Abstract
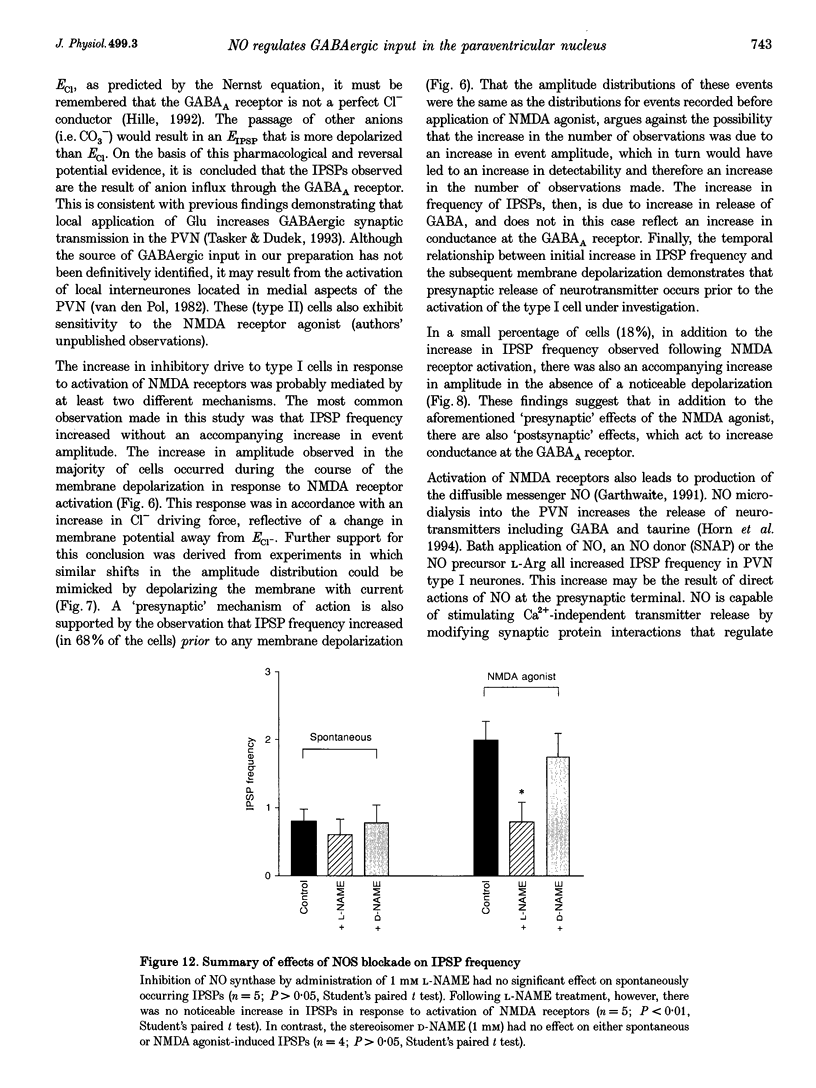
1. Whole-cell recordings were obtained from type I paraventricular nucleus (PVN) neurones in coronal slices of rat hypothalamus to study the involvement of nitric oxide (NO) in the modulation of inhibitory transmission resulting from the activation of N-methyl-D-aspartate (NMDA) receptors by the high affinity receptor agonist D,L-tetrazol-5-ylglycine. 2. A brief pulse of NMDA agonist (0.1-10 microM) faithfully elicited increases in action potential firing frequency in all type I cells tested (n = 55). In cells with membrane potentials positive to -75 mV, this excitation was accompanied by an underlying depolarization (> 2 mV) in the majority of cases (n = 45). At membrane potentials negative to -75 mV, NMDA agonist application elicited an initial monotonie depolarization, which was auxiliary to profound, rhythmic oscillations of the membrane potential, resulting in the emergence of burst-like activity in these cells (n = 8). 3. In addition to depolarizing the neurones, the NMDA agonist also elicited inhibitory postsynaptic potentials (IPSPs) in 40% (n = 22) of the cells tested. The IPSPs were inhibited by the GABAA receptor antagonist bicuculline methiodide (BMI). 4. Microdialysis of NO into the PVN has been shown to increase local levels of inhibitory neurotransmitters, including GABA. The possibility that NO-induced increases in GABA lead to an increase in inhibitory synaptic activity in PVN was investigated by administering NO by three different methods. Bath application of the donor compound, S-nitroso-N-acetyl-penicillamine (SNAP; n = 7), bubbled NO solution (n = 5), or the NO precursor L-arginine (n = 6) all elicited increases in IPSP frequency. 5. Production of NO in other brain centres has been linked to the activation of the NMDA receptor. In order to determine whether the increase in IPSPs following NMDA was the result of activation of NO, the production of NO was blocked with the NO synthase inhibitor N omega-nitro-L-arginine methylester (L-NAME). Subsequent NMDA receptor activation elicited more pronounced depolarizations, but there was no accompanying increase in IPSP frequency (n = 5). 6. This study demonstrates that GABAergic inhibition resulting from NMDA receptor activation can be regulated profoundly by NO. By increasing inhibitory transmission within a nucleus, NO may serve as an important intermediary in the regulation of neuronal excitability in the central nervous system.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Leinders-Zufall T., Kocsis J. D., Shepherd G. M., Zufall F., Barnstable C. J. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994 Jan;12(1):155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Andrew R. D., MacVicar B. A., Dudek F. E., Hatton G. I. Dye transfer through gap junctions between neuroendocrine cells of rat hypothalamus. Science. 1981 Mar 13;211(4487):1187–1189. doi: 10.1126/science.7466393. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Warach S., Hatton G. I., McNeill T. H. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Bains J. S., Ferguson A. V. Angiotensin II neurotransmitter actions in paraventricular nucleus are potentiated by a nitric oxide synthase inhibitor. Regul Pept. 1994 Feb 3;50(1):53–59. doi: 10.1016/0167-0115(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Smithson K. G., Hatton G. I. Dye-coupled magnocellular peptidergic neurons of the rat paraventricular nucleus show homotypic immunoreactivity. Neuroscience. 1985 Dec;16(4):885–895. doi: 10.1016/0306-4522(85)90103-4. [DOI] [PubMed] [Google Scholar]
- Cui L. N., Inenaga K., Nagatomo T., Yamashita H. Sodium nitroprusside modulates NMDA response in the rat supraoptic neurons in vitro. Brain Res Bull. 1994;35(3):253–260. doi: 10.1016/0361-9230(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Decavel C., Dubourg P., Leon-Henri B., Geffard M., Calas A. Simultaneous immunogold labeling of GABAergic terminals and vasopressin-containing neurons in the rat paraventricular nucleus. Cell Tissue Res. 1989 Jan;255(1):77–80. doi: 10.1007/BF00229068. [DOI] [PubMed] [Google Scholar]
- Decavel C., van den Pol A. N. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol. 1992 Feb 1;316(1):104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Fagni L., Olivier M., Lafon-Cazal M., Bockaert J. Involvement of divalent ions in the nitric oxide-induced blockade of N-methyl-D-aspartate receptors in cerebellar granule cells. Mol Pharmacol. 1995 Jun;47(6):1239–1247. [PubMed] [Google Scholar]
- Ferguson A. V., Loucks C. Cardiovascular and single-unit responses to subfornical organ stimulation are abolished by pentobarbital anesthesia. Can J Physiol Pharmacol. 1994 Sep;72(9):1031–1034. doi: 10.1139/y94-144. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Boulton C. L. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Hoffman N. W., Tasker J. G., Dudek F. E. Immunohistochemical differentiation of electrophysiologically defined neuronal populations in the region of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 1991 May 15;307(3):405–416. doi: 10.1002/cne.903070306. [DOI] [PubMed] [Google Scholar]
- Horn T., Smith P. M., McLaughlin B. E., Bauce L., Marks G. S., Pittman Q. J., Ferguson A. V. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994 Jan;266(1 Pt 2):R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- Hu B., Bourque C. W. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992 Dec;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Oomura Y., Yakushiji T., Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986 Nov 13;324(6093):156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Kiss J. Z., Martos J., Palkovits M. Hypothalamic paraventricular nucleus: a quantitative analysis of cytoarchitectonic subdivisions in the rat. J Comp Neurol. 1991 Nov 22;313(4):563–573. doi: 10.1002/cne.903130403. [DOI] [PubMed] [Google Scholar]
- Li Z., Ferguson A. V. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: modulation of IA by angiotensin II. Neuroscience. 1996 Mar;71(1):133–145. doi: 10.1016/0306-4522(95)00434-3. [DOI] [PubMed] [Google Scholar]
- Liposits Z. Ultrastructure of hypothalamic paraventricular neurons. Crit Rev Neurobiol. 1993;7(2):89–162. [PubMed] [Google Scholar]
- Lipton S. A., Choi Y. B., Pan Z. H., Lei S. Z., Chen H. S., Sucher N. J., Loscalzo J., Singel D. J., Stamler J. S. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993 Aug 12;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lupica C. R. Delta and mu enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995 Jan;15(1 Pt 2):737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni O., Bockaert J. Nitric oxide synthase activity endogenously modulates NMDA receptors. J Neurochem. 1993 Jul;61(1):368–370. doi: 10.1111/j.1471-4159.1993.tb03580.x. [DOI] [PubMed] [Google Scholar]
- Manzoni O., Prezeau L., Marin P., Desagher S., Deshager S., Bockaert J., Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992 Apr;8(4):653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Martin D. S., Segura T., Haywood J. R. Cardiovascular responses to bicuculline in the paraventricular nucleus of the rat. Hypertension. 1991 Jul;18(1):48–55. doi: 10.1161/01.hyp.18.1.48. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Calakos N. C., Scheller R. H., Schulman H. Nitric oxide modulates synaptic vesicle docking fusion reactions. Neuron. 1996 Jun;16(6):1229–1236. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- Raber J., Bloom F. E. IL-2 induces vasopressin release from the hypothalamus and the amygdala: role of nitric oxide-mediated signaling. J Neurosci. 1994 Oct;14(10):6187–6195. doi: 10.1523/JNEUROSCI.14-10-06187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Characterization of spontaneous and evoked inhibitory postsynaptic potentials in rat supraoptic neurosecretory neurons in vitro. J Neurophysiol. 1986 Dec;56(6):1703–1717. doi: 10.1152/jn.1986.56.6.1703. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Renaud L. P. Actions of gamma-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol. 1987 Jun;387:629–647. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C., Shen G. H. In the rat, endogenous nitric oxide modulates the response of the hypothalamic-pituitary-adrenal axis to interleukin-1 beta, vasopressin, and oxytocin. J Neurosci. 1994 Apr;14(4):1985–1993. doi: 10.1523/JNEUROSCI.14-04-01985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C. B., Loewy A. D., Swanson L. W., Cowan W. M. Direct hypothalamo-autonomic connections. Brain Res. 1976 Nov 26;117(2):305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Summy-Long J. Y., Bui V., Mantz S., Koehler E., Weisz J., Kadekaro M. Central inhibition of nitric oxide synthase preferentially augments release of oxytocin during dehydration. Neurosci Lett. 1993 Apr 2;152(1-2):190–193. doi: 10.1016/0304-3940(93)90515-m. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Kuypers H. G. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980 Dec 1;194(3):555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991 Mar;434:271–293. doi: 10.1113/jphysiol.1991.sp018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1993 Sep;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W. The regulation of quantal size. Prog Neurobiol. 1991;36(2):93–130. doi: 10.1016/0301-0082(91)90019-w. [DOI] [PubMed] [Google Scholar]
- Yasin S., Costa A., Trainer P., Windle R., Forsling M. L., Grossman A. Nitric oxide modulates the release of vasopressin from rat hypothalamic explants. Endocrinology. 1993 Sep;133(3):1466–1469. doi: 10.1210/endo.133.3.7689960. [DOI] [PubMed] [Google Scholar]
- Zarri I., Bucossi G., Cupello A., Rapallino M. V., Robello M. Modulation by nitric oxide of rat brain GABAA receptors. Neurosci Lett. 1994 Oct 24;180(2):239–242. doi: 10.1016/0304-3940(94)90529-0. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol. 1982 Apr 20;206(4):317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]






