Abstract
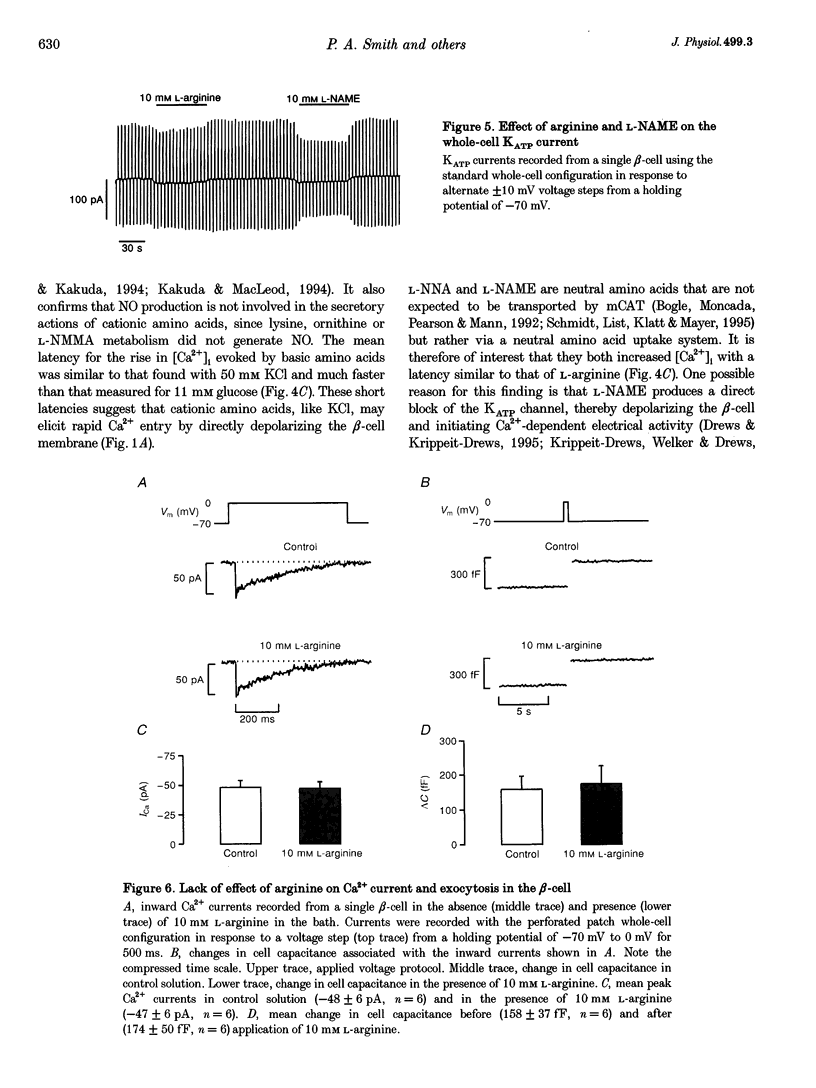
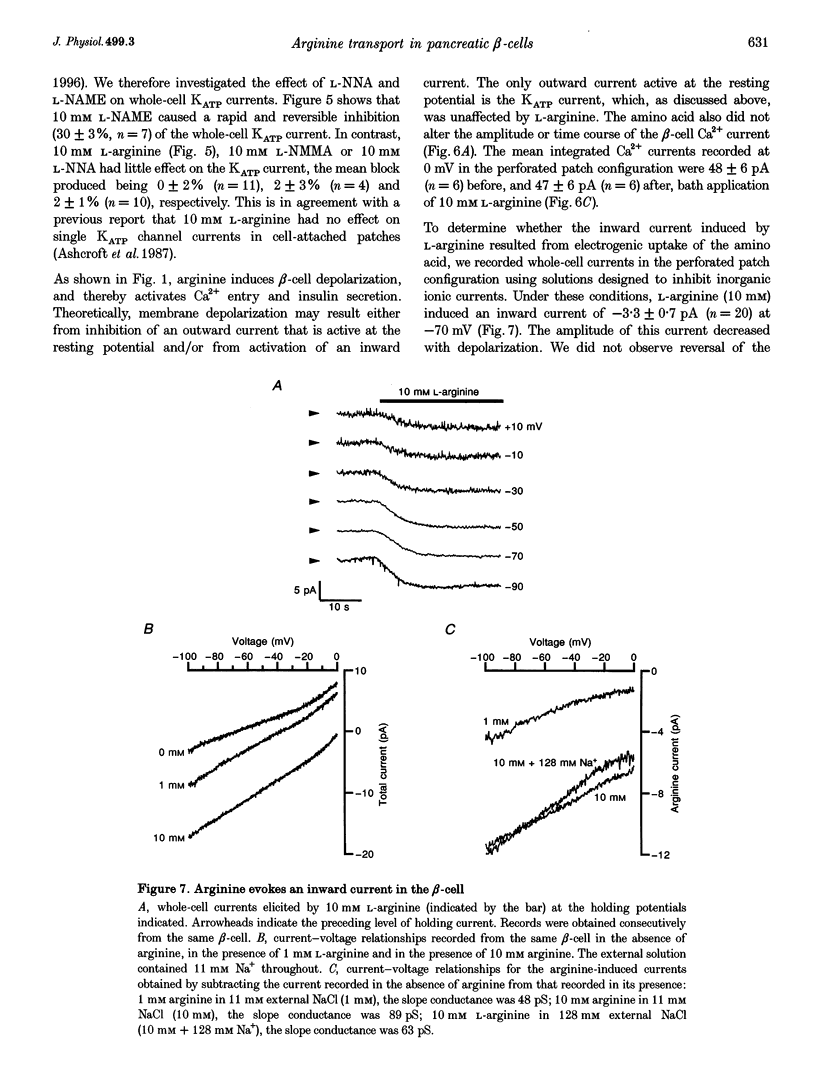
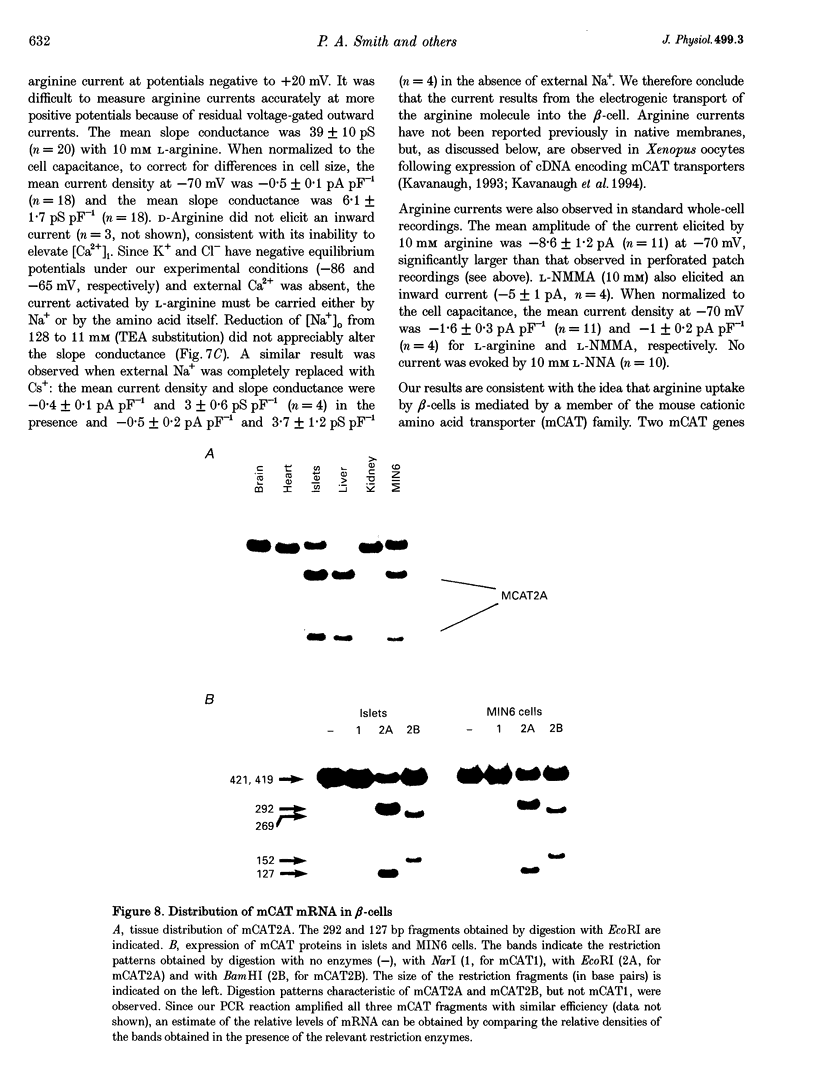
1. We have investigated the mechanism by which L-arginine stimulates membrane depolarization, an increase of intracellular calcium ([Ca2+]i) and insulin secretion in pancreatic beta-cells. 2. L-Arginine failed to affect beta-cell metabolism, as monitored by NAD(P)H autofluorescence. 3. L-Arginine produced a dose-dependent increase in [Ca2+]i, which was dependent on membrane depolarization and extracellular calcium. 4. The cationic amino acids L-ornithine, L-lysine, L-homoarginine (which is not metabolized) and NG-monomethyl-L-arginine (L-NMMA, a nitric oxide synthase inhibitor) produced [Ca2+]i responses similar to that produced by L-arginine. The neutral nitric oxide synthase inhibitors NG-nitro-L-arginine (L-NNA) and N omega-monomethyl-L-arginine (L-NAME) also increased [Ca2+]i. D-Arginine was ineffective. 5. L-Arginine did not affect whole-cell Ca2+ currents or ATP-sensitive K+ currents, but produced an inward current that was carried by the amino acid. 6. The reverse transcriptase-polymerase chain reaction demonstrated the presence of messenger RNA for the murine cationic amino acid transporters mCAT2A and mCAT2B within the beta-cell. 7. L-Arginine did not affect beta-cell exocytosis as assayed by changes in cell capacitance. 8. Our data suggest that L-arginine elevates [Ca2+]i and stimulates insulin secretion as a consequence of its electrogenic transport into the beta-cell. This uptake is mediated by the mCAT2A transporter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Eliasson L., Bokvist K., Larsson O., Ashcroft F. M., Rorsman P. Exocytosis elicited by action potentials and voltage-clamp calcium currents in individual mouse pancreatic B-cells. J Physiol. 1993 Dec;472:665–688. doi: 10.1113/jphysiol.1993.sp019966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Ashcroft S. J., Harrison D. E. Effects of 2-ketoisocaproate on insulin release and single potassium channel activity in dispersed rat pancreatic beta-cells. J Physiol. 1987 Apr;385:517–529. doi: 10.1113/jphysiol.1987.sp016505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Blachier F., Leclercq-Meyer V., Marchand J., Woussen-Colle M. C., Mathias P. C., Sener A., Malaisse W. J. Stimulus-secretion coupling of arginine-induced insulin release. Functional response of islets to L-arginine and L-ornithine. Biochim Biophys Acta. 1989 Sep 19;1013(2):144–151. doi: 10.1016/0167-4889(89)90042-6. [DOI] [PubMed] [Google Scholar]
- Blachier F., Mourtada A., Sener A., Malaisse W. J. Stimulus-secretion coupling of arginine-induced insulin release. Uptake of metabolized and nonmetabolized cationic amino acids by pancreatic islets. Endocrinology. 1989 Jan;124(1):134–141. doi: 10.1210/endo-124-1-134. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Moncada S., Pearson J. D., Mann G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. Br J Pharmacol. 1992 Apr;105(4):768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L., Klöppel G. Cytochemical localization of NADPH-diaphorase in the four types of pancreatic islet cell. Histochemistry. 1994 Mar;101(3):209–214. doi: 10.1007/BF00269546. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Charles S., Tamagawa T., Henquin J. C. A single mechanism for the stimulation of insulin release and 86Rb+ efflux from rat islets by cationic amino acids. Biochem J. 1982 Nov 15;208(2):301–308. doi: 10.1042/bj2080301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs E. I., Albritton L. M., Kim J. W., Cunningham J. M. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem. 1993 Apr 5;268(10):7538–7544. [PubMed] [Google Scholar]
- Dimitriadis G. D., Pehling G. B., Gerich J. E. Abnormal glucose modulation of islet A- and B-cell responses to arginine in non-insulin-dependent diabetes mellitus. Diabetes. 1985 Jun;34(6):541–547. doi: 10.2337/diab.34.6.541. [DOI] [PubMed] [Google Scholar]
- Drews G., Krippeit-Drews P. NO synthase activity does not influence electrical activity of mouse pancreatic B-cells. Biochem Biophys Res Commun. 1995 May 25;210(3):914–920. doi: 10.1006/bbrc.1995.1744. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Smith P. A., Ashcroft F. M. Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Biochem J. 1993 Aug 15;294(Pt 1):35–42. doi: 10.1042/bj2940035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. C., Jenkinson D. H. The effect of noradrenaline on the ion permeability of isolated mammalian hepatocytes, studied by intracellular recording. J Physiol. 1987 Nov;392:493–512. doi: 10.1113/jphysiol.1987.sp016793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. Uptake of alanine, arginine and leucine by mammalian pancreatic beta-cells. Endocrinology. 1971 Dec;89(6):1432–1439. doi: 10.1210/endo-89-6-1432. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Effects of amino acids on membrane potential and 86Rb+ fluxes in pancreatic beta-cells. Am J Physiol. 1981 Mar;240(3):E245–E252. doi: 10.1152/ajpendo.1981.240.3.E245. [DOI] [PubMed] [Google Scholar]
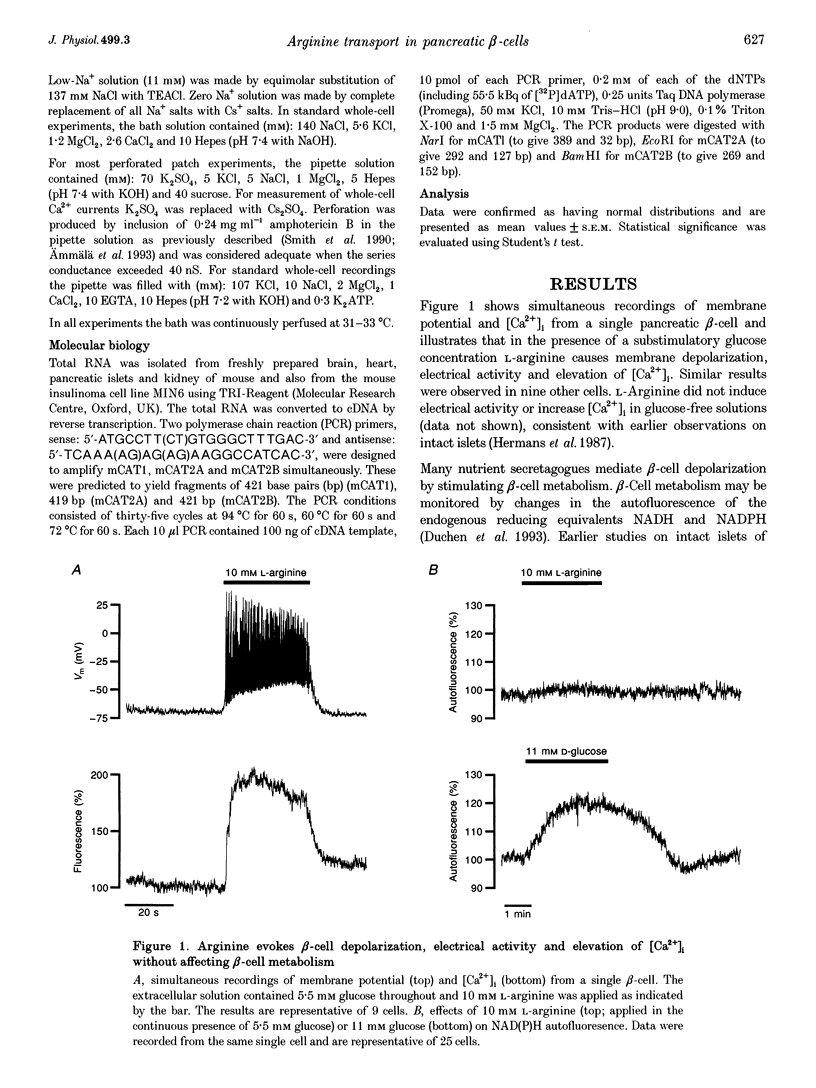
- Hermans M. P., Schmeer W., Henquin J. C. The permissive effect of glucose, tolbutamide and high K+ on arginine stimulation of insulin release in isolated mouse islets. Diabetologia. 1987 Aug;30(8):659–665. doi: 10.1007/BF00277325. [DOI] [PubMed] [Google Scholar]
- Jansson L., Sandler S. The nitric oxide synthase II inhibitor NG-nitro-L-arginine stimulates pancreatic islet insulin release in vitro, but not in the perfused pancreas. Endocrinology. 1991 Jun;128(6):3081–3085. doi: 10.1210/endo-128-6-3081. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Bjaaland T., Pearson J. D., Howell S. L. Nitric oxide is not involved in the initiation of insulin secretion from rat islets of Langerhans. Diabetologia. 1992 Nov;35(11):1020–1027. doi: 10.1007/BF02221676. [DOI] [PubMed] [Google Scholar]
- Kakuda D. K., MacLeod C. L. Na(+)-independent transport (uniport) of amino acids and glucose in mammalian cells. J Exp Biol. 1994 Nov;196:93–108. doi: 10.1242/jeb.196.1.93. [DOI] [PubMed] [Google Scholar]
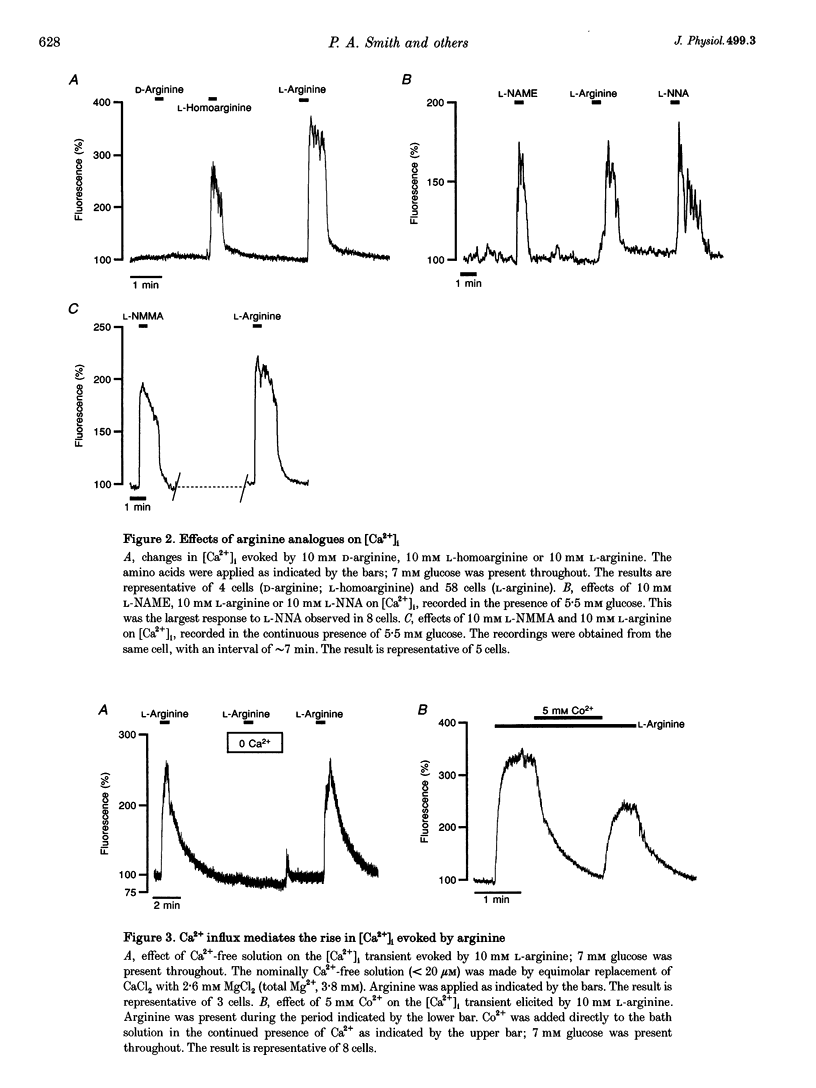
- Kavanaugh M. P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry. 1993 Jun 8;32(22):5781–5785. doi: 10.1021/bi00073a009. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Wang H., Zhang Z., Zhang W., Wu Y. N., Dechant E., North R. A., Kabat D. Control of cationic amino acid transport and retroviral receptor functions in a membrane protein family. J Biol Chem. 1994 Jun 3;269(22):15445–15450. [PubMed] [Google Scholar]
- Krippeit-Drews P., Welker S., Drews G. Effects of the nitric oxide synthase inhibitor N omega nitro-L-arginine methyl ester on electrical activity and ion channels of mouse pancreatic B cells. Biochem Biophys Res Commun. 1996 Jul 5;224(1):199–205. doi: 10.1006/bbrc.1996.1007. [DOI] [PubMed] [Google Scholar]
- MacLeod C. L., Finley K. D., Kakuda D. K. y(+)-type cationic amino acid transport: expression and regulation of the mCAT genes. J Exp Biol. 1994 Nov;196:109–121. doi: 10.1242/jeb.196.1.109. [DOI] [PubMed] [Google Scholar]
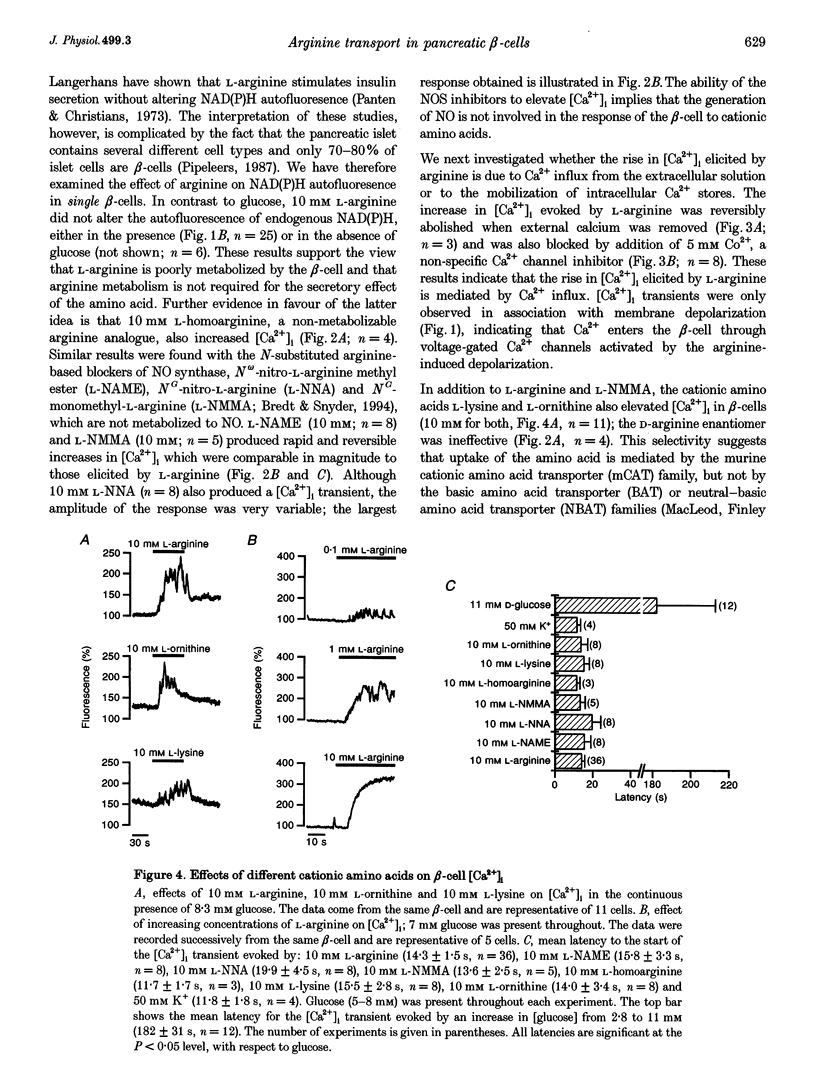
- Panagiotidis G., Akesson B., Rydell E. L., Lundquist I. Influence of nitric oxide synthase inhibition, nitric oxide and hydroperoxide on insulin release induced by various secretagogues. Br J Pharmacol. 1995 Jan;114(2):289–296. doi: 10.1111/j.1476-5381.1995.tb13225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotidis G., Alm P., Lundquist I. Inhibition of islet nitric oxide synthase increases arginine-induced insulin release. Eur J Pharmacol. 1992 Dec 15;229(2-3):277–278. doi: 10.1016/0014-2999(92)90568-o. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. The biosociology of pancreatic B cells. Diabetologia. 1987 May;30(5):277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Ashcroft F. M., Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. FEBS Lett. 1990 Feb 12;261(1):187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- Southern C., Schulster D., Green I. C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990 Dec 10;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- Toback F. G., Mayers A. M., Lowenstein L. M. Alterations in renal and plasma amino acid concentrations during renal compensatory growth. Am J Physiol. 1973 Nov;225(5):1247–1251. doi: 10.1152/ajplegacy.1973.225.5.1247. [DOI] [PubMed] [Google Scholar]
- Ward W. K., Beard J. C., Halter J. B., Porte D., Jr Pathophysiology of insulin secretion in diabetes mellitus. Adv Exp Med Biol. 1985;189:137–158. doi: 10.1007/978-1-4757-1850-8_9. [DOI] [PubMed] [Google Scholar]
- Ward W. K., Bolgiano D. C., McKnight B., Halter J. B., Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984 Oct;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott N. J., Galione A., Smith P. A. Nitric oxide induces intracellular Ca2+ mobilization and increases secretion of incorporated 5-hydroxytryptamine in rat pancreatic beta-cells. FEBS Lett. 1995 Sep 4;371(2):99–104. doi: 10.1016/0014-5793(95)00848-4. [DOI] [PubMed] [Google Scholar]
- Wörl J., Wiesand M., Mayer B., Greskötter K. R., Neuhuber W. L. Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in rat and human pancreas: influence of fixation. Histochemistry. 1994 Nov;102(5):353–364. doi: 10.1007/BF00268906. [DOI] [PubMed] [Google Scholar]