Abstract
1. The hypothalamic arcuate nucleus (ARC) contains neuroendocrine neurons that regulate endocrine secretions by releasing substances which control anterior pituitary hormonal release into the portal blood stream. Many neuroactive substances have been identified in the ARC, but the existence of excitatory neurons in the ARC and the identity of an excitatory transmitter have not been investigated physiologically. 2. In the present experiments using whole-cell current- and voltage-clamp recording of neurons from cultures and slices of the ARC, we demonstrate for the first time that some of the neurons in the ARC secrete glutamate as their transmitter. 3. Using microdrop stimulation of presynaptic neurons in ARC slices, we found that local axons from these glutamatergic neurons make local synaptic contact with other neurons in the ARC and that all evoked excitatory postsynaptic potentials could be blocked by the selective ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 microM) and D,L-2-amino-5-phosphonovalerate (AP5; 100 microM). To determine the identity of ARC neurons postsynaptic to local glutamatergic neurons, we used antidromic stimulation to reveal that many of these cells were neuroendocrine neurons by virtue of their maintaining axon terminals in the median eminence. 4. In ARC cultures, postsynaptic potentials, both excitatory and inhibitory, were virtually eliminated by the glutamate receptor antagonists AP5 and CNQX, underlining the functional importance of glutamate within this part of the neuroendocrine brain. 5. GABA was secreted by a subset of ARC neurons from local axons. The GABAA receptor antagonist bicuculline released glutamatergic neurons from chronic inhibition mediated by synaptically released GABA, resulting in further depolarization and an increase in the amplitude and frequency of glutamate-mediated excitatory postsynaptic potentials.
Full text
PDF

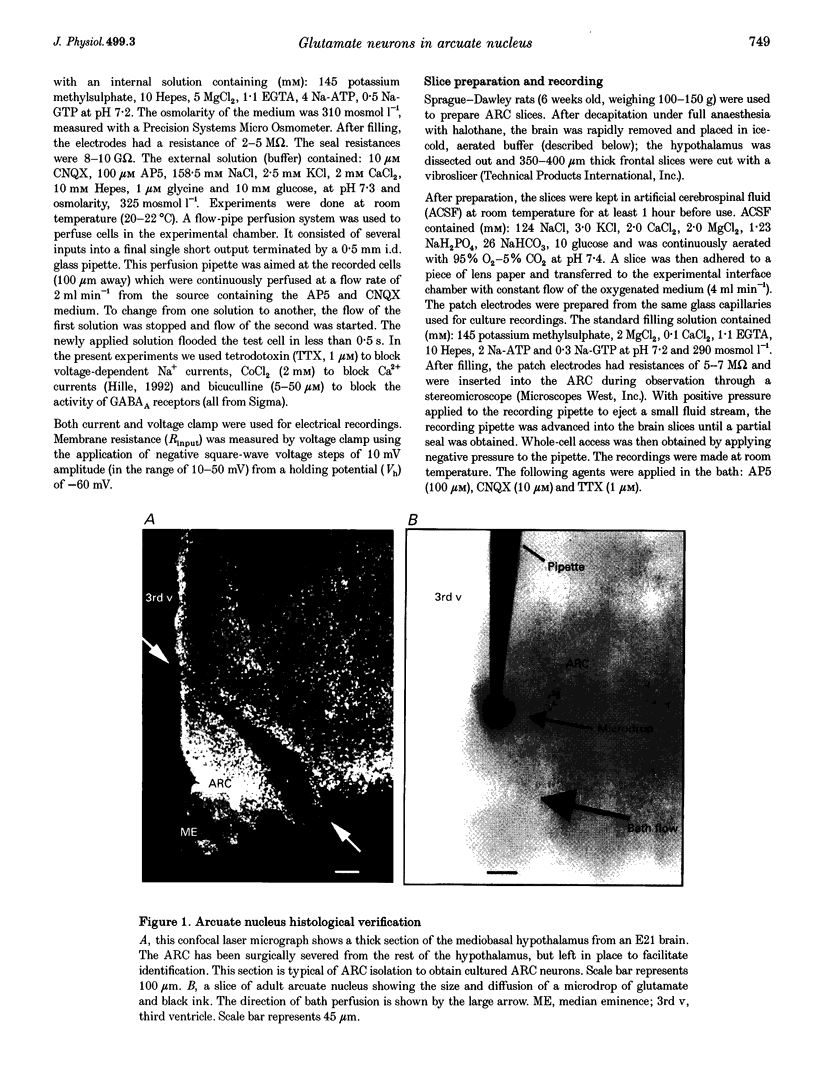
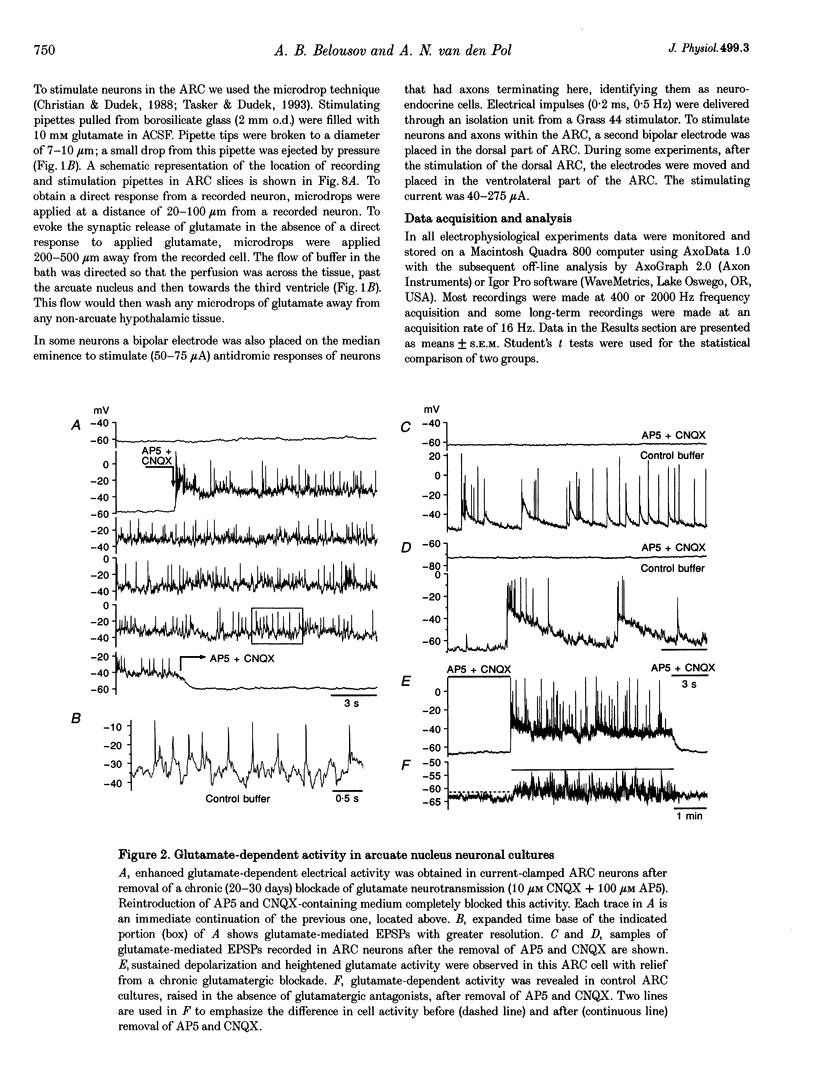
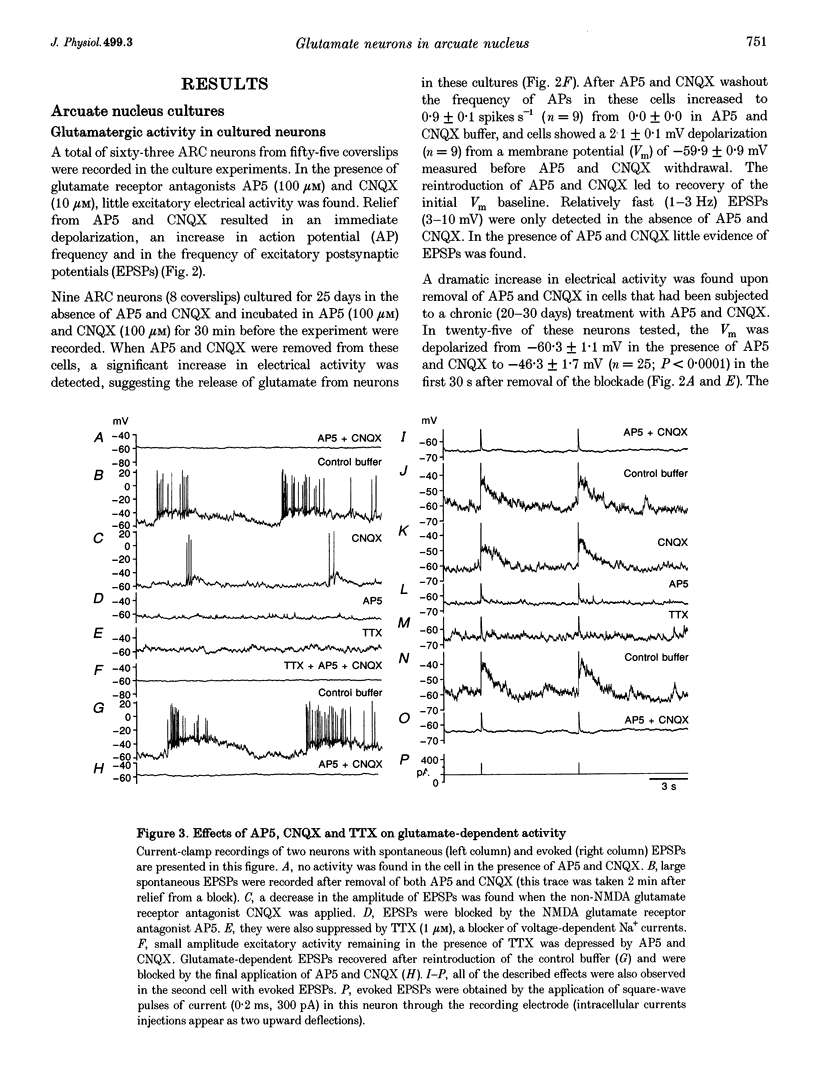
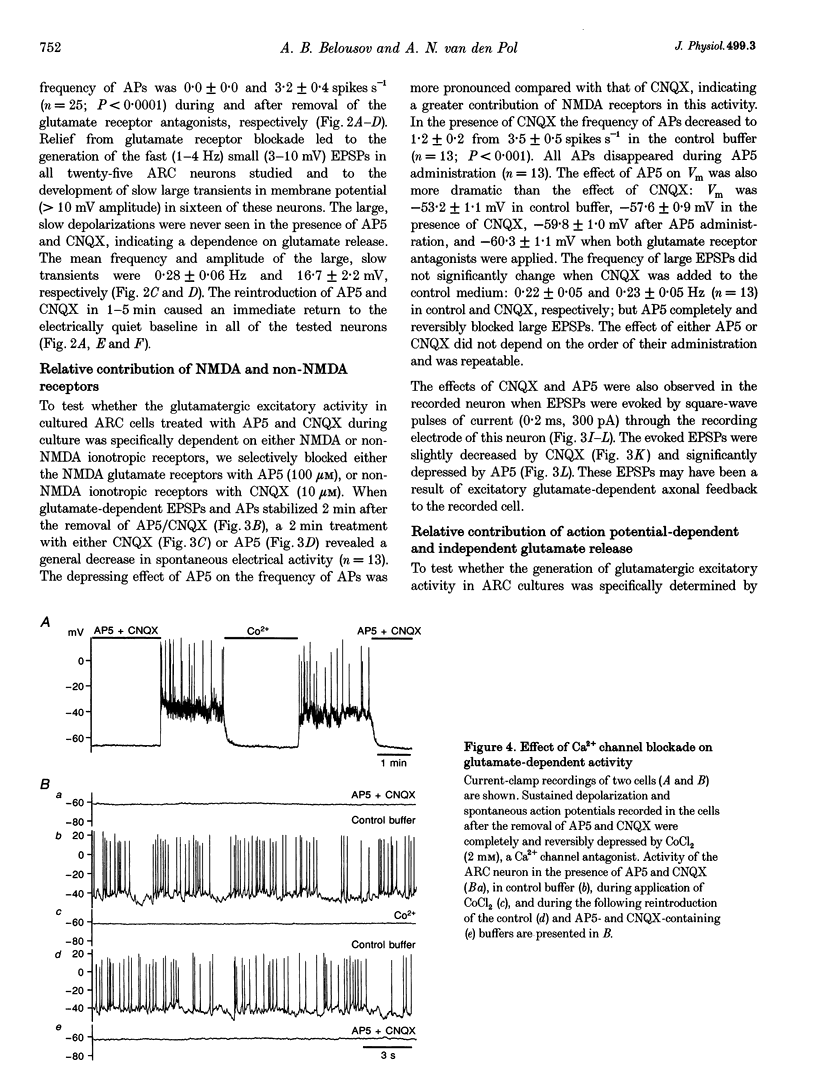
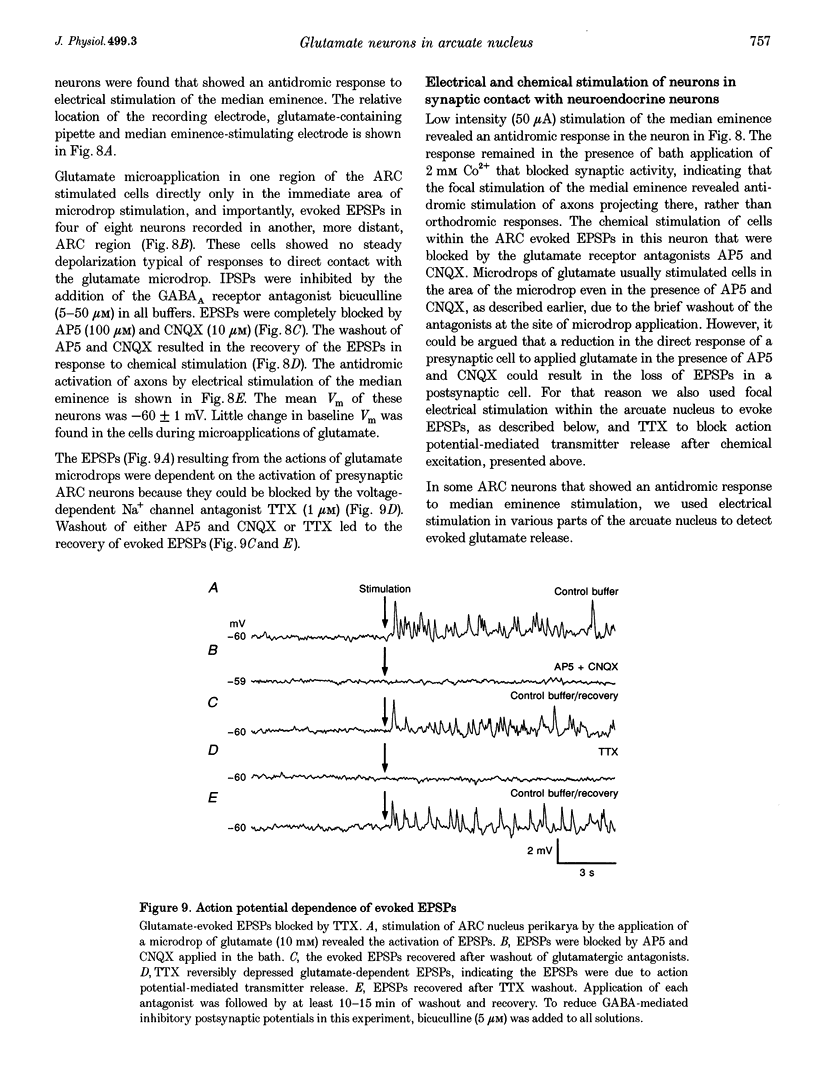
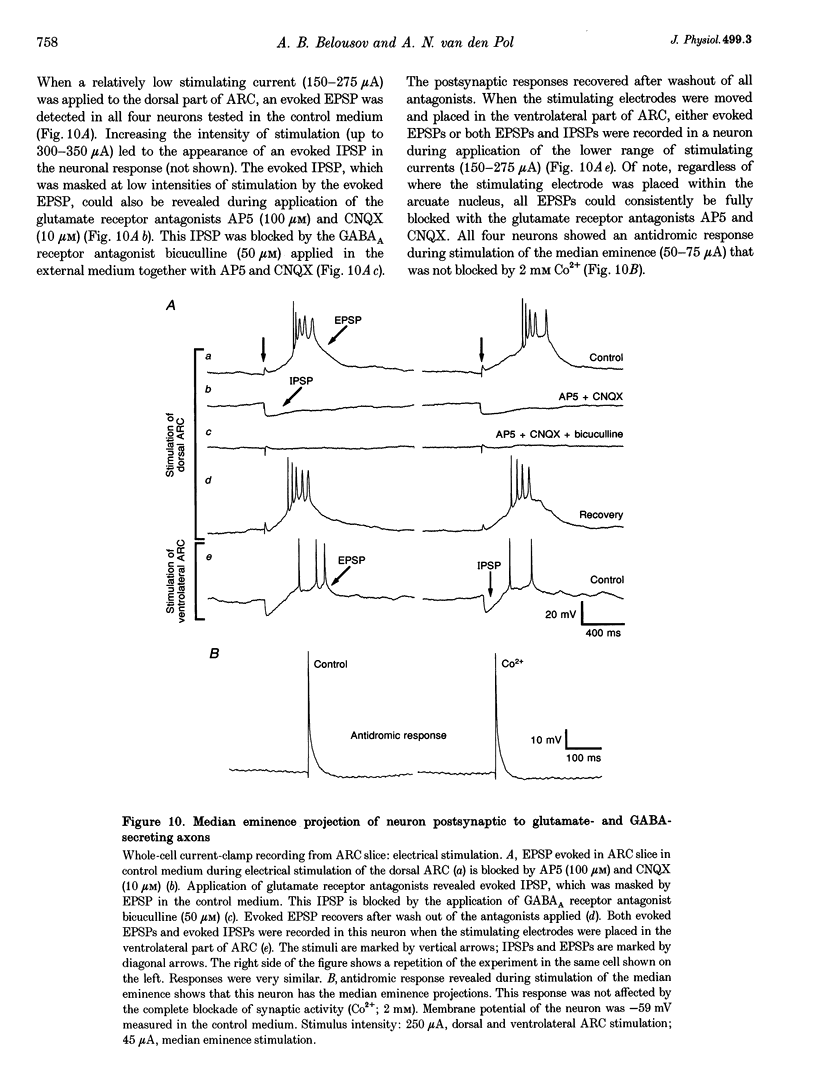



Images in this article
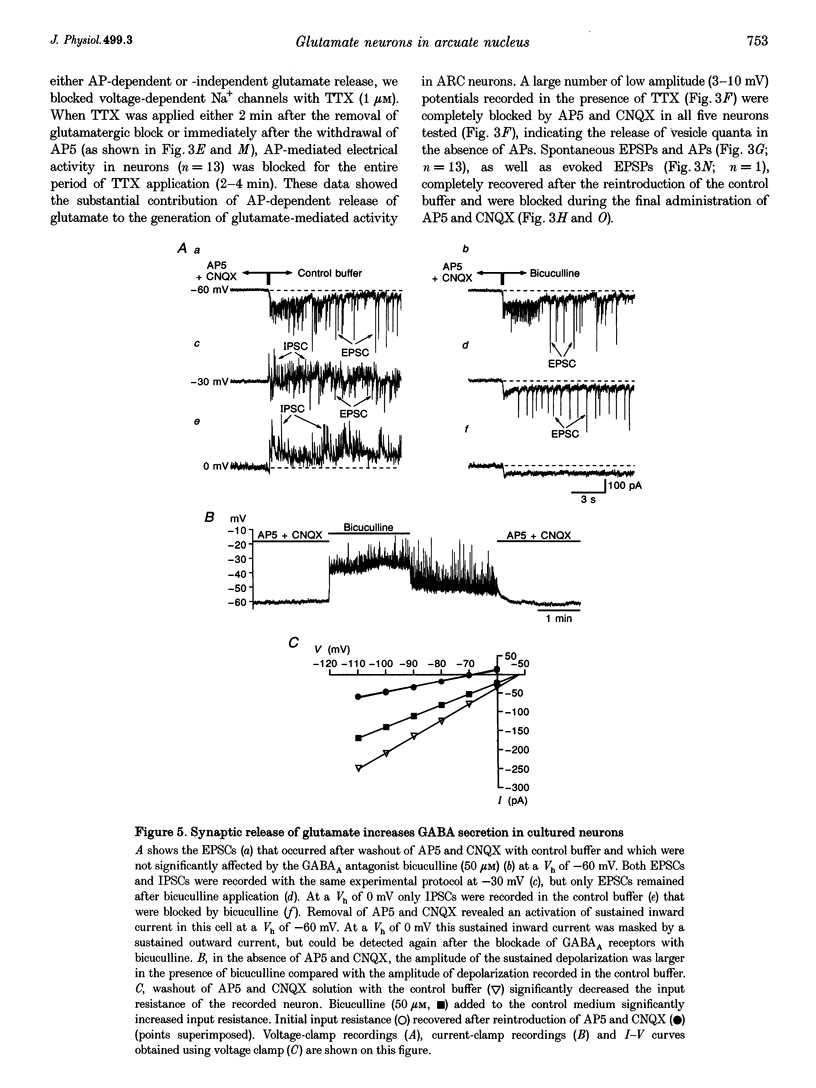
Selected References
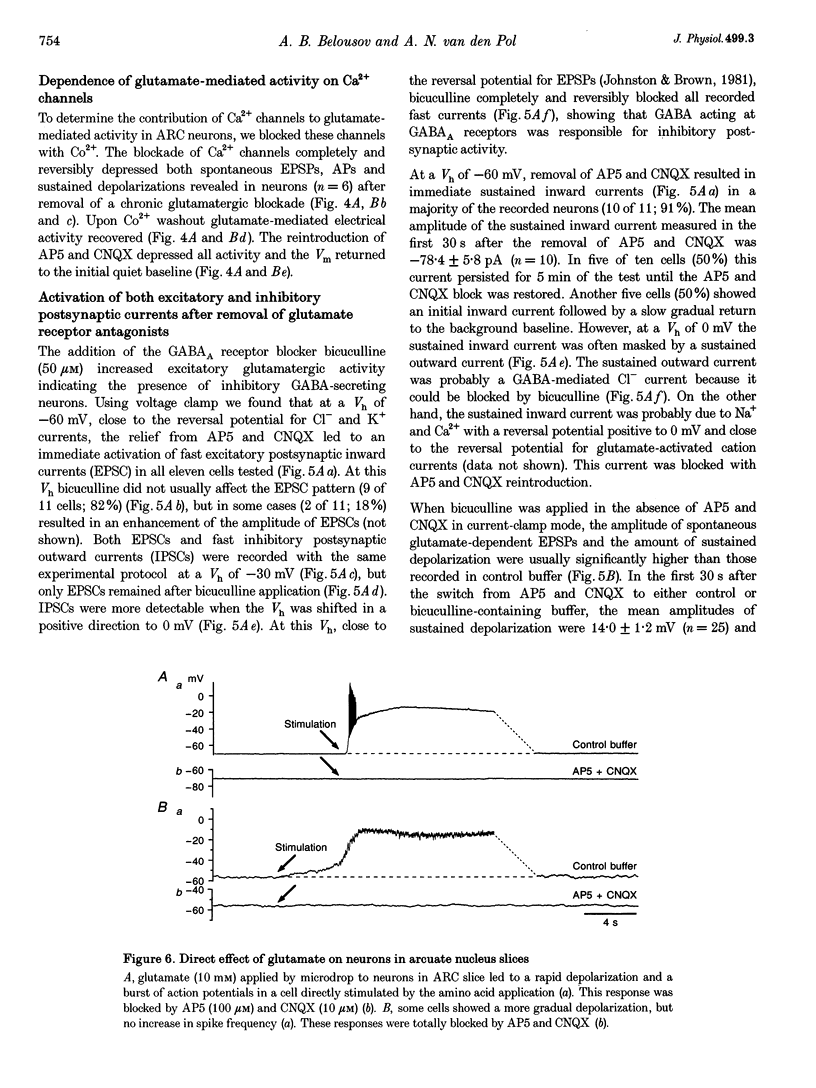
These references are in PubMed. This may not be the complete list of references from this article.
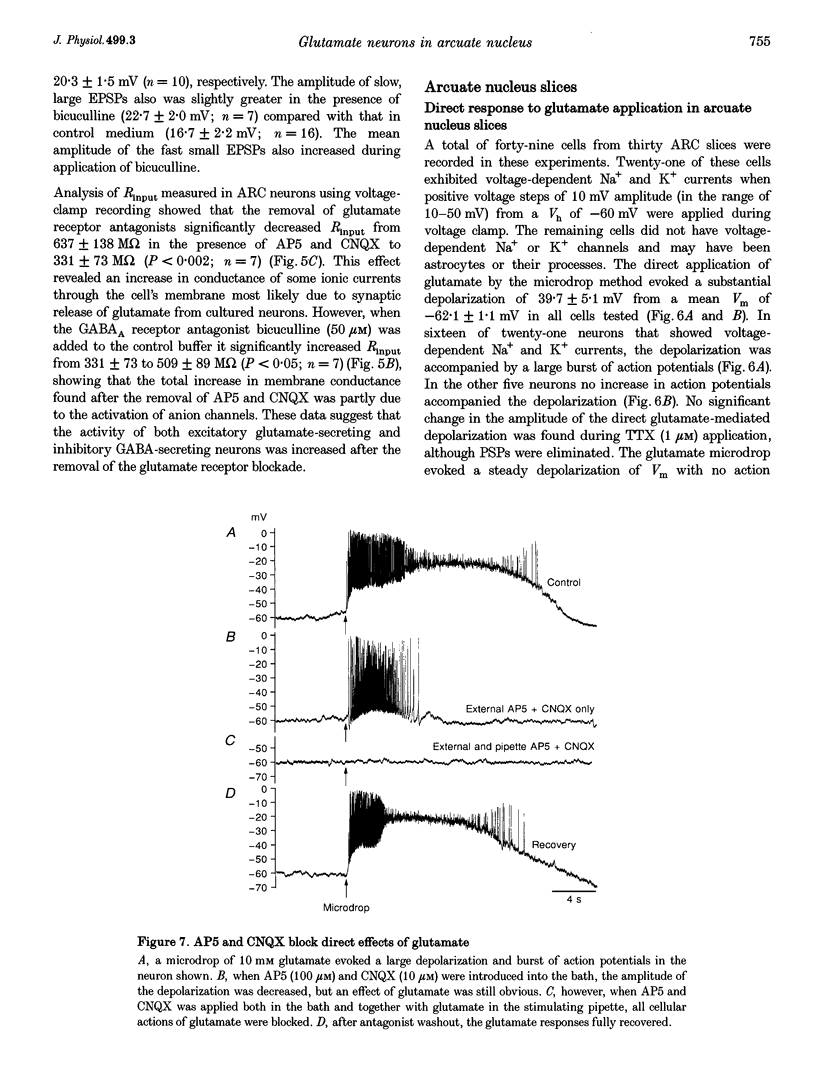
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
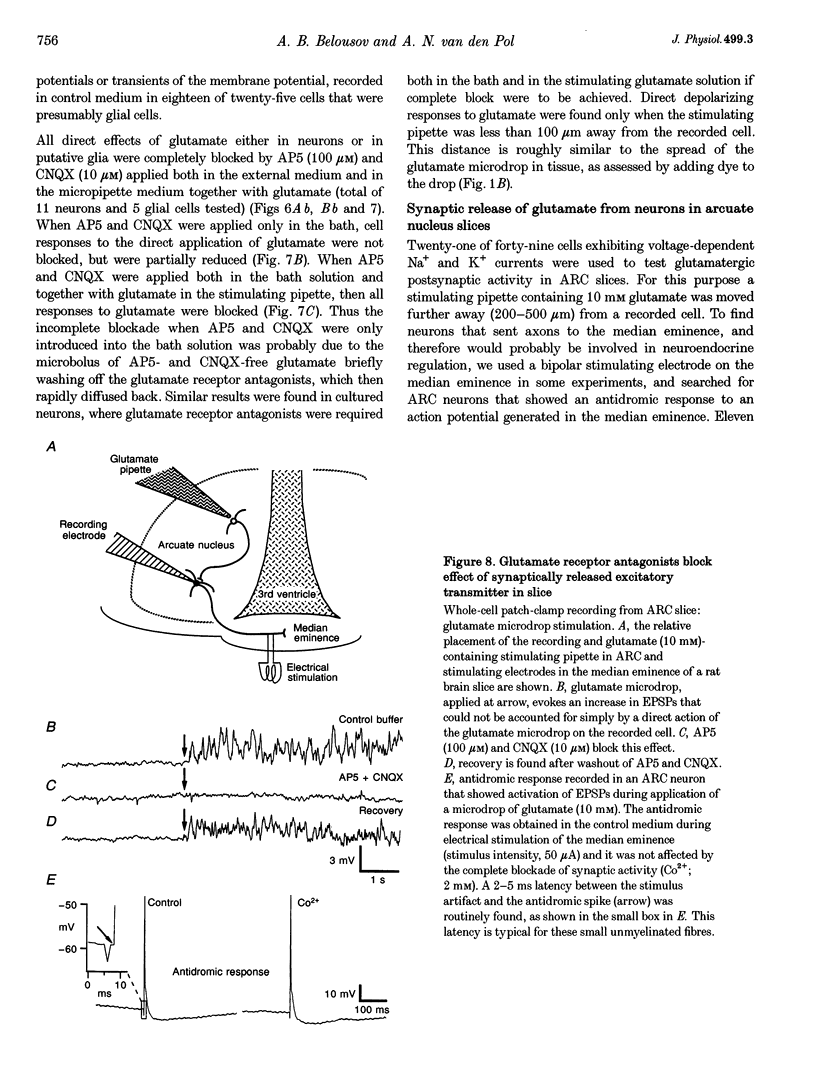
- Bach F. W., Yaksh T. L. Release of beta-endorphin immunoreactivity from brain by activation of a hypothalamic N-methyl-D-aspartate receptor. Neuroscience. 1995 Apr;65(3):775–783. doi: 10.1016/0306-4522(94)00528-d. [DOI] [PubMed] [Google Scholar]
- Brann D. W. Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 1995 Mar;61(3):213–225. doi: 10.1159/000126843. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989 Feb 6;479(1):76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Dudek F. E. Characteristics of local excitatory circuits studied with glutamate microapplication in the CA3 area of rat hippocampal slices. J Neurophysiol. 1988 Jan;59(1):90–109. doi: 10.1152/jn.1988.59.1.90. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6 (Suppl 2):1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Costa A., Yasin S. A., Hucks D., Forsling M. L., Besser G. M., Grossman A. Differential effects of neuroexcitatory amino acids on corticotropin-releasing hormone-41 and vasopressin release from rat hypothalamic explants. Endocrinology. 1992 Dec;131(6):2595–2602. doi: 10.1210/endo.131.6.1359961. [DOI] [PubMed] [Google Scholar]
- Decavel C., van den Pol A. N. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol. 1992 Feb 1;316(1):104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Hökfelt T., Wu J. Y., Goldstein M. Coexistence of tyrosine hydroxylase-like and gamma-aminobutyric acid-like immunoreactivities in neurons of the arcuate nucleus. Neuroendocrinology. 1984 Aug;39(2):189–191. doi: 10.1159/000123977. [DOI] [PubMed] [Google Scholar]
- Furshpan E. J., Potter D. D. Seizure-like activity and cellular damage in rat hippocampal neurons in cell culture. Neuron. 1989 Aug;3(2):199–207. doi: 10.1016/0896-6273(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Plant T. M. N-methyl-D,L-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatta). Endocrinology. 1987 Jun;120(6):2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- Goldsmith P. C., Thind K. K., Perera A. D., Plant T. M. Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology. 1994 Feb;134(2):858–868. doi: 10.1210/endo.134.2.7905410. [DOI] [PubMed] [Google Scholar]
- Gribkoff V. K., Dudek F. E. Effects of excitatory amino acid antagonists on synaptic responses of supraoptic neurons in slices of rat hypothalamus. J Neurophysiol. 1990 Jan;63(1):60–71. doi: 10.1152/jn.1990.63.1.60. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987 Oct;23(1):1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981 Jan 16;211(4479):294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Dudek F. E. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol. 1991 Dec;444:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López F. J., Donoso A. O., Negro-Vilar A. Endogenous excitatory amino acids and glutamate receptor subtypes involved in the control of hypothalamic luteinizing hormone-releasing hormone secretion. Endocrinology. 1992 Apr;130(4):1986–1992. doi: 10.1210/endo.130.4.1312433. [DOI] [PubMed] [Google Scholar]
- Mason G. A., Bissette G., Nemeroff C. B. Effects of excitotoxic amino acids on pituitary hormone secretion in the rat. Brain Res. 1983 Dec 19;289(1-2):366–369. doi: 10.1016/0006-8993(83)90044-6. [DOI] [PubMed] [Google Scholar]
- Meeker R. B., Greenwood R. S., Hayward J. N. Glutamate receptors in the rat hypothalamus and pituitary. Endocrinology. 1994 Feb;134(2):621–629. doi: 10.1210/endo.134.2.7905409. [DOI] [PubMed] [Google Scholar]
- Obrietan K., Belousov A. B., Heller H. C., van den Pol A. N. Adenosine pre- and postsynaptic modulation of glutamate-dependent calcium activity in hypothalamic neurons. J Neurophysiol. 1995 Nov;74(5):2150–2162. doi: 10.1152/jn.1995.74.5.2150. [DOI] [PubMed] [Google Scholar]
- Obrietan K., Van den Pol A. N. Calcium hyperexcitability in neurons cultured with glutamate receptor blockade. J Neurophysiol. 1995 Apr;73(4):1524–1536. doi: 10.1152/jn.1995.73.4.1524. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Ho O. L., Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14(1):61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Sharpe L. G. Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science. 1969 Oct 17;166(3903):386–388. doi: 10.1126/science.166.3903.386. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Crowley W. R. Stimulation of oxytocin release in the lactating rat by a central interaction of alpha 1-adrenergic and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-sensitive excitatory amino acid mechanisms. Endocrinology. 1993 Dec;133(6):2855–2860. doi: 10.1210/endo.133.6.7694847. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tasker J. G., Dudek F. E. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1993 Sep;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski H. F., Ojeda S. R. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990 Mar;126(3):1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Van Den Pol A. N., Obrietan K., Belousov A. Glutamate hyperexcitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture. Neuroscience. 1996 Oct;74(3):653–674. doi: 10.1016/0306-4522(96)00153-4. [DOI] [PubMed] [Google Scholar]
- Wayman C. P., Wilson J. F. Endogenous glutamate stimulates release of alpha-melanocyte-stimulating hormone from the rat hypothalamus. Neuropeptides. 1992 Oct;23(2):93–97. doi: 10.1016/0143-4179(92)90084-a. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Knobil E. Acute effects of N-methyl-DL-aspartate on the release of pituitary gonadotropins and prolactin in the adult female rhesus monkey. Brain Res. 1982 Sep 23;248(1):177–179. doi: 10.1016/0006-8993(82)91160-x. [DOI] [PubMed] [Google Scholar]
- Wuarin J. P., Dudek F. E. Excitatory amino acid antagonists inhibit synaptic responses in the guinea pig hypothalamic paraventricular nucleus. J Neurophysiol. 1991 Apr;65(4):946–951. doi: 10.1152/jn.1991.65.4.946. [DOI] [PubMed] [Google Scholar]
- Záborszky L., Makara G. B. Intrahypothalamic connections: an electron microscopic study in the rat. Exp Brain Res. 1979 Jan 15;34(2):201–215. doi: 10.1007/BF00235668. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Cassidy J. R. The hypothalamic arcuate nucleus of rat--a quantitative Golgi analysis. J Comp Neurol. 1982 Jan 1;204(1):65–98. doi: 10.1002/cne.902040108. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Hermans-Borgmeyer I., Hofer M., Ghosh P., Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. J Comp Neurol. 1994 May 15;343(3):428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Obrietan K., Cao V., Trombley P. Q. Embryonic hypothalamic expression of functional glutamate receptors. Neuroscience. 1995 Jul;67(2):419–439. doi: 10.1016/0306-4522(95)96912-w. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Obrietan K., Chen G., Belousov A. B. Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci. 1996 Sep 15;16(18):5883–5895. doi: 10.1523/JNEUROSCI.16-18-05883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Romano C., Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995 Nov 6;362(1):134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N., Trombley P. Q. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993 Jul;13(7):2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N., Wuarin J. P., Dudek F. E. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990 Nov 30;250(4985):1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]



