty studies from 14 countries, including 6159 patients, were analyzed, with three studies from France, two from the U.S., and four from China. The population had a slight male preponderance, with a mean age range of 35–73 years and a BMI range of 24.4–41.1%. The diagnostic accuracy for detecting any fibrosis (≥F1) was reported in four studies (n = 210) with an AUC of 0.74, sensitivity of 69.5%, and specificity of 70.6%. For significant fibrosis (≥F2), eight studies (n = 650) reported an AUC of 0.69, sensitivity of 81.7%, and specificity of 64.6%. Advanced fibrosis (≥F3) was evaluated in 10 studies (n = 619), with an AUC of 0.84, sensitivity of 88.1%, and specificity of 63.8%. Cirrhosis (F4) was assessed in nine studies (n = 533), with an AUC of 0.65, sensitivity of 87.5%, and specificity of 62.6%. Steatosis diagnoses across stages S1 to S3 showed increasing diagnostic accuracies, with AUCs of 0.85, 0.76, and 0.80, respectively. Probe type and BMI were significant covariates influencing diagnostic performance for both fibrosis and steatosis, while the percentage of male participants also showed significant associations. Conclusions: VCTE shows high diagnostic accuracy for fibrosis and steatosis in MASLD patients at the point of care. Future research should assess its implementation in fibroscan settings.
Keywords: point of care, MASLD, MASH, liver cirrhosis, liver transplant, Fibroscan
1. Introduction
Point-of-care tests are well established, offering benefits like real-time decision-making, cost-effectiveness, shorter hospital stays, and better patient satisfaction [1]. While liver biopsy remains the gold standard for diagnosing liver fibrosis, it has drawbacks such as invasiveness, sampling errors, and observer variability, especially at lower fibrosis stages [2]. This has led to growing interest in non-invasive diagnostic tools like Vibration-Controlled Transient Elastography (VCTE), performed by FibroScan, which is precise, quick, and suitable for point-of-care use [3]. Despite VCTE’s validation, the accuracy, yield, and adherence in point-of-care settings for Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) remain underexplored [4].
Although meta-analyses have evaluated VCTE’s accuracy in diagnosing nonalcoholic fatty liver disease (NAFLD), now reclassified as MASLD, research on its use in point-of-care settings is limited [5]. MASLD is a growing global health issue, with guidelines recommending initial blood test screenings followed by VCTE for those at risk [6]. However, the need for multiple appointments and referrals can hinder compliance [7]. While VCTE’s role in predicting liver-related events is increasingly studied, there is a lack of comparative research on its effectiveness in point-of-care settings versus traditional referrals [8]. This systematic review aims to fill that gap by evaluating point-of-care VCTE for MASLD and metabolic dysfunction-associated steatohepatitis (MASH), offering a comprehensive analysis of its diagnostic metrics.
2. Materials and Methods
2.1. Literature Search
A comprehensive literature search was conducted in Pubmed, EMBASE, and Cochrane Library with the assistance of a librarian in January 2023. The results were limited to the English language only. The following terms were searched: “Point of care tests gastroenterology, bedside tests in gastroenterology, liver stiffness measurement (LSM), transient elastography, VCTE, Fibro Scan, MASLD, MASH, fibrosis, and cirrhosis”. Additional studies were identified via a manual search for referenced studies and review articles. EndNote 20 software was used to manage the references. Duplicates were removed. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
From the initial identification of 1302 studies in the databases, 19 and 214 studies were excluded, leaving 869 for screening. Of these, 823 were further removed for not meeting the inclusion criteria or being off-topic, resulting in 46 studies remaining. After a detailed review, 31 studies were excluded for not meeting the criteria or meeting the exclusion criteria, leaving 15 studies. Additionally, 31 hand-searched articles were included, totaling 49 studies. However, 17 of these were excluded for not being point-of-care studies, leaving 32 studies for analysis.
2.2. Selection Criteria
Studies were included based on the following criteria: (1) evaluation of the performance of transient elastography in predicting fibrosis, steatosis, and/or cirrhosis in MASLD/MASH at the point of care, published in peer-reviewed journals; (2) the use of liver biopsy as the gold standard for diagnosing liver fibrosis; (3) a focus on adult populations (≥18 years); (4) reporting of estimates for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), or receiver operating characteristic (ROC) curves for diagnosing fibrosis stages and differentiating MASH from simple steatosis.
2.3. Exclusion Criteria
Studies were excluded if they (i) were not point-of-care tests, (ii) addressed a different context of use, (iii) used an alternative index test as the gold standard, (iv) had insufficient data to calculate the diagnostic accuracy estimates, (v) included pediatric patients, (vi) were not published in English, or (vii) involved patients with coexisting liver diseases (e.g., MASLD and viral hepatitis in the same patient) or other causes of liver disease.
2.4. Data Extraction and Quality Assessment
Two reviewers (TSD and AS) independently evaluated a study’s eligibility, graded the study’s quality, and extracted data from the study. Any disagreements between the reviewers were resolved with detailed discussions between them, along with a third reviewer (RS). The parameters of our literature search included the author, year of publication, country, type of study region, patient gender, age, body mass index (BMI), number of patients, ultrasound-based transient elastography, % of diabetes, % of hypertension, % hyperlipidemia, prevalence of the fibrosis stage (as well as cutoff values to identify the fibrosis and steatosis stage), details of index text, performance indices of index test (cutoff values, sensitivity, specificity, PPV, NPV, AUROC), and histological fibrosis stages. The necessary data to calculate the number of true positives, false positives, true negatives, and false negatives were extracted. The quality of the included studies was independently appraised by two reviewers (TSD and AS) using the quality assessment of diagnostic accuracy studies (QUADAS) questionnaire. This could estimate the internal and external validity of diagnostic accuracy studies used in systematic reviews (Table 1).
Table 1.
Risk of Bias.
| Study Author | Was a Consecutive or Random Sample of Patients Enrolled? | Was a Case–Control Design Avoided? | Did the Study Avoid Inappropriate Exclusions? | Were the Index Test Results (VCTE) Interpreted Without Knowledge of the Results of the Reference Standard (Liver Biopsy)? | If a Threshold Was Used, Was It Pre-Specified? | Did All Patients Receive a Reference Standard (Liver Biopsy)? | Were All Patients Included in the Analysis? | Risk of Bias for Patient Selection? High, Low, or Unclear | Risk of Bias for Result Interpretion? High, Low, or Uncertain | Risk of Bias for Interpretion of Test and Reference Test? High, Low, or Unclear |
|---|---|---|---|---|---|---|---|---|---|---|
| Boursier et al. [9] | consecutive | yes | yes | yes | yes | no, 594 underwent biopsy | yes | |||
| Siddiqui et al. [10] | consecutive | yes | yes | yes | yes | yes | yes | |||
| Lai et al. [11] | consecutive | yes | yes | yes | yes | No, 171 who had LSM ≥ 8, 71 underwent biopsy | yes | low | low | high |
| Chan et al. 2014 [12] | consecutive | yes | yes | yes | yes | yes | yes | low | low | high |
| Chan et al. 2017 [13] | consecutive | yes | yes | yes | yes | yes | yes | low | low | high |
| Jung et al. [14] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Kwok et al. [15] | consecutive | yes | yes | yes | yes | no, only patients with advanced fibrosis or cirrhosis on VCTE | yes | low | low | high |
| Sasso et al. [16] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Shen et al. [17] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Karlas et al. [18] | consecutive | yes | yes | yes | yes | yes | yes | low | high | low |
| Chon et al. [19] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Masaki [20] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Kumar et al. [21] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Friedrich-Rust et al. [22] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Caussy et al. [23] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Wong et al. [24] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Tapper et al. [25] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Bertrot et al. [26] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Lee et al. [27] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
| Liu et al. [28] | consecutive | yes | yes | yes | yes | yes | yes | low | low | low |
2.5. Target Conditions
Liver fibrosis, steatosis, and MASH were the target conditions. Liver fibrosis was defined according to the MASH Clinical Research Network’s (CRN’s) histological classification. The diagnostic accuracy of VCTE was assessed in the following dichotomized groups: F0 vs. F1–4, F0–1 vs. F2–4, F0–2 vs. F3–4, F0–3 vs. F4, and MASH vs. simple steatosis. For this review, any definition of MASH was accepted. The following index tests were assessed in this review: VCTE (FibroScan®, Echosens, Paris, France) and its application as a point-of-care test.
2.6. Quality of Evidence Assessment
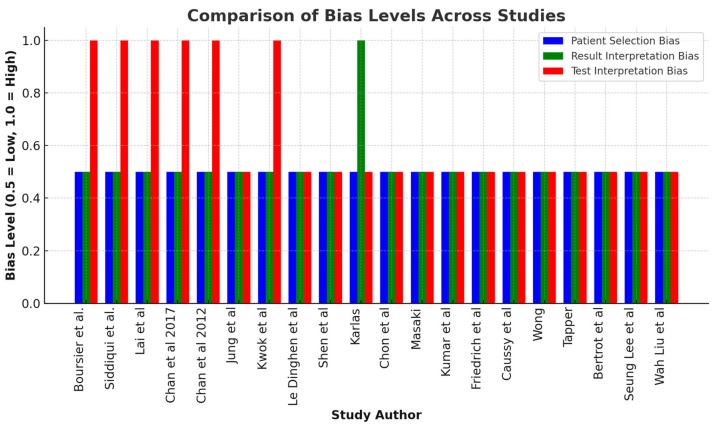
Table 1 and Figure 1 summarize the risk of bias in the studies. Most studies reported a low risk of patient selection bias, indicating representative samples with consecutive enrollment and enhancing their generalizability. However, some studies, like those by Karlas et al. [18], Boursier et al. [9], and Chan et al. [12,13], showed a high bias in VCTE result interpretation compared to liver biopsy, potentially affecting their accuracy. Additionally, studies such as Boursier et al. [9] and Lai et al. [11] introduced bias by not performing liver biopsies on all patients. Fortunately, no studies showed exclusion bias, ensuring comprehensive patient inclusion.
Figure 1.
The bar chart above visualizes the comparison of bias levels across different studies. Blue bars represent the risk of bias in patient selection, which is consistently low across all studies. Green bars indicate the risk of bias in result interpretation, with only Karlas et al. [18] showing a high level of bias in this category. Red bars represent the risk of bias in test and reference test interpretation. Several studies, including Boursier et al. [9], Siddiqui et al. [10], Lai et al. [11], and both Chan et al. [12,13] studies, exhibit high bias here, while others, like Jung et al. [14] and Wong et al. [24], show low bias [15,17,18,19,20,21,22,23,25,27,28,29].
2.7. Evaluation of Diagnostic Accuracy
Classification tables were extracted and reconstructed to assess the diagnostic performance of the index test for each predefined target condition. For binary classifications, study-specific estimates of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and both positive and negative likelihood ratios, along with their corresponding 95% confidence intervals, were computed. Forest plots were employed for a visual representation of sensitivity for each dichotomized group. Bivariate logit-normal random-effects models were used to estimate the average sensitivity, average specificity, and their corresponding variances and covariance. Summary receiver operating characteristic (sROC) curves were also generated. The I2 statistic was used to determine the heterogeneity between the studies, with significant heterogeneity defined with a value above 50% or p-value < 0.1.
2.8. Data Analysis
Covariate meta-regressions were performed to explore potential sources of heterogeneity. The degree of fibrosis, degree of steatosis, and probe type were evaluated as potential covariates for each dichotomized group. Additionally, the mean BMI and percentage of male participants were assessed as potential covariates for each study. Reitsma models were constructed, both with and without the inclusion of these covariates for each fibrosis stage group, and were compared using the likelihood ratio test statistic. All analyses were conducted using the statistical software R (Version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study Characteristics
Twenty studies from 14 different countries were included in the analysis. Three studies were from France, two from the US, and four from China. One was a cross-sectional study, while seventeen were cohort studies (Table 2).
Table 2.
Characteristics of Studies Included in the Meta-Analysis.
| Study | N | Type of Study | Location | % Male | Mean/ Median Age |
BMI | % Completed Test | % with Diabetes | % with Hyper-Tension | % with Dyslipidemia | Type of Probe | Why People Refused |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boursier et al. [9] | 1057 | multicenter cohort | France, Sweden, Spain | 62 | 55 | 76.8 | 37 | 44 | 27 | M and XL | No show | |
| Siddiqui et al. [10] | 393 | prospective | USA | 32 | 51 | 34 | not reported | 44 | 57 | |||
| Lai et al. [11] | 557 | prospective cross-sectional | Malaysia | 40.6 | 61 | 28.2 | not reported | 100 | 36.4 | 52.4 | M and XL | NA |
| Chan et al. 2014 [12] | 101 | prospective cohort | Malaysia | 51.5 | 50.3 | 29.4 | not reported | 52.5 | 88.1 | 95 | M only | |
| Chan et al. 2017 [13] | 57 | prospective cross-sectional | Malaysia, Hong Kong | 49 | 50.1 | 30.2 | not reported | M | ||||
| Jung et al. [14] | 161 | prospective | Korea | 63.4 | 49 | 24.4 | not reported | 17.4 | M | |||
| Kwok et al. [15] | 1918 | prospective cohort study | Hong Kong | 54.3 | 61 | 26.6 | 90.4 | 100 | 69.9 | 67.6 | M and XL | |
| Sasso et al. [16] | 112 | prospective study | France | 54 | 53.8 | 25.8 | not reported | 24 | 37 | M | ||
| Shen et al. [17] | 152 | multicenter prospective | China | 69.3 | 35 | 26 | not reported | M | ||||
| Karlas et al. [18] | 50 | prospective cohort study | Germany | 25 | 54.7 +/− 9.1 | 33.0 +/− 4.9 | not reported | 50 | 67 | M | ||
| Chon et al. [19] | 135 | prospective study | Korea | 64 | 51 | 24.4 | not reported | M | ||||
| Masaki et al. [20] | 150 | Japan | 61.3 | 55 | 24.4 | not reported | M | |||||
| Kumar et al. [21] | 317 | India | 73 | 37 | 25.1 +/− 2.0 | not reported | M | |||||
| Friedrich-Rust et al. [22] | 57 | Germany | 52.6 | 45 +/− 14 | 28 +/− 5.5 | not reported | M and XL | |||||
| Caussy et al. [23] | 119 | cross-section prospective | California, San Diego (UCSD) | 41.2 | 52.4 | 29.9 | 95 | X and M | No show | |||
| Wong et al. [24] | 246 | prospective cohort | France, China | 54.9 | 51 +/− 11 | 28 +/− 4.5 | not reported | 36.2 | 40.2 | M | ||
| Tapper et al. [25] | 164 | prospective cohort | USA | 91.4 (3 m) 53 (6 m) |
M | |||||||
| Bertrot et al. [26] | 271 | retrospective cohort | Australia | 40 | 52 +/− 12 | 38 +/− 8 | not reported | 49 | 45 | 27 | M and XL | |
| Lee et al. [27] | 251 | multi-center retrospective cohort | Korea | 52.6 | 44 | 28.64 | 87.2 | 46.6 | 31.1 | M and XL | ||
| Liu et al. [28] | 101 | prospective cohort study | China | 16.8 | 38.9 +/− 10.8 | 41.1 +/− 5.6 | 94.6 (1 y) 100 (2 y) 94.6 (3 y) 91.9 (4 y) 64.9 (5 y) |
48.6 | 51.4 | 43.2 | M and XL |
3.2. Patient Characteristics
In total, 6369 patients were included in the analysis (Table 2). There was a slight preponderance of males, a mean age range 35–61 years, and a BMI range of 24.4–41.1%.
3.3. Diagnosis of Any Fibrosis (F0 vs. F1–4)
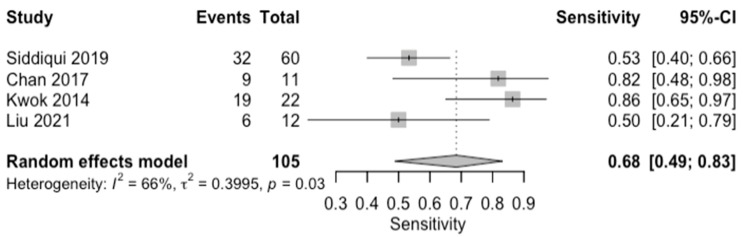
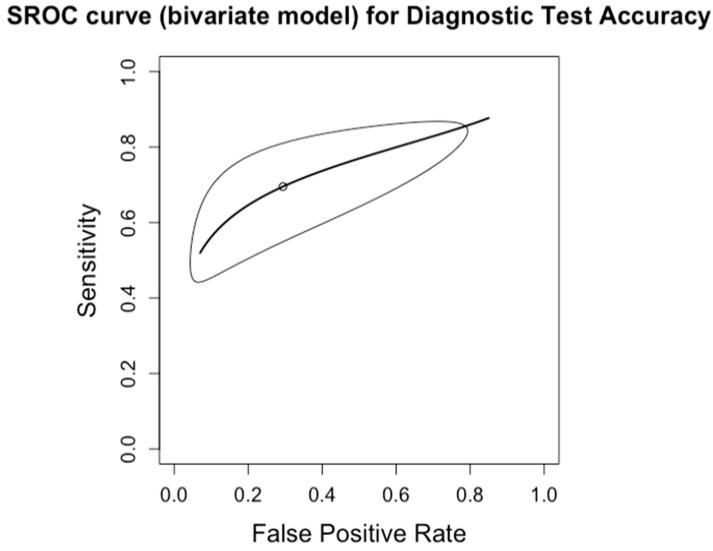
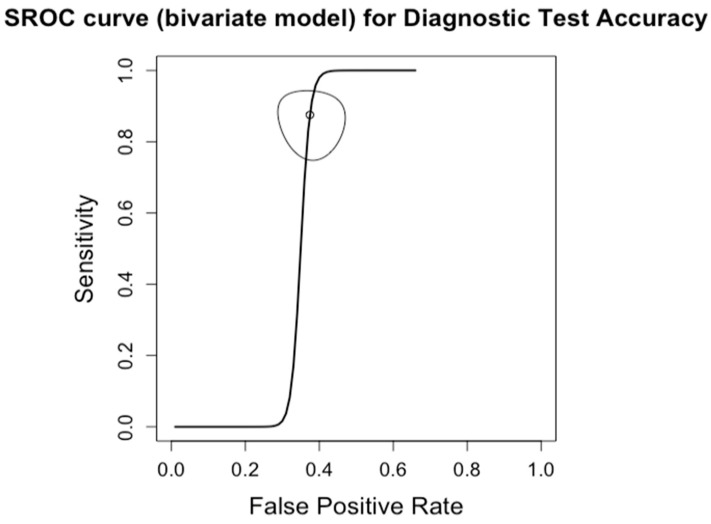
The diagnostic accuracy in detecting any degree of fibrosis (F1) was investigated by four studies (n = 210) (Table 3; Figure 2 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for a diagnosing stage F1 were as follows: 0.74, 69.5%, 70.6%, 2.93, 0.47, and 6.62. The summary point estimate of the mean is shown in Figure 3.
Table 3.
Summary Diagnostic Performance of Point-of-Care Fibroscan for the Detection of Fibrosis Stages and CAP.
| Studies, (Patients; n) | AUC | Sensitivity (95%CI) | Specificity (95%CI) | Negative Likelihood Ratio (95% CI) | Positive Likelihood Ratio (95% CI) | Diagnostic Odds Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| F ≥ 1 | 4 (210) | 0.74 | 69.5% (0.49–0.84) | 70.6% (0.29–0.93) | 0.47 (0.31–0.71) | 2.93 (1.13–8.23) | 6.62 (1.64–18.30) |
| F ≥ 2 | 8 (650) | 0.69 | 81.7% (0.62–0.92) | 64.6% (0.56–0.73) | 0.30 (0.12–0.58) | 2.30 (1.67–3.02) | 9.28 (2.96–22.30) |
| F ≥ 3 | 10 (619) | 0.84 | 88.1% (0.78–0.94) | 63.8% (0.49–0.77) | 0.20 (0.10–0.36) | 2.50 (1.68–3.77) | 14.60 (5.02–33.50) |
| F ≥ 4 | 9 (543) | 0.65 | 87.5% (0.78–0.93) | 62.6% (0.55–0.70) | 0.21 (0.11–0.36) | 2.34 (1.86–2.92) | 12.70 (5.36–25.70) |
| CAP < 33% | 10 (510) | 0.85 | 84.3% (0.81–0.94) | 70.3% (0.55–0.82) | 0.23 (0.17–0.31) | 2.94 (1.88–4.65) | 13.40 (6.59–24.40) |
| CAP 34–66% | 12 (309) | 0.75 | 76.5% (0.68–0.83) | 54.0% (0.40–0.68) | 0.45 (0.29–0.68) | 1.70 (1.23–2.40) | 4.11 (1.83–8.00) |
| CAP ≥ 67% | 12 (518) | 0.80 | 80.6% (0.75–0.85) | 57.9% (0.45–0.70) | 0.35 (0.23–0.51) | 1.95 (1.42–2.71) | 6.07 (2.85–11.40) |
Figure 2.
Forest plots for sensitivity of any degree of fibrosis (F0 vs. F1–4) along with their 95% confidence intervals (CIs). The plot includes four studies: Siddiqui 2019 [9], Chan 2017 [13], Kwok 2015 [15], and Liu 2021 [28]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (66%) and τ2 (0.3995), with a p-value of 0.03 indicating significant heterogeneity.
Figure 3.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of any degree of fibrosis (F0 vs. F1–4). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
3.4. Diagnosis of Significant Fibrosis (F0–1 vs. F2–4)
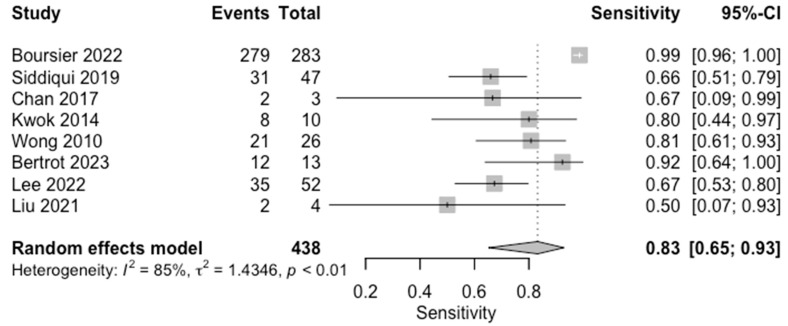
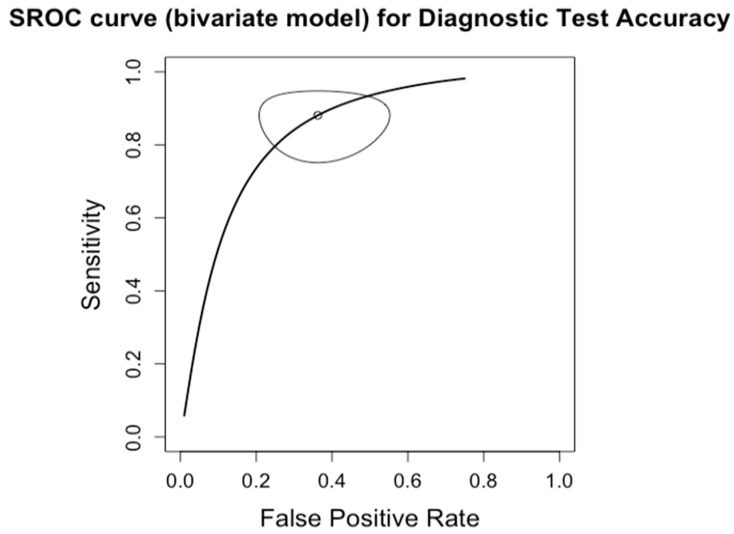
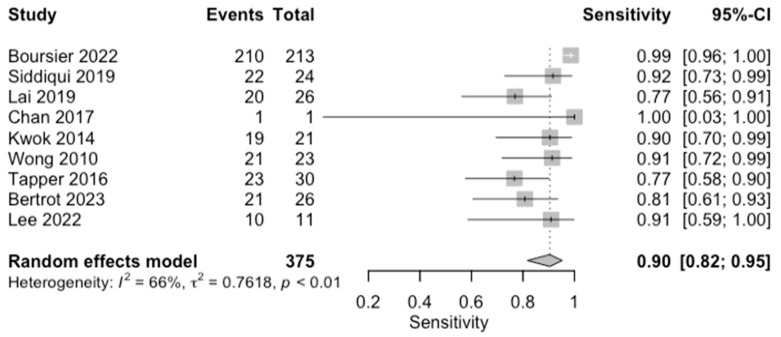
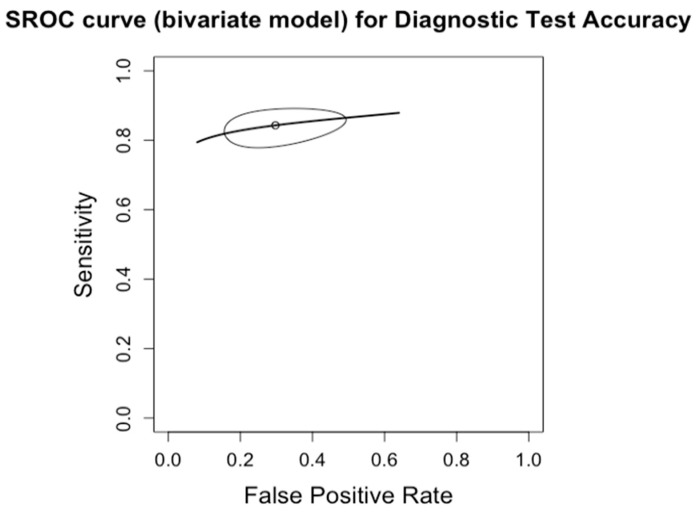
The diagnostic accuracy in detecting significant fibrosis (≥F2) was investigated by eight studies (n = 650) (Table 3; Figure 4 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for a diagnosing stage ≥ F2 were as follows: 0.69, 81.7%, 64.6%, 2.30, 0.30, and 9.28. The summary point estimate of the mean is shown in Figure 5.
Figure 4.
Forest plots for sensitivity of significant fibrosis (F0–1 vs. F2–4) along with their 95% confidence intervals (CIs). The plot includes four studies: Boursier 2022 [9], Siddiqui 2019 [10], Chan 2017 [13], Kwok 2014 [15], Wong 2010 [24], Bertrot 2023 [26], Lee 2022 [27], and Liu 2021 [28]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (85%) and τ2 (1.4346), with a p-value of 0.01 indicating significant heterogeneity.
Figure 5.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of fignificant fibrosis (F0–1 vs. F2–4). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
3.5. Diagnosis of Advanced Fibrosis (F0–2 vs. F3–4)
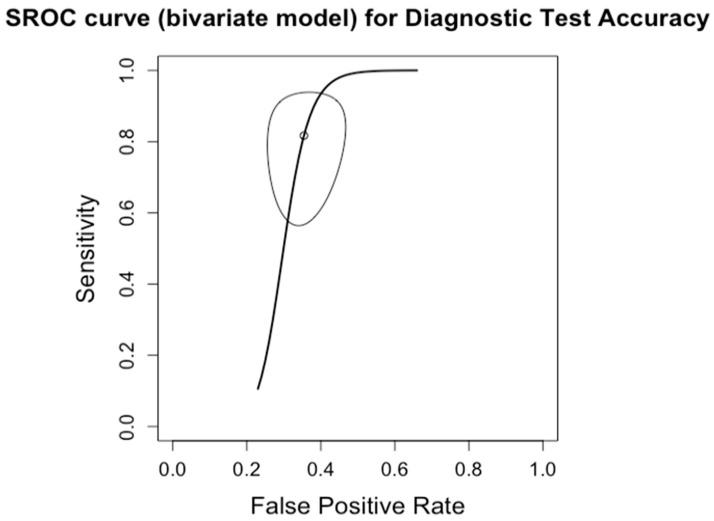
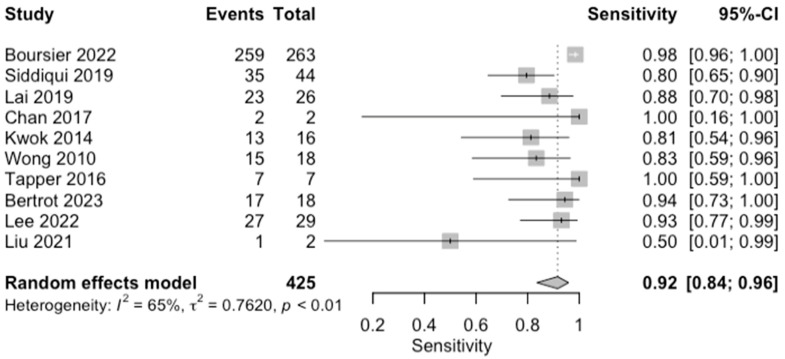
The diagnostic accuracy in detecting advanced fibrosis (F3) was investigated by ten studies (n = 619) (Table 3; Figure 6 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for a diagnosing stage F3 were as follows: 0.84, 88.1%, 63.8%, 2.50, 0.20, and 14.60. The summary point estimate of the mean is shown in Figure 7.
Figure 6.
Forest plots for sensitivity of advanced fibrosis (F0–2 vs. F3–4) along with their 95% confidence intervals (CIs). The plot includes four studies: Boursier 2022 [9], Siddiqui 2019 [10], Lai 2019 [11], Chan 2017 [13], Kwok 2014 [15], Wong 2010 [24], Tapper 2016 [25], Bertrot 2023 [26], Lee 2022 [27], and Liu 2021 [28]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (65%) and τ2 (0.7620), with a p-value of 0.01 indicating significant heterogeneity.
Figure 7.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of advanced fibrosis (F0–2 vs. F3–4). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
3.6. Diagnosis of Cirrhosis (F0–3 vs. F4)
The diagnostic accuracy in detecting cirrhosis (F4) was investigated by nine studies (n = 543) (Table 3; Figure 8 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for diagnosing stage F4 were as follows: 0.65, 87.5%, 62.6%, 2.34, 0.21, and 12.70. The summary point estimate of the mean is shown in Figure 9.
Figure 8.
Forest plots for sensitivity of cirrhosis (F0–3 vs. F4) along with their 95% confidence intervals (CIs). The plot includes four studies: Boursier 2022 [9], Siddiqui 2019 [10], Lai 2019 [11], Chan 2017 [13], Kwok 2014 [15], Wong 2010 [24], Tapper 2016 [25], Bertrot 2023 [26], Lee 2022 [27], and Liu 2021 [28]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (65%) and τ2 (0.7620), with a p-value of 0.01 indicating significant heterogeneity.
Figure 9.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of cirrhosis (F0–3 vs. F4). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
3.7. Diagnosis of Mild Steatosis (CAP < 33%, S1)
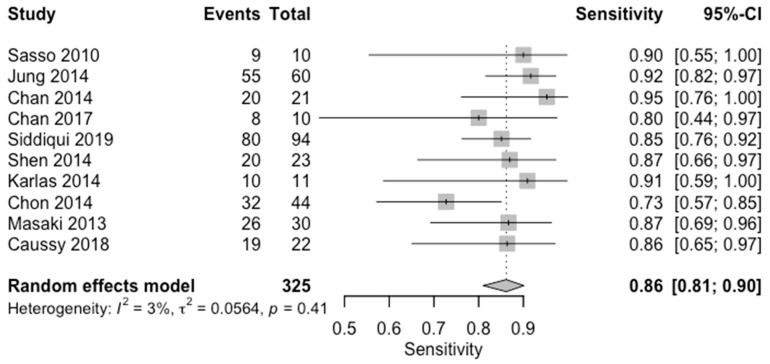
The diagnostic accuracy in detecting mild steatosis (S1) was investigated by ten studies (n = 510) (Table 3; Figure 10 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for diagnosing stage S1 were as follows: 0.85, 84.3%, 70.3%, 2.94, 0.23, and 13.40. The summary point estimate of the mean is shown in Figure 11.
Figure 10.
Forest plots for sensitivity of mild steatosis (CAP < 33%, S1) along with their 95% confidence intervals (CIs). The plot includes four studies: Sasso 2010 [16], Jung 2014 [14], Chan 2014 [12], Chan 2017 [13], Siddiqui 2019 [10], Shen 2014 [17], Karlas 2014 [18], Chon 2014 [19], Masaki 2013 [20], and Caussy 2018 [23]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (3%) and τ2 (0.0564), with a p-value of indicating low heterogeneity.
Figure 11.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of mild steatosis (CAP < 33%, S1). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
3.8. Diagnosis of Moderate Steatosis (CAP 34–66%, S2)
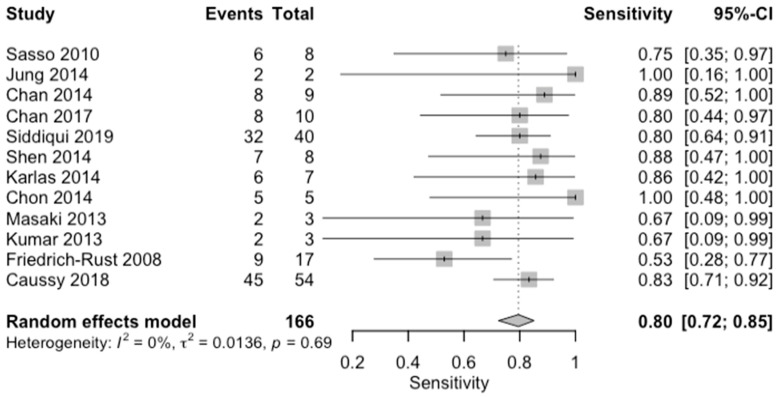
The diagnostic accuracy in detecting moderate steatosis (S2) was investigated by thirteen studies (n = 309) (Table 3; Figure 12 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for diagnosing stage S2 were as follows: 0.75, 76.8%, 54.0%, 1.70, 0.45, and 4.11. The summary point estimate of the mean is shown in Figure 13.
Figure 12.
Forest plots for sensitivity of moderate steatosis (CAP 34–66%, S2) along with their 95% confidence intervals (CIs). The plot includes four studies: Sasso 2010 [16], Jung 2014 [14], Chan 2014 [12], Chan 2017 [13], Siddiqui 2019 [10], Shen 2014 [17], Karlas 2014 [18], Chon 2014 [19], Masaki 2013 [20], Kumar 2013 [21], Friedrich-Rust 2008 [22], and Caussy 2018 [23]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (0%) and τ2 (0.0136), with a p-value of indicating low heterogeneity.
Figure 13.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of moderate steatosis (CAP 34–66%, S2). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
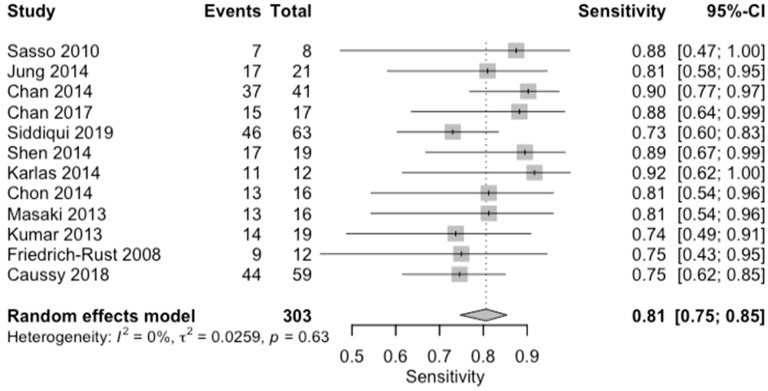
3.9. Diagnosis of Severe Steatosis (CAP > 67%, S3)
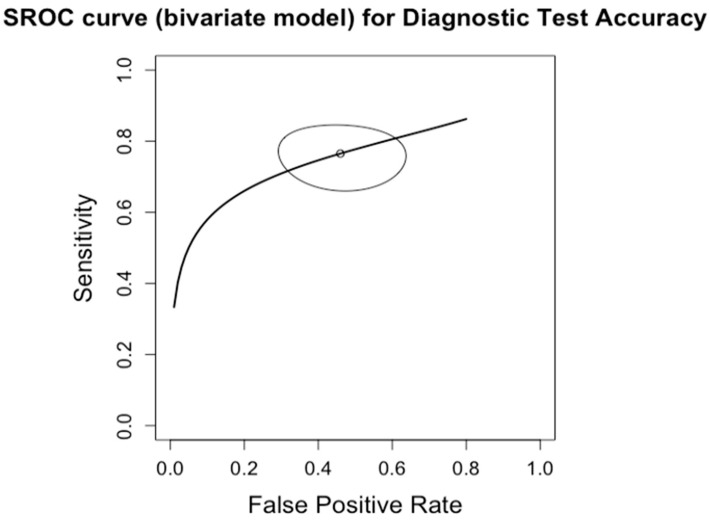
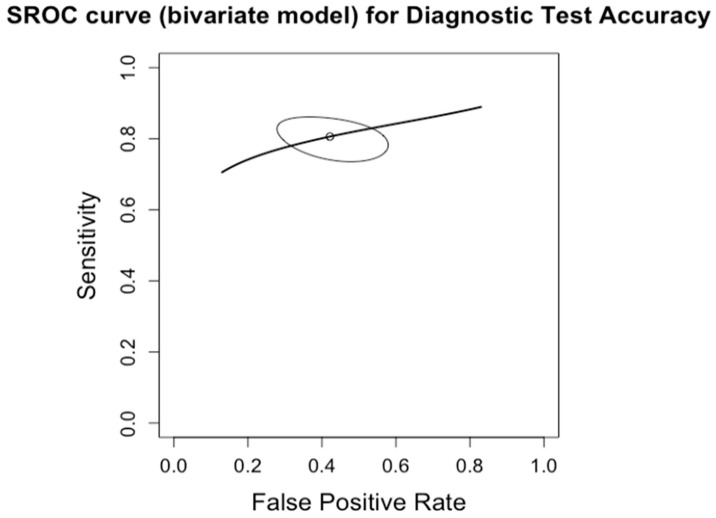
The diagnostic accuracy in detecting severe steatosis (S3) was investigated by twelve studies (n = 518) (Table 3; Figure 14 for forest plot). The respective AUCs, sensitivities, specificities, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratios for diagnosing stage S3 were as follows: 0.80, 80.6%, 57.9%, 1.95, 0.35, and 6.07. The summary point estimate of the mean is shown in Figure 15.
Figure 14.
Forest plots for sensitivity of severe steatosis (CAP > 67%, S3) along with their 95% confidence intervals (CIs). The plot includes four studies: Sasso 2010 [16], Jung 2014 [14], Chan 2014 [12], Chan 2017 [13], Siddiqui 2019 [10], Shen 2014 [17], Karlas 2014 [18], Chon 2014 [19], Masaki 2013 [20], Kumar 2013 [21], Friedrich-Rust 2008 [22], and Caussy 2018 [23]. Each study’s sensitivity is represented by a square, with the square size reflecting the study weight in the random effects model. Horizontal lines indicate the 95% CI for each study. The diamond at the bottom represents the pooled sensitivity estimate and its 95% CI, based on the random effects model. Heterogeneity among studies is quantified by I2 (0%) and τ2 (0.0259), with a p-value of indicating low heterogeneity.
Figure 15.
Summary Receiver Operating Characteristic (SROC) curve for diagnostic test accuracy of moderate steatosis (CAP >67%, S3). The curve plots sensitivity (y-axis) against false positive rate (x-axis), providing an overall measure of test performance across studies. The central curve represents the relationship between sensitivity and false positive rate, while the surrounding shaded region illustrates the 95% confidence region, indicating the variability in diagnostic accuracy.
The probe type was identified as a significant covariate influencing the diagnostic performance for both any fibrosis and significant fibrosis, as well as for mild and severe steatosis (Table 4). BMI was a significant covariate for steatosis but not for fibrosis (Table 5). The percentage of male participants demonstrated a significant association with both fibrosis and steatosis (Table 6). However, the degree of fibrosis and steatosis did not have a statistically significant impact on the diagnostic performance of VCTE (Table 7).
Table 4.
Probe Type (M, M, and XL or Not Specified Studies) as a Covariate of the Diagnostic Performance.
| M | M and XL | Not Specified | Chi-Square | p-Value | |
|---|---|---|---|---|---|
| Any fibrosis (F 1) | 11 | 34 | 60 | 12.13 | 0.0164 |
| Significant fibrosis (F 2) | 29 | 362 | 47 | 10.536 | 0.0323 |
| Advanced fibrosis (F 3) | 27 | 354 | 44 | 8.2596 | 0.0825 |
| Cirrhosis (F = 4) | 54 | 297 | 24 | 9.0037 | 0.0610 |
| CAP < 33% | 209 | 22 | 94 | 11.0670 | 0.0258 |
| CAP 34–66% | 55 | 71 | 40 | 7.3715 | 0.1175 |
| CAP >= 67% | 169 | 71 | 63 | 9.8008 | 0.0439 |
Table 5.
BMI as a Covariate of the Diagnostic Performance.
| Chi-Square | p-Value | |
|---|---|---|
| Fibrosis | 0.3842 | 0.8252 |
| Steatosis | 7.292 | 0.0261 |
Table 6.
Male Percentage as a Covariate of the Diagnostic Performance.
| Chi-Square | p-Value | |
|---|---|---|
| Fibrosis | 9.0215 | 0.0110 |
| Steatosis | 10.9400 | 0.0042 |
Table 7.
Fibrosis or Steatosis Level as a Covariate of the Diagnostic Performance.
| Chi-Square | p-Value | |
|---|---|---|
| Figure 7 | 7.0286 | 0.3182 |
| Steatosis | 3.032 | 0.5525 |
3.10. Adherence to POC VCTE
Table 2 summarizes the findings from six studies that evaluated the completion rate of Vibration-Controlled Transient Elastography (VCTE) in patients who were offered this option. The adherence rates were predominantly high, exceeding 90% in the majority of studies.
4. Discussion
Numerous meta-analyses have assessed the accuracy of Vibration-Controlled Transient Elastography (VCTE) in diagnosing NAFLD [30], but none have focused on its use in a point-of-care setting. MASLD, formerly known as NAFLD, is a rapidly growing global health issue. Current guidelines recommend using simple blood tests to screen at-risk populations for fibrosis, followed by referral for VCTE if elevated scores are detected [31]. However, challenges such as the need for multiple appointments and traveling to urban centers for VCTE or MRE may result in non-compliance. To date, no comparative study has examined VCTE performed at the point of care versus the traditional referral route.
VCTE is being increasingly explored for its potential to predict liver-related events (LREs) [32,33]. In this systematic review, we investigate the use of point-of-care VCTE for diagnosing MASLD/MASH. This synthesis represents the most comprehensive pooled analysis of diagnostic metrics for point-of-care VCTE in MASLD to date. Our findings indicate that point-of-care VCTE exhibits high diagnostic accuracy, with sensitivities ranging from 76% to 89% and specificities from 67% to 73% for F1 to F4, underscoring its value as a critical point-of-care test.
The AASLD (AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease) has recently updated its guidelines on high-risk populations [34].
Clinicians are advised to screen patients with MASLD for type 2 diabetes, particularly advanced fibrosis, due to the increased risk of nonalcoholic steatohepatitis (MASH). Screening for advanced fibrosis is also recommended in patients with obesity. The adherence rate for Vibration-Controlled Transient Elastography (VCTE) is notably high in comparison to other tests used for diagnosing fibrosis and steatosis. In a large multicenter prospective study that utilized Vibration-Controlled Transient Elastography (VCTE) for diagnosing nonalcoholic fatty liver disease (MASLD), there was a high compliance rate. Out of 1696 scans performed, only 11 patients did not show up for the procedure. This translates to a compliance rate of approximately 99%. This high acceptance rate indicates the feasibility and patient acceptance of VCTE in clinical settings for MASLD diagnosis [35]. According to research, VCTE’s compliance rate was found to be 93.4% (142 out of 152 cases), which is higher than the 85% compliance rate observed for MRI–Proton Density Fat Fraction (MRI-PDFF) in one study [36]. In another study, the compliance rate for MRI-PDFF was reported to be 85% (103 out of 120 cases) [37,38].
Kan et al. conducted a study to determine patient preferences between VCTE and liver biopsy (LB), along with their willingness to pay for VCTE services. In British Columbia, where liver biopsy is covered by public funding, the study found that VCTE was the more favored method for assessing liver fibrosis among patients. Furthermore, a majority of these patients were willing to cover the costs of VCTE themselves [39].
The current prices of various diagnostic tools for liver fibrosis are as follows: MRE scan costs USD 250.00, VCTE (FibroScan) is USD 140.33, and biopsy amounts to USD 1372.45.
Additionally, a study by Gomez et al. demonstrated that VCTE is more cost-effective than both MRE and liver biopsy over a five-year period [40].
Research indicates that the initial attendance for Vibration-Controlled Transient Elastography (VCTE) is high, but attendance tends to decrease with follow-up appointments. One study showed a follow-up response rate of 50.9% (59 out of 116) [41]. Tapper et al. reported a 91.4% (169 out of 185) adherence at 3 months, dropping to 53% (87 out of 164) at 6 months [25]. Wah Liu et al. observed adherence rates of 94.6% at 1 year, 100% at 2 years, 94.6% at 3 years, 91.9% at 4 years, and 64.9% at 5 years [28]. Another study reported a 6-mnth follow-up rate of 86% (43 out of 50). This trend highlights a decline in follow-up engagement over time [42].
Integrating point-of-care testing for Vibration-Controlled Transient Elastography (VCTE) during primary care physician/general gastroenterologist visits could minimize barriers such as the need for multiple appointments. This approach would streamline the completion of VCTE without compromising the test’s diagnostic accuracy. We found adherence rates to POC VCTE ranging from 54 to 98.3% but mostly above 90%.
This finding underscores the significance of VCTE (Vibration-Controlled Transient Elastography) as a valuable tool in the point-of-care setting for evaluating MASLD [43].
Several multivariate analyses have been initiated to enhance the sensitivity and predictive efficacy of simple laboratory tests for liver fibrosis. These include the FIB-4 index, the MASLD fibrosis score, the ELF (Enhanced Liver Fibrosis) test, the BARD score, the FibroTest, and the Hepascore. The ELF test, a commercially available algorithm, incorporates three serum biomarkers: hyaluronic acid (HA), the N-terminal pro-peptide of collagen type III (PIIINP), and tissue inhibitor of metalloproteinase-1 (TIMP1). A recent prospective study compared the ELF test with FibroTest and Elastography in 289 patients. While the results from the ELF test and FibroTest were not significantly different from those of liver stiffness measurements in intention-to-diagnose analyses (AUROC for transient elastography, 0.90), discrepancies were observed in the per-protocol analysis (AUROC for transient elastography, 0.97). The cutoff value of 10.5 for the ELF test was excellent for ruling out advanced fibrosis, with a negative predictive value (NPV) of 98%. However, it could not definitively confirm a diagnosis of advanced fibrosis due to a positive predictive value (PPV) of 60% [44,45]. Considering that VCTE is notably the most cost-effective among the non-invasive tests [46,47] and surpasses blood fibrosis tests in accuracy [48], point-of-care VCTE emerges as an economical option with the potential to address healthcare disparities by enabling the early diagnosis and treatment of MASLD within underserved, unrepresented, and marginalized communities. Its user and resource-friendly nature with easy portability allows for wider implementation in primary care settings and community settings, facilitating large-scale screening and monitoring. This enables primary care physicians and gastroenterologists alike to assess liver fibrosis at the point of care and allows for the early implementation of awareness and lifestyle modifications. It can streamline referrals by reducing multiple appointments, minimizing scheduling complexities and the risk of missing work multiple times. It can avoid unnecessary specialist consultations for mild fibrosis. Incorporating TE could lead to better early MASLD management and increased patient education and engagement. It can help in liver disease monitoring at the point of care to avoid MASH, cirrhosis, and hepatocellular carcinoma and help in healthcare cost savings. It is particularly advantageous for underserved populations, given its cost-effectiveness compared to alternatives such as MRI or liver biopsy. It is important to note that resistance toward MR elastography is often driven by insurance coverage or patient preferences. Additionally, this approach eliminates the requirement for additional travel, which can be a significant obstacle, especially when traveling to a city is not feasible. Yoneda et al. highlighted that referring patients to hepatology for elastographic examinations can decrease patient follow-up and attendance [48]. Thus, employing a point-of-care (POC) VCTE during clinic appointments could additionally diminish the necessity for recurring visits, minimize delays, and prevent loss of follow-up. Introducing visual reports during patients’ visits, VCTE can enhance their engagement in liver care by providing a pictorial representation of their liver condition. This can lead to improved follow-up appointments, better compliance with treatment plans, and increased overall involvement of patients in managing their liver health. It has been seen that combining TE with visual reports enhances MASLD patient satisfaction by improving clarity and understanding [49].
Our study has several limitations that warrant consideration. Several of the included studies were retrospective, observational, and population-based. It has been observed that CAP’s accuracy diminishes at higher BMIs, and although using an XL probe can partially mitigate this issue, it still remains less accurate compared to individuals with lower BMIs.
Moreover, CAP may not be sufficiently reliable for grading steatosis in patients with MASLD. Its diagnostic performance in identifying severe steatosis is sub-optimal, and its ability to differentiate between steatosis grade 2 and grade 3 was found to be unsatisfactory, similar to evidence by studies conducted by Sasso et al. and Ledinghen et al. [29,50]. Our research revealed that steatosis grade 3 or high CAP values serve as independent risk factors for discordant results between a liver biopsy and CAP. Another limitation arises from the lack of universally standardized cutoffs for MASLD. As a result, our study encountered a range of cutoff values being used, which could potentially impact the overall accuracy and consistency of the results. Also, there was variability among the included studies and technical differences that were not fully addressed, which could have influenced the heterogeneity observed in the results. Nevertheless, this study offers significant insights into the combined diagnostic accuracy of point-of-care VCTE for MASLD.
5. Conclusions
In conclusion, point-of-care Vibration-Controlled Transient Elastography (VCTE) emerges as a valuable tool for the non-invasive assessment of liver fibrosis in patients with Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) and Metabolism-Associated Steatohepatitis (MASH). This systematic review highlights the high diagnostic accuracy of VCTE, with sensitivity and specificity levels that make it a reliable option for staging liver fibrosis at the point of care. The integration of VCTE into routine clinical practice could significantly reduce the barriers associated with traditional referral-based approaches, such as the need for multiple appointments and the resulting non-compliance. Moreover, the high adherence rates observed in point-of-care settings underscore the feasibility and acceptance of VCTE among patients.
By streamlining the diagnostic process and enabling earlier intervention, point-of-care VCTE has the potential to improve patient outcomes, particularly in high-risk populations. As the global burden of MASLD continues to grow, the adoption of VCTE in primary care and community settings could play a crucial role in large-scale screening and monitoring efforts. Ultimately, the findings from this review support the broader implementation of point-of-care VCTE as a cost-effective, accessible, and efficient strategy for managing liver disease in diverse clinical environments.
Author Contributions
Conceptualization, T.S.D. and A.S.; methodology, T.S.D. and A.S.; software, X.M. and M.A.; validation, A.S., M.B., and R.S.; writing—T.S.D. and A.S.; writing—review and editing— A.S., M.B. and R.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The study was supported by Steve and Alex Cohen Foundation.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Luppa P.B., Muller C., Schlichtiger A., Schlebusch H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Analyt Chem. 2011;30:887–898. doi: 10.1016/j.trac.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey D.C., Caldwell S.H., Goodman Z.D., Nelson R.C., Smith A.D., American Association for the Study of Liver D. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 3.Castera L., Forns X., Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Wong V.W., Adams L.A., de Ledinghen V., Wong G.L., Sookoian S. Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 5.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.Harman D.J., Ryder S.D., James M.W., Jelpke M., Ottey D.S., Wilkes E.A., Card T.R., Aithal G.P., Guha I.N. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: A cross-sectional diagnostic study utilising transient elastography. BMJ Open. 2015;5:e007516. doi: 10.1136/bmjopen-2014-007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddowes P.J., Sasso M., Allison M., Tsochatzis E., Anstee Q.M., Sheridan D., Guha I.N., Cobbold J.F., Deeks J.J., Paradis V., et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 9.Boursier J., Hagstrom H., Ekstedt M., Moreau C., Bonacci M., Cure S., Ampuero J., Nasr P., Tallab L., Canivet C.M., et al. Non-invasive tests accurately stratify patients with NAFLD based on their risk of liver-related events. J. Hepatol. 2022;76:1013–1020. doi: 10.1016/j.jhep.2021.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui M.S., Vuppalanchi R., Van Natta M.L., Hallinan E., Kowdley K.V., Abdelmalek M., Neuschwander-Tetri B.A., Loomba R., Dasarathy S., Brandman D., et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019;17:156–163 e152. doi: 10.1016/j.cgh.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai L.L., Wan Yusoff W.N.I., Vethakkan S.R., Nik Mustapha N.R., Mahadeva S., Chan W.K. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J. Gastroenterol. Hepatol. 2019;34:1396–1403. doi: 10.1111/jgh.14577. [DOI] [PubMed] [Google Scholar]
- 12.Chan W.K., Nik Mustapha N.R., Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2014;29:1470–1476. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 13.Chan W.K., Nik Mustapha N.R., Wong G.L., Wong V.W., Mahadeva S. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol. J. 2017;5:76–85. doi: 10.1177/2050640616646528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung K.S., Kim B.K., Kim S.U., Chon Y.E., Chun K.H., Kim S.B., Lee S.H., Ahn S.S., Park J.Y., Kim D.Y., et al. Factors affecting the accuracy of controlled attenuation parameter (CAP) in assessing hepatic steatosis in patients with chronic liver disease. PLoS ONE. 2014;9:e98689. doi: 10.1371/journal.pone.0098689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok R., Tse Y.K., Wong G.L., Ha Y., Lee A.U., Ngu M.C., Chan H.L., Wong V.W. Systematic review with meta-analysis: Non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 16.Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., Sandrin L., Miette V. Controlled attenuation parameter (CAP): A novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Shen F., Zheng R.D., Mi Y.Q., Wang X.Y., Pan Q., Chen G.Y., Cao H.X., Chen M.L., Xu L., Chen J.N., et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J. Gastroenterol. 2014;20:4702–4711. doi: 10.3748/wjg.v20.i16.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlas T., Petroff D., Garnov N., Bohm S., Tenckhoff H., Wittekind C., Wiese M., Schiefke I., Linder N., Schaudinn A., et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS ONE. 2014;9:e91987. doi: 10.1371/journal.pone.0091987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chon Y.E., Jung K.S., Kim S.U., Park J.Y., Park Y.N., Kim D.Y., Ahn S.H., Chon C.Y., Lee H.W., Park Y., et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native Korean population. Liver Int. 2014;34:102–109. doi: 10.1111/liv.12282. [DOI] [PubMed] [Google Scholar]
- 20.Masaki K., Takaki S., Hyogo H., Kobayashi T., Fukuhara T., Naeshiro N., Honda Y., Nakahara T., Ohno A., Miyaki D., et al. Utility of controlled attenuation parameter measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol. Res. 2013;43:1182–1189. doi: 10.1111/hepr.12094. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R., Rastogi A., Sharma M.K., Bhatia V., Tyagi P., Sharma P., Garg H., Chandan Kumar K.N., Bihari C., Sarin S.K. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: Diagnostic performance and clinicopathological correlation. Dig. Dis. Sci. 2013;58:265–274. doi: 10.1007/s10620-012-2306-1. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich-Rust M., Ong M.F., Martens S., Sarrazin C., Bojunga J., Zeuzem S., Herrmann E. Performance of transient elastography for the staging of liver fibrosis: A meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Caussy C., Chen J., Alquiraish M.H., Cepin S., Nguyen P., Hernandez C., Yin M., Bettencourt R., Cachay E.R., Jayakumar S., et al. Association Between Obesity and Discordance in Fibrosis Stage Determination by Magnetic Resonance vs Transient Elastography in Patients With Nonalcoholic Liver Disease. Clin. Gastroenterol. Hepatol. 2018;16:1974–1982 e1977. doi: 10.1016/j.cgh.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong V.W., Vergniol J., Wong G.L., Foucher J., Chan H.L., Le Bail B., Choi P.C., Kowo M., Chan A.W., Merrouche W., et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 25.Tapper E.B., Challies T., Nasser I., Afdhal N.H., Lai M. The Performance of Vibration Controlled Transient Elastography in a US Cohort of Patients With Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016;111:677–684. doi: 10.1038/ajg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertot L.C., Jeffrey G.P., de Boer B., Wang Z., Huang Y., Garas G., MacQuillan G., Wallace M., Smith B.W., Adams L.A. Comparative Accuracy of Clinical Fibrosis Markers, Hepascore and Fibroscan(R) to Detect Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2023;68:2757–2767. doi: 10.1007/s10620-023-07896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J.S., Lee H.W., Kim B.K., Park J.Y., Kim D.Y., Ahn S.H., Jang J.Y., Park S.Y., Lee H.W., Lee C.K., et al. Comparison of FibroScan-Aspartate Aminotransferase (FAST) Score and Other Non-invasive Surrogates in Predicting High-Risk Non-alcoholic Steatohepatitis Criteria. Front. Med. 2022;9:869190. doi: 10.3389/fmed.2022.869190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y.-W., Wong V.W., Wong S.K., Wong G.L., Lai C.M., Lam C.C., Shu S.S., Chan H.L., Ng E.K. A prospective 5-year study on the use of transient elastography to monitor the improvement of non-alcoholic fatty liver disease following bariatric surgery. Sci. Rep. 2021;11:5416. doi: 10.1038/s41598-021-83782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lédinghen V., Vergniol J., Capdepont M., Chermak F., Hiriart J.-B., Cassinotto C., Merrouche W., Foucher J. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: A prospective study of 5323 examinations. J. Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Laurence C.O., Gialamas A., Bubner T., Yelland L., Willson K., Ryan P., Beilby J., the Point of Care Testing in General Practice Trial Management Group Patient satisfaction with point-of-care testing in general practice. Br. J. Gen. Pract. 2010;60:e98–e104. doi: 10.3399/bjgp10X483508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regev A., Berho M., Jeffers L.J., Milikowski C., Molina E.G., Pyrsopoulos N.T., Feng Z.-Z., Reddy K.R., Schiff E.R. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 32.Bota S., Herkner H., Sporea I., Salzl P., Sirli R., Neghina A.M., Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–1147. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 33.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302.e4. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Rinella M.E., Neuschwander-Tetri B.A., Siddiqui M.S., Abdelmalek M.F., Caldwell S., Barb D., Kleiner D.E., Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tapper E.B., Castera L., Afdhal N.H. FibroScan (vibration-controlled transient elastography): Where does it stand in the United States practice. Clin. Gastroenterol. Hepatol. 2015;13:27–36. doi: 10.1016/j.cgh.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 36.Vuppalanchi R., Siddiqui M.S., Van Natta M.L., Hallinan E., Brandman D., Kowdley K., Neuschwander-Tetri B.A., Loomba R., Dasarathy S., Abdelmalek M., et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen A.M., Shah V.H., Therneau T.M., Venkatesh S.K., Mounajjed T., Larson J.J., Mara K.C., Schulte P.J., Kellogg T.A., Kendrick M.L., et al. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery. Hepatology. 2020;71:510–521. doi: 10.1002/hep.30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garteiser P., Castera L., Coupaye M., Doblas S., Calabrese D., Dioguardi Burgio M., Ledoux S., Bedossa P., Esposito-Farèse M., Msika S., et al. Prospective comparison of transient elastography, MRI and serum scores for grading steatosis and detecting non-alcoholic steatohepatitis in bariatric surgery candidates. JHEP Rep. 2021;3:100381. doi: 10.1016/j.jhepr.2021.100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kan V.Y., Marquez Azalgara V., Ford J.A., Peter Kwan W.C., Erb S.R., Yoshida E.M. Patient preference and willingness to pay for transient elastography versus liver biopsy: A perspective from British Columbia. Can. J. Gastroenterol. Hepatol. 2015;29:72–76. doi: 10.1155/2015/169190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilar-Gomez E., Lou Z., Kong N., Vuppalanchi R., Imperiale T.F., Chalasani N. Cost Effectiveness of Different Strategies for Detecting Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Based on United States Health Care System. Clin. Gastroenterol. Hepatol. 2020;18:2305–2314 e2312. doi: 10.1016/j.cgh.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Reinson T., Byrne C.D., Patel J., El-Gohary M., Moore M. Transient elastography in patients at risk of liver fibrosis in primary care: A follow-up study over 54 months. BJGP Open. 2021;5:145. doi: 10.3399/BJGPO.2021.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul J., Venugopal R.V., Peter L., Hussain S., Naresh Kumar Shetty K., Shetti M.P. Effects of lifestyle modification on liver enzyme and Fibroscan in Indian patients with non-alcoholic fatty liver disease. Gastroenterol. Rep. 2018;6:49–53. doi: 10.1093/gastro/gox020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leong W.L., Lai L.L., Nik Mustapha N.R., Vijayananthan A., Rahmat K., Mahadeva S., Chan W.K. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020;35:135–141. doi: 10.1111/jgh.14782. [DOI] [PubMed] [Google Scholar]
- 44.Wang J., Qin T., Sun J., Li S., Cao L., Lu X. Non-invasive methods to evaluate liver fibrosis in patients with non-alcoholic fatty liver disease. Front. Physiol. 2022;13:1046497. doi: 10.3389/fphys.2022.1046497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiele M., Madsen B.S., Hansen J.F., Detlefsen S., Antonsen S., Krag A. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and Indirect Markers in Detection of Advanced Fibrosis in Patients with Alcoholic Liver Disease. Gastroenterology. 2018;154:1369–1379. doi: 10.1053/j.gastro.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Choo B.P., Goh G.B.B., Chia S.Y., Oh H.C., Tan N.C., Tan J.Y.L., Ang T.L., Bee Y.M., Wong Y.J. Non-alcoholic fatty liver disease screening in type 2 diabetes mellitus: A cost-effectiveness and price threshold analysis. Ann. Acad. Med. Singap. 2022;51:686–694. doi: 10.47102/annals-acadmedsg.2022284. [DOI] [PubMed] [Google Scholar]
- 47.Staufer K., Halilbasic E., Spindelboeck W., Eilenberg M., Prager G., Stadlbauer V., Posch A., Munda P., Marculescu R., Obermayer-Pietsch B., et al. Evaluation and comparison of six noninvasive tests for prediction of significant or advanced fibrosis in nonalcoholic fatty liver disease. United Eur. Gastroenterol. J. 2019;7:1113–1123. doi: 10.1177/2050640619865133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoneda M., Imajo K., Nakajima A. Non-invasive diagnosis of nonalcoholic fatty liver disease. Off. J. Am. Coll. Gastroenterol.|ACG. 2018;113:1409–1411. doi: 10.1038/s41395-018-0170-0. [DOI] [PubMed] [Google Scholar]
- 49.McKay A., Pantoja C., Hall R., Matthews S., Spalding P., Banerjee R. Patient understanding and experience of non-invasive imaging diagnostic techniques and the liver patient pathway. J. Patient-Rep. Outcomes. 2021;5:89. doi: 10.1186/s41687-021-00363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasso K.E., Strunk D.R., Braun J.D., DeRubeis R.J., Brotman M.A. Identifying moderators of the adherence-outcome relation in cognitive therapy for depression. J. Consult. Clin. Psychol. 2015;83:976. doi: 10.1037/ccp0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study.