Abstract
Mutation analysis of latent membrane protein 1 (LMP1) in Epstein-Barr virus (EBV)-induced B-cell immortalization revealed two transformation effector sites, TES1 and TES2. TES2 mediates the interaction with tumor necrosis factor receptor-associated death domain protein (TRADD) and plays a key role in transactivating NF-κB and AP-1. Recombinant EBV containing LMP1 with TES2 deleted induces a limited proliferation of B cells. The present study shows that a mutant with an LMP1 site-specific mutation at TES2, LMP1TRADD, initially stimulates cell growth and significantly extends the life span of MEF. However, it is not sufficient for the immortalization of MEF, and MEF-LMP1TRADD cells eventually enter growth arrest. Further analysis reveals that although LMP1TRADD promotes cell growth, it does not prevent the eventual onset of senescence and the expression of tumor suppressor p16Ink4a.
Epstein-Barr virus (EBV) is a prevalent human gamma herpesvirus. It is frequently associated with a number of human cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's lymphoma, and gastric carcinoma (31). EBV adopts highly restricted patterns of latent gene expression in these cancers. In particular, only the nuclear protein EBNA 1 and latent membrane proteins LMP1 and LMP2 are present in nasopharyngeal carcinoma and Hodgkin's lymphoma (31). In vitro, EBV induces a continuous proliferation of infected B cells, resulting in the outgrowth of immortal lymphoblastoid cell lines (LCLs) (23). While EBV-induced B-cell proliferation serves as a good functional model system for studying EBV-associated proliferative diseases such as infectious mononucleosis, it may have limitations for analyzing EBV-associated malignancies. It is clear that EBV-mediated B-cell immortalization requires several EBV genes encoding latent membrane protein 1 (LMP1) and nuclear antigens EBNA 2, EBNA 3A, EBNA 3C, and EBNA LP (23), whereas of these five antigens, only LMP1 is consistently present in most EBV-associated cancers (31).
The viral transforming protein LMP1 is composed of six transmembrane domains and a long carboxy-terminal cytoplasmic segment (9). The region containing the six transmembrane domains mediates its oligomerization in the cytoplasmic membrane, resulting in the constitutive activation of the downstream signal (10). There are at least two functional domains in the cytoplasmic tail of LMP1 that interact with tumor necrosis factor receptor-associated factors (TRAF) (29) and tumor necrosis factor receptor-associated death domain protein (TRADD) (19), resulting in the activation of transcription factors NF-κB (17) (28) and AP-1 through c-Jun N-terminal kinase (JNK) (7, 24). In parallel, genetic studies of EBV-mediated B-cell immortalization revealed two transformation effector sites (TES1 and TES2) correlated with both TRAF and TRADD binding sites of LMP1. A mutant with a TES1 (TRAF site) deletion retains 75% of NF-κB activation activity but is insufficient for B-lymphocyte transformation (18). Interestingly, an EBV recombinant (MS231) with a LMP1-TES2 (TRADD) deletion, while retaining a low level of NF-κB activation activities, is capable of effectively inducing an initial primary B-cell proliferation (22) but not long-term LCL growth (21). It is not known why MS231 supports a limited proliferation but not the long-term outgrowth of B lymphocytes.
Our recent work indicates that LMP1 alone, when transduced into primary mouse embryonic fibroblasts (MEF) via a recombinant retrovirus, induces the proliferation of MEF (40). Our data further indicate that LMP1 may suppress replicative senescence and premature senescence induced by the ras oncogene (39). As LMP1-mediated MEF immortalization may provide another model system for analyzing the roles of LMP1 in the control of cell growth and the development of malignant diseases, it is important to correlate the functions of LMP1 in MEF immortalization with that in EBV-mediated B-cell immortalization. In addition, the activities of LMP1 in inducing cell proliferation and suppressing senescence and the mechanism of LMP1-mediated MEF immortalization need further exploration through genetic analysis.
An LMP1TRADD site-specific mutant induces the initial proliferation of MEF.
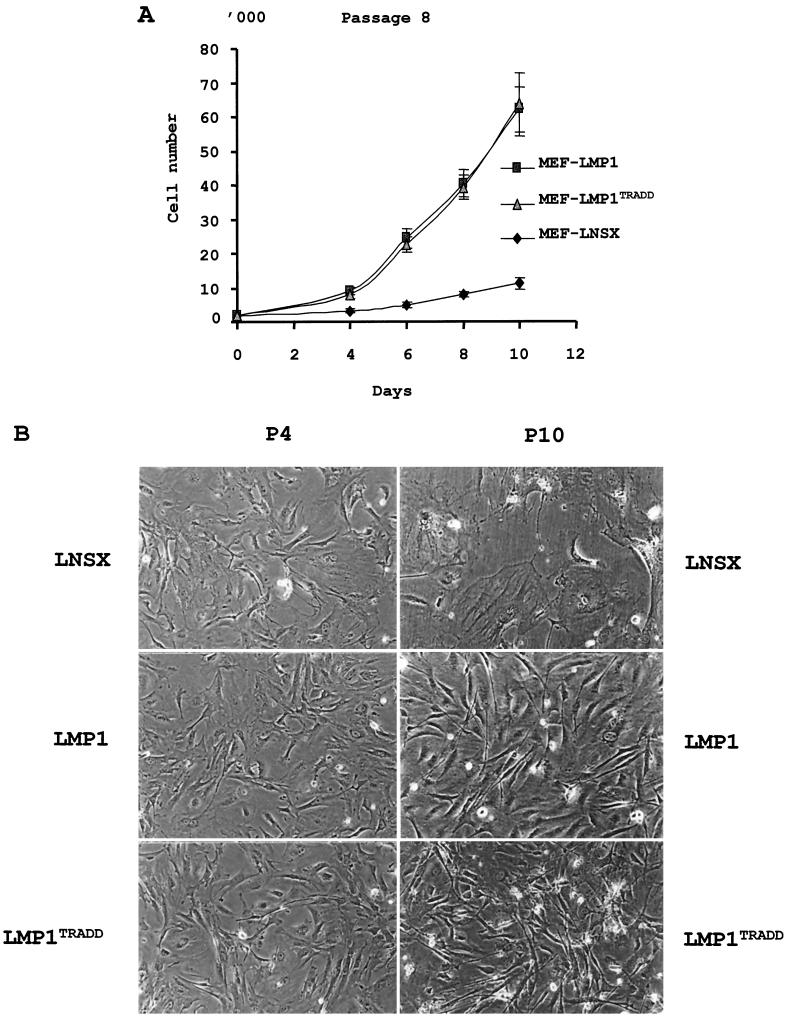
A site-specific mutant of LMP1 with a defective TRADD binding site (LMP1TRADD) was chosen for its roles in NF-κB and AP-1 activation, and for its interesting phenotype in inducing a limited B-cell proliferation (19, 21, 22). The LMP1TRADD mutant was constructed with a substitution of ID for YYD in the last three amino acid residues of the protein (positions 384 to 386) as previously described (14). This particular LMP1TRADD is completely defective in AP-1 activation, 80% defective in NF-κB activation, and partially defective in Rat-1 transformation (14). Previous studies indicated that this change affects the ability of LMP1 to bind to TRADD and the capacity of recombinant EBV to sustain a long-term LCL outgrowth (19). To evaluate the ability of LMP1TRADD in stimulating cell proliferation, MEF were infected with retroviruses containing genes for LNSX, LMP1, or LMP1TRADD at passage 3, and they were passaged without drug selection. The expression of LMP1 was confirmed at passage 4 by both immunofluorescence and immunoblotting with S12 antibody (data not shown). An immunofluorescence assay at passage 4 also revealed that approximately 25 to 30% of the cells were LMP1 positive. Due to concerns about the effect of the cell density on the growth of primary MEF, the infected cells were split 1:2 only when they reached confluence. MEF-LMP1 and MEF-LMP1TRADD cells started to exhibit a growth rate significantly higher than that of MEF-LNSX cells three passages later, at passage 6. To demonstrate the ability of LMP1TRADD to stimulate proliferative growth, all three types of infected cells were plated out for growth analysis in triplicate at passage 8. Subsequently, the number of cells per well was counted every other day, and the data indicated that both MEF-LMP1 and MEF-LMP1TRADD cells grew much faster than the control MEF-LNSX cells (Fig. 1A). At this early passage, no visible difference in the growth rate was detected between MEF-LMP1 and MEF-LMP1TRADD cells. Further cytological examination supports the above observation. While all cells were identical in morphology immediately following the infections at passage 4, they were very different at passage 10 (Fig. 1B). The MEF-LNSX control cells were large and flat, reminiscent of replicative senescence (36). In contrast, both MEF-LMP1 and MEF-LMP1TRADD cells presented fibroblast morphology. Thus, it appears that LMP1TRADD also has a similar activity in stimulating the initial cell growth of MEF despite the fact that the mutant is completely defective in AP-1 activation and 75% defective in NF-κB activation.
FIG. 1.
LMP1TRADD induces an immediate proliferation of MEF comparable to that of wild-type LMP1. (A) Growth analysis of MEF infected with retroviruses carrying genes for LMP1TRADD and wild-type LMP1 (14, 40). The infected cells were seeded at 2,000/well into 24-well dishes at passage 8. They were removed by trypsin digestion and counted at the indicated time points. The experiment was done in triplicate to determine the mean and standard deviation. (B) Morphology of MEF infected with retroviruses carrying genes for LNSX, LMP1, or LMP1TRADD at passages 4 and 10 (P4 and P10). At passage 10, the MEF-LNSX cells exhibited the flat and enlarged morphology that was absent in MEF with either LMP1 or LMP1TRADD. Magnification, ×100.
LMP1TRADD is not sufficient to sustain long-term growth of MEF.
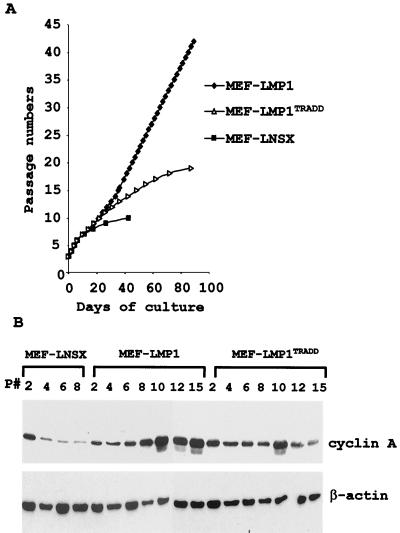
Previous work with recombinant EBV carrying the LMP1TRADD gene shows that such mutant virus, although capable of inducing the initial proliferation of resting B cells, is insufficient to sustain the long-term outgrowth of B cells (21, 22). To investigate the long-term effect of the LMP1TRADD gene as the sole EBV gene on the proliferation of MEF, MEF-LMP1 and MEF-LMP1TRADD cells were continuously passaged at 1:2 per split whenever they reached confluence. They began to exhibit a small difference in the growth rate at passages 10 (also population doubling 10), the last obtainable passage for MEF-LNSX control cells (Fig. 2A), when MEF-LMP1 cells started to grow faster (3 days per passage) than MEF-LMP1TRADD cells (4 days per passage). Four passages later, MEF-LMP1 cells again decreased the doubling time to a steady rate of 2 days for the next 30 passages without any sign of growth restriction in 3 months. Up to the present time, the LMP1 retrovirus-infected MEF have been passaged for 80 doublings and they are immortalized. In contrast, MEF-LMP1TRADD cells had a doubling time of 4 days till passage 11, after which their rate of doubling gradually decreased. MEF-LMP1TRADD cells reached passage 19 in 3 months and could not be passaged further. Similar results were reproduced in three independent experiments. Thus, LMP1TRADD prolongs the life span of MEF but is not sufficient to induce their immortalization.
FIG. 2.
LMP1TRADD is not sufficient for MEF immortalization. (A) MEF were infected with retroviruses carrying genes for LNSX, LMP1, or LMP1TRADD at passage 3. They were subsequently split 1:2 whenever they reached confluence in the absence of any drug selection. MEF-LNSX cells had a limited life span of 10 passages (10 population doublings) and did not reach confluence afterwards. MEF-LMP1TRADD cells had an extended passage number of 19 in about 3 months but ceased proliferating afterwards. (B) Transient induction of S-phase-specific cyclin A by LMP1TRADD. Cell lysates were obtained from the infected MEF at the indicated passages and examined for the expression of cyclin A by Western blotting. A blot against β-actin was used to control loading.
Cell growth analysis further confirms that MEF-LMP1TRADD cells stop dividing as they reach a later passage. When both MEF-LMP1 and MEF-LMP1TRADD cells were seeded for growth analysis at passage 16, it became apparent that while MEF-LMP1 cells proliferated continuously, MEF-LMP1TRADD cells exhibited little growth (data not shown). Cells of both types were collected and analyzed for cell cycle distribution with a fluorescence-activated cell sorter. The results revealed that 50% of MEF-LMP1 cells were in G1 phase of the cell cycle, whereas over 90% of MEF-LMP1TRADD cells were in G1 (data not shown). Thus, after extended passages, MEF-LMP1TRADD cells eventually came to cell growth arrest.
The transduction of LMP1 into MEF also had a clear effect on the expression of cyclin A, specific for the S and G2 phases of the cell cycle (15, 33). In the control MEF-LNSX cells, decreased expression of cyclin A was observed at passage 4 and subsequent passages (Fig. 2B). On the other hand, the level of cyclin A was never reduced in MEF-LMP1 cells. Instead, it was significantly induced from passage 10 and stayed at this higher level throughout the experiment. Interestingly, MEF-LMP1TRADD cells exhibited a transient induction of cyclin A expression at passage 10, but it quickly subsided in subsequent passages. The data further support the idea that LMP1TRADD initially induces cell proliferation but such induction is short-lived.
LMP1TRADD cannot suppress the replicative senescence of MEF.
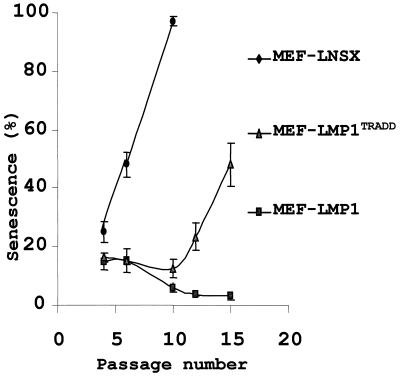
During in vitro passage, MEF go through a process known as replicative senescence, in which the expression of senescence-associated acidic β-galactosidase (SA-β-Gal) was increased (5). Our previous results reveal that LMP1 suppresses replicative senescence of MEF (39). To understand the factors underlying the inability of LMP1TRADD to sustain long-term proliferation of MEF, the occurrence of cell senescence at different passages was examined. MEF infected with LNSX, LMP1, and LMP1TRADD retroviruses were examined for senescent cells with a SA-β-Gal assay. The results showed that MEF-LNSX cells quickly entered senescence. Nearly 45% of the cells were positive for SA-β-Gal at passage 6, and virtually all of them were positive at passage 10 (Fig. 3). In contrast, about 15% of MEF-LMP1 cells were in senescence at passages 4 and 6. The low percentage of senescence in MEF-LMP1 cells may be due to the fact that a significant population of MEF (70%) was not infected by LMP1 retrovirus. The percentage of senescent cells was significantly decreased at passage 10. By passage 13, the MEF were nearly free from senescent cells. Interestingly, the transduction of LMP1TRADD into MEF initially prevented replicative senescence. By passage 10, while virtually all MEF-LNSX cells were in senescence, MEF-LMP1TRADD cells showed little sign of it. However, MEF-LMP1TRADD cells exhibited increasing levels of replicative senescence in the subsequent passages. By passage 15, half of MEF-LMP1TRADD cells were positive for SA-β-Gal. Therefore, the data suggest that MEF-LMP1TRADD cells, while displaying a delayed onset of replicative senescence, cannot escape from normal cellular mechanisms against proliferation and eventually enter senescence.
FIG. 3.
LMP1TRADD delays the onset of replicative senescence of MEF. Replicative senescence was examined in MEF infected with retroviruses carrying genes for LNSX, LMP1, or LMP1TRADD at the indicated passage numbers with SA-β-Gal staining (39). The mean and standard deviation of the percentage of blue cells were determined in three independent areas with about 300 cells each.
LMP1TRADD fails to suppress the expression of senescence-associated p16Ink4a.
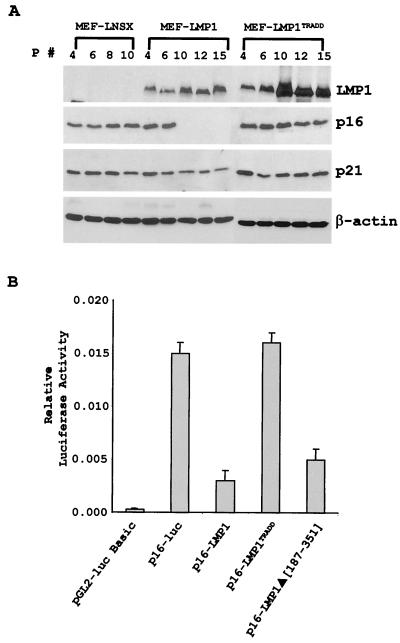
The gene for the tumor suppressor p16INK4a was implicated to have a critical role in replicative senescence in both rodent and human fibroblasts (1, 20, 35, 41). Our previous results revealed that LMP1 suppressed replicative senescence associated with the inhibition of p16 expression (39). Similarly, the data presented here indicate that LMP1 inhibits p16 expression. No p16 was detected in MEF-LMP1 cells at passage 8 and subsequent passages (Fig. 4A). The presence of p16 in the earlier passages (passages 4 and 6) could be due to the absence of LMP1 in some of the MEF, as only about 25% of the MEF were positive for LMP1 immediately after infection. It is also apparent that the levels of LMP1 at passage 8 and later passages were higher than those in earlier passages, suggesting an increasing percentage of LMP1-positive cells in late passages. In comparison, p16 was consistently detected in all passages of MEF-LNSX cells. Interestingly, contrary to the effect of wild-type LMP1, LMP1TRADD never affected the expression of p16. The inhibition of p16 by LMP1 appears to be specific, as no obvious inhibitory effect was observed for another cyclin-dependent kinase inhibitor, p21Waf1, in the infected MEF. In our previous report, LMP1 was shown to inhibit the expression of p16 associated with suppression of p16 promoter transactivation (39). To further examine the effect of LMP1TRADD on the p16 promoter activity, a similar promoter reporter study was carried out in rat embryonic fibroblasts (REF52). The results indicated that while LMP1 significantly inhibited the p16 promoter, consistent with the previous report, LMP1TRADD was not capable of inhibiting this promoter (Fig. 4B). Interestingly, an LMP1Δ(187–351) mutant with most of the cytoplasmic domain deleted but with the TRADD domain intact (8) exhibited an inhibitory effect comparable to that of wild-type LMP1, suggesting that the suppression of p16 promoter by LMP1 may be dependent on a functional TRADD binding domain. Thus, the data suggest that although LMP1TRADD induces a limited proliferation of MEF and temporarily suppresses the onset of replicative senescence, it cannot suppress the expression of p16 the way wild-type LMP1 does.
FIG. 4.
LMP1TRADD fails to suppress the expression of p16Ink4a. (A) Cell lysates were prepared from MEF infected with various retroviruses at the indicated passages and examined for the expression of LMP1, p16Ink4a, and p21Waf1 by immunoblotting with specific antibodies as previously described (39). (B) p16 promoter reporter assay. REF52 rat embryonic fibroblasts were cotransfected with 5 ng of pRL-SV40 reporter, 100 ng of pGL2-p16 promoter reporter (−1214 to −1), and 1,000 ng of the indicated LMP1 constructs by FuGENE 6 (GIBCO). pGL2-basic plasmid was used as a control in the reporter assay. Cells were harvested 48 h after transfection, and a dual luciferase assay was performed (Promega). The results shown here are representative of at least three separate experiments performed in triplicate. The error bars represent calculated standard deviations.
Conclusions.
The present results indicate that although the LMP1TRADD mutant stimulates an initial proliferative growth of MEF and significantly extends their life span, it is not sufficient for their immortalization. In contrast to wild-type LMP1, which completely inhibits replicative senescence, LMP1TRADD only delays the onset of such senescence. After extended passages in culture, MEF-LMP1TRADD cells eventually come to growth arrest and replicative senescence. Furthermore, while LMP1 specifically down-regulates the expression of tumor suppressor p16, LMP1TRADD is defective in such suppression.
Our previous study shows that a single LMP1 is capable of inducing the proliferation and immortalization of MEF. While MEF provide a simpler model system to study the roles of oncogenes and tumor suppressor genes in cell growth regulation, such a system needs validation to establish its biological relevance for EBV-associated cancers. Genetic analysis of LMP1TRADD mutants revealed a remarkable resemblance between EBV-mediated B-cell growth transformation (19, 21, 22) and LMP1-mediated primary fibroblast immortalization in this study. Therefore, the genetic analysis of LMP1TRADD mutants provides important evidence on the biological relevance of the MEF system for studying the function of LMP1 in cell proliferation and immortalization. Consequently, the system of LMP1-induced MEF immortalization provides a more direct way to examine the critical functions of LMP1 in cell growth control and immortalization.
This study further explored the process of LMP1-induced cell immortalization. It is well known that normal cells have a limited number of population doublings before reaching a stable and permanent growth arrest known as replicative senescence (4, 11, 13). MEF can normally be passaged for 10 to 12 doublings before they enter senescence (41). Previous LMP1 mutation analysis suggests that the TES1 domain is involved in the initial B-cell proliferation and TES2 is required for its long-term outgrowth (18, 22). However, the differences between the initial proliferation and long-term outgrowth are not clear, nor are the different roles of LMP1 in these processes understood. The present study indicates that LMP1TRADD stimulates the initial growth of MEF in the first 10 passages, similar to that of wild-type LMP1. However, the induction of cyclin A is short-lived, and it is followed by the onset of replicative senescence associated with cell growth arrest. Thus, although LMP1TRADD effectively induces the initial MEF proliferation, it does not suppress replicative senescence and fails to induce MEF immortalization. It is tempting to suggest that LMP1-mediated cell immortalization may involve two functions, growth stimulation and prevention of the programmed cell growth arrest, or senescence. The relationships between these two functions in inducing cell immortalization need to be further investigated.
The cyclin-dependent kinase inhibitor p16Ink4a is a tumor suppressor that exclusively binds and inhibits the D-type cyclin-dependent kinases, and it is an important cell cycle checkpoint protein (34). Deletion of INK4a results in the development of an extensive number of tumors in mice (32), similar to that of p53 null mice. Studies further indicate an important role of p16 in regulating cellular senescence and aging; while its expression is not detectable during mouse embryonic development, it is up-regulated as the mouse ages (41). Primary mouse fibroblasts lacking p16 do not become senescent and can readily be established as immortalized cells (32). Furthermore, the overexpression of the oncogene bmi-1 induces the immortalization of MEF associated with the down-regulation of p16 (20). Our previous results suggest that wild-type LMP1 inhibits the induction of senescence-associated p16 in MEF (39). In contrast, LMP1TRADD does not have any inhibitory effect on p16 expression. Thus, it appears that the removal of the cell cycle checkpoint regulator p16 maybe important for LMP1-mediated MEF immortalization, as the LMP1TRADD mutant is incapable of inhibiting p16 expression and suppressing replicative senescence and is deficient in inducing MEF immortalization.
The TRADD binding site of LMP1 is also critical for the LMP1 signaling process. By interacting with TRADD, it leads to the aggregation of TRAF proteins into complexes, which in turn accounts for 75% of LMP1-mediated NF-κB activation and all of its AP-1 activation (3). It is not clear how a mutation at this site affects LMP1's ability to induce cell immortalization or suppress replicative senescence. However, the activation of NF-κB and JNK is important for cell proliferation and cancer development. It is clear that the amplification and rearrangement of Rel and NF-κB genes are common in leukemias, lymphomas, and some solid tumors (30). Conversely, mutation in IκBα seems to be the most common event for Hodgkin's disease (2, 25, 38). In addition, there is ample evidence demonstrating that Tax protein of human T-cell leukemia virus type 1 activates IKK kinase and leads to a persistent NF-κB activation (30). Furthermore, our recent study suggests that NF-κB activation is involved in LMP1-mediated transformation and tumorigenesis of Rat-1 fibroblasts (14). NF-κB induces the expression of a variety of genes involved in cell proliferation and cell cycle progression, including myc (6, 26) and the gene encoding cyclin D1 (12, 16). Alternatively, activated JNK leads to the phosphorylation and activation of c-Jun and the up-regulation of AP-1 activity. c-Jun is capable of regulating cell proliferation (27) and may regulate the transcription of genes such as cyclin D1 (37). Interestingly, primary fibroblasts derived from c-Jun null mice have a severe proliferation defect and undergo premature cell growth arrest in vitro (37). All the evidence suggests the importance of NF-κB and AP-1 activation in cell proliferation and cancer development, and further examination of each pathway may help us to understand the mechanism of LMP1-mediated cell immortalization.
Acknowledgments
We are very grateful to F. A. Grasser for the LMP1 plasmid and D. Thorley-Lawson for S12 antibody against LMP1. We thank J. Zhong and J. D. Huang for comments on the manuscript.
This work was supported by grants from CRCG and RGC of Hong Kong and from the Croucher Foundation to L. Cao.
REFERENCES
- 1.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabannes E, Khan G, Aillet F, Jarrett R F, Hay R T. Mutations in the IκBa gene in Hodgkin's disease suggest a tumour suppressor role for IκBα. Oncogene. 1999;18:3063–3070. doi: 10.1038/sj.onc.1202893. [DOI] [PubMed] [Google Scholar]
- 3.Cahir E, McFarland D, Izumi K M, Mosialos G. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 4.Campisi J. Replicative senescence: an old lives' tale? Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 5.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duyao P M, Buckler A J, Sonenshein G E. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci USA. 1990;87:4727–4731. doi: 10.1073/pnas.87.12.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos A G, Young L S. Activation of the c-Jun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos A G, Gallagher N J, Blake S M S, Dawson C W, Young L S. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 9.Fennewald S, van Santen V, Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984;51:411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 12.Guttridge D C, Albanese C, Reuther J Y, Pestell R G, Baldwin A S., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayflick L, Moorhead P S. The serial cultivation of human diploid cell stains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 14.He Z, Xin B, Yang X, Chan C, Cao L. Nuclear factor-κB activation is involved in LMP1-mediated transformation and tumorigenesis of rat-1 fibroblasts. Cancer Res. 2000;60:1845–1848. [PubMed] [Google Scholar]
- 15.Heichman K A, Roberts J M. Rules to replicate by. Cell. 1994;79:556–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 16.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 18.Izumi K M, Kaye K M, Kieff E D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs J J, Kieboom K, Marino S, DePinho R A, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 21.Kaye K M, Izumi K M, Li H, Johannsen E, Davidson D, Longnecker R, Kieff E. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. J Virol. 1999;73:10525–10530. doi: 10.1128/jvi.73.12.10525-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye K M, Izumi K M, Mosialos G, Kieff E. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation; fibroblast cocultivation complements a critical function within the terminal 155 residues. J Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieff E. Epstein Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 24.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, Dorken B, Scheidereit C. Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene. 1999;18:943–953. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 26.La Rosa F A, Pierce J W, Sonenshein G E. Differential regulation of the c-myc oncogene promoter by the NF-κB rel family of transcription factors. Mol Cell Biol. 1994;14:1039–1044. doi: 10.1128/mcb.14.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell T, Sugden B. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 30.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 31.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 32.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 33.Sherr C J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 34.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 35.Stein G H, Drullinger L F, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein G H, Dulic V. Origins of G1 arrest in senescent human fibroblasts. Bioessays. 1995;17:537–543. doi: 10.1002/bies.950170610. [DOI] [PubMed] [Google Scholar]
- 37.Wisdom R, Johnson R S, Moore C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999;18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood K M, Roff M, Hay R T. Defective IκBα in Hodgkin cell lines with constitutively active NF-κB. Oncogene. 1998;16:2131–2139. doi: 10.1038/sj.onc.1201735. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, He Z, Xin B, Cao L. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16INK4a expression. Oncogene. 2000;19:2002–2013. doi: 10.1038/sj.onc.1203515. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Sham J S, Ng M H, Tsao S W, Zhang D, Lowe S W, Cao L. LMP1 of Epstein-Barr virus induces proliferation of primary mouse embryonic fibroblasts and cooperatively transforms the cells with a p16-insensitive CDK4 oncogene. J Virol. 2000;74:883–891. doi: 10.1128/jvi.74.2.883-891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zindy F, Quelle D E, Roussel M F, Sherr C J. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]