Abstract
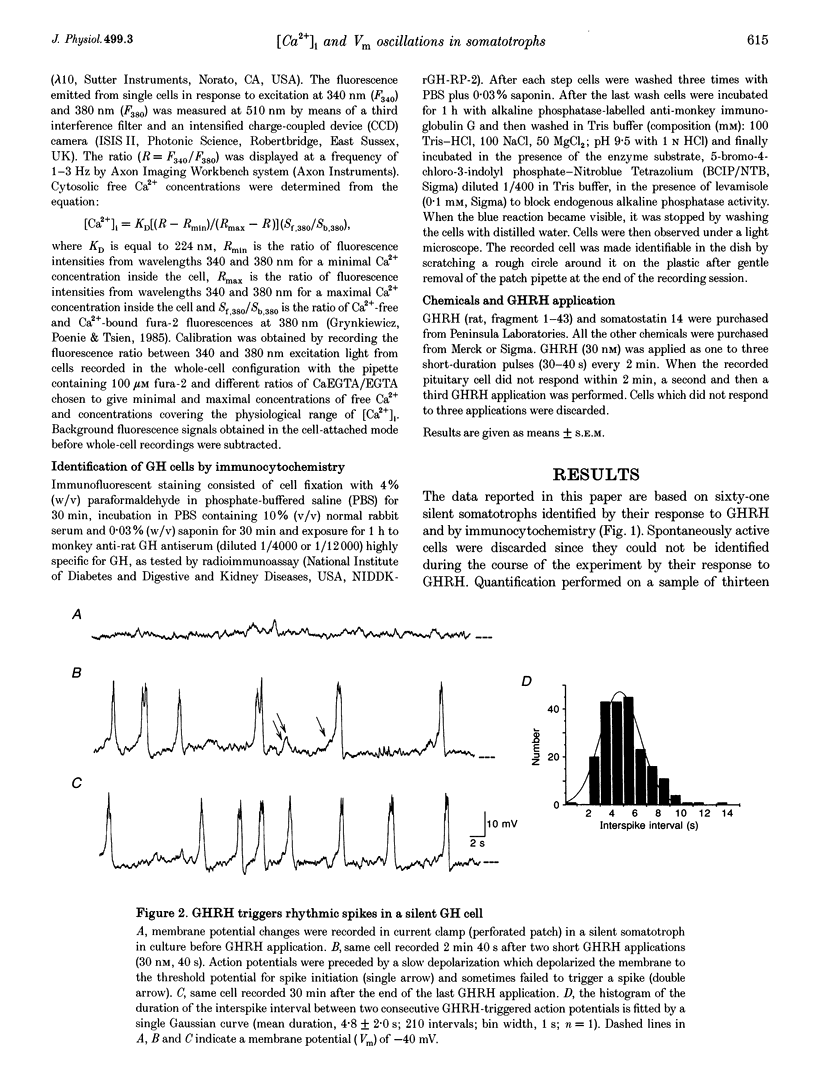
1. The effects of brief applications of growth hormone-releasing hormone (GHRH) to male rat somatotrophs in culture were analysed with the perforated patch clamp technique to record changes in potential or with fura-2 imaging techniques to measure variations of cytosolic Ca2+ concentration ([Ca2+]i). 2. Silent somatotrophs (n = 61) had a mean resting potential of -37 +/- 1 mV and a mean basal [Ca2+]i of 30 +/- 4 nM. Brief GHRH applications (30 nM, 40 s) triggered rhythmic action potentials (23.6 +/- 0.9 mV, 613 +/- 82 ms, 0.21 +/- 0.02 Hz) and [Ca2+]i increase (to 352 +/- 30 nM) followed by rhythmic [Ca2+]i transients (to 138 +/- 6 nM) that persisted up to 90 min after the last GHRH application. Both action potentials and [Ca2+]i transients were totally and reversibly blocked by removing external Ca2+ or Na+ or by adding inorganic Ca2+ channel blockers or nifedipine (3 microM). 3. Somatostatin (1-300 nM), carbamylcholine (0.1-1 microM) and muscarine (0.1-1 microM) each had a dose-dependent inhibitory effect, from a decrease of Ca2+ spike duration and frequency to a complete block of the GHRH-evoked action potentials. 4. The present results show that somatotrophs in culture have intrinsic membrane properties that allow them to sustain a pacemaker activity and subsequent long-lasting sequences of [Ca2+]i oscillations triggered by short pulses of GHRH and inhibited by somatostatin and muscarinic agonists.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertherat J., Bluet-Pajot M. T., Epelbaum J. Neuroendocrine regulation of growth hormone. Eur J Endocrinol. 1995 Jan;132(1):12–24. doi: 10.1530/eje.0.1320012. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Denef C. Immunocytochemical and pharmacological evidence for an intrinsic cholinomimetic system modulating prolactin and growth hormone release in rat pituitary. Endocrinology. 1988 Aug;123(2):1128–1139. doi: 10.1210/endo-123-2-1128. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Denef C. Synthesis and release of acetylcholine by normal and tumoral pituitary corticotrophs. Endocrinology. 1989 May;124(5):2218–2227. doi: 10.1210/endo-124-5-2218. [DOI] [PubMed] [Google Scholar]
- Chen C., Clarke I. J. Ion channels in the regulation of growth hormone secretion from somatotrophs by somatostatin. Growth Regul. 1992 Dec;2(4):167–174. [PubMed] [Google Scholar]
- Chen C., Clarke I. J. Modulation of Ca2+ influx in the ovine somatotroph by growth hormone-releasing factor. Am J Physiol. 1995 Feb;268(2 Pt 1):E204–E212. doi: 10.1152/ajpendo.1995.268.2.E204. [DOI] [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses of rat pituitary cells in somatotroph-enriched primary culture to human growth-hormone releasing factor. Neuroendocrinology. 1989 Dec;50(6):679–687. doi: 10.1159/000125299. [DOI] [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses to somatostatin of rat hypophysial cells in somatotroph-enriched primary cultures. J Physiol. 1989 Jan;408:493–510. doi: 10.1113/jphysiol.1989.sp017472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang J., Vincent J. D., Israel J. M. Sodium and calcium currents in action potentials of rat somatotrophs: their possible functions in growth hormone secretion. Life Sci. 1990;46(14):983–989. doi: 10.1016/0024-3205(90)90021-i. [DOI] [PubMed] [Google Scholar]
- Cuttler L., Glaum S. R., Collins B. A., Miller R. J. Calcium signalling in single growth hormone-releasing factor-responsive pituitary cells. Endocrinology. 1992 Feb;130(2):945–953. doi: 10.1210/endo.130.2.1733736. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Holl R. W., Thorner M. O., Leong D. A. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988 Jun;122(6):2927–2932. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- Kato M., Hattori M. A., Suzuki M. Inhibition by extracellular Na+ replacement of GRF-induced GH secretion from rat pituitary cells. Am J Physiol. 1988 Apr;254(4 Pt 1):E476–E481. doi: 10.1152/ajpendo.1988.254.4.E476. [DOI] [PubMed] [Google Scholar]
- Kato M., Hoyland J., Sikdar S. K., Mason W. T. Imaging of intracellular calcium in rat anterior pituitary cells in response to growth hormone releasing factor. J Physiol. 1992 Feb;447:171–189. doi: 10.1113/jphysiol.1992.sp018997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Suzuki M. Growth hormone releasing factor depolarizes rat pituitary cells in Na+-dependent mechanism. Brain Res. 1989 Jan 2;476(1):145–148. doi: 10.1016/0006-8993(89)91547-3. [DOI] [PubMed] [Google Scholar]
- Kato M., Suzuki M. The time course and extracellular Ca2+ involvement of growth hormone (GH) releasing factor-induced GH secretion in perifused dispersed rat pituitary cells. Jpn J Physiol. 1986;36(6):1225–1239. doi: 10.2170/jjphysiol.36.1225. [DOI] [PubMed] [Google Scholar]
- LaBella F. S., Shin S. Estimation of cholinesterase and choline acetyltransferase in bovine anterior pituitary, posterior pituitary, and pineal body. J Neurochem. 1968 Apr;15(4):335–342. doi: 10.1111/j.1471-4159.1968.tb11618.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. L., Goodman M. B., St John P. A., Barker J. L. Calcium currents and fura-2 signals in fluorescence-activated cell sorted lactotrophs and somatotrophs of rat anterior pituitary. Endocrinology. 1988 Jul;123(1):611–621. doi: 10.1210/endo-123-1-611. [DOI] [PubMed] [Google Scholar]
- Lussier B. T., Wood D. A., French M. B., Moor B. C., Kraicer J. Free intracellular Ca2+ concentration ([Ca2+]i) and growth hormone release from purified rat somatotrophs. II. Somatostatin lowers [Ca2+]i by inhibiting Ca2+ influx. Endocrinology. 1991 Jan;128(1):583–591. doi: 10.1210/endo-128-1-583. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Rawlings S. R. Whole-cell recordings of ionic currents in bovine somatotrophs and their involvement in growth hormone secretion. J Physiol. 1988 Nov;405:577–593. doi: 10.1113/jphysiol.1988.sp017349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Snyder G., McCann S. M. Characterization of muscarinic cholinergic receptors on intact rat anterior pituitary cells. Life Sci. 1980 Aug 11;27(6):475–482. doi: 10.1016/0024-3205(80)90128-9. [DOI] [PubMed] [Google Scholar]
- Naumov A. P., Herrington J., Hille B. Actions of growth-hormone-releasing hormone on rat pituitary cells: intracellular calcium and ionic currents. Pflugers Arch. 1994 Jul;427(5-6):414–421. doi: 10.1007/BF00374255. [DOI] [PubMed] [Google Scholar]
- Nussinovitch I. Growth hormone releasing factor evokes rhythmic hyperpolarizing currents in rat anterior pituitary cells. J Physiol. 1988 Jan;395:303–318. doi: 10.1113/jphysiol.1988.sp016920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch I. Somatostatin inhibits two types of voltage-activated calcium currents in rat growth-hormone secreting cells. Brain Res. 1989 Dec 11;504(1):136–138. doi: 10.1016/0006-8993(89)91610-7. [DOI] [PubMed] [Google Scholar]
- Selverston A. I., Miller J. P., Wadepuhl M. Cooperative mechanisms for the production of rhythmic movements. Symp Soc Exp Biol. 1983;37:55–87. [PubMed] [Google Scholar]
- Sheppard M. S., Moor B. C., Kraicer J. Release of growth hormone (GH) from purified somatotrophs: interaction of GH-releasing factor and somatostatin and role of adenosine 3',5'-monophosphate. Endocrinology. 1985 Dec;117(6):2364–2370. doi: 10.1210/endo-117-6-2364. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Lussier B. T., Kraicer J. Somatostatin activates an inwardly rectifying K+ conductance in freshly dispersed rat somatotrophs. J Physiol. 1991 Sep;441:615–637. doi: 10.1113/jphysiol.1991.sp018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum G. S., Martin J. B. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology. 1976 Mar;98(3):562–570. doi: 10.1210/endo-98-3-562. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Burt D. R. Pituitary cell cultures contain muscarinic receptors. Eur J Pharmacol. 1980 Jul 25;65(2-3):305–308. doi: 10.1016/0014-2999(80)90407-0. [DOI] [PubMed] [Google Scholar]
- Weiss J., Cronin M. J., Thorner M. O. Periodic interactions of GH-releasing factor and somatostatin can augment GH release in vitro. Am J Physiol. 1987 Nov;253(5 Pt 1):E508–E514. doi: 10.1152/ajpendo.1987.253.5.E508. [DOI] [PubMed] [Google Scholar]



