Abstract
As an early response to viral infection, cells express a number of cellular genes that play a role in innate immunity, including alpha/beta interferons (IFN). IFN-α/β are encoded by a single IFNB gene and multiple, closely related IFNA genes. The induction of these IFN genes in infected cells occurs at the transcriptional level, and two transcription factors of the IRF family, IRF-3 and IRF-7, were shown to play a role in their activation. While the expression of IRF-3 alone was shown to be sufficient for induction of the IFNB gene, induction of all the IFNA subtypes in human cells required the presence of IRF-7. Since IRF-3 is expressed constitutively in all cells examined, the role of IRF-3 in the induction of IFNA genes has not been clarified. Using ribozyme targeted to IRF-3 mRNA, we found that the downregulation of IRF-3 levels in the infected cells inhibited not only the induction of IFNB gene but also the expression of IFNA genes. Furthermore, downmodulation of IRF-3 levels altered the expression profile of IFNA subtypes induced by viral infection. These studies suggest that the ratio between the relative levels of IRF-3 and IRF-7 is a critical determinant for the induction of the individual IFNA subtypes in infected cells.
As an early response to viral infection, cells express large numbers of cellular genes, some of which are important modulators of innate and adaptive immunity. Among these, the alpha/beta interferons (IFN-α/β) play a unique role, since they elicit direct antiviral effects as well as multiple biological responses that activate the immune system. IFN-α/β are encoded by a single IFNB gene and a family of closely related IFNA genes. Induction of IFN-α/β gene expression in infected cells occurs at the transcriptional level, and while IFNB is expressed in a large variety of cell types, human IFNA genes are expressed preferentially in cells of lymphoid origin. Virus-responsive elements (VRE) were identified in promoters of IFNA and IFNB genes that alone can confer virus-mediated activation. The VRE show sequence motifs that are highly conserved in both IFNA and IFNB promoters (2, 28). The molecular mechanisms of activation of IFNB gene expression by virus infection or double-stranded (ds) RNA have been studied extensively (4, 6, 8, 9, 17, 25, 26, 27, 28). It has been shown that IFNB VRE contains multiple regulatory domains that serve as binding sites for NFκB, ATF-2, c-Jun, and HMG I(Y) (4). In addition, this VRE contains two IRF-Es (PRDI and -III) which are recognized by transcription factors of the interferon regulatory factor (IRF) family (6, 20, 24). Although it was initially assumed that IRF-1 binds to these IRF-E sites (6, 24), it was shown recently that viral infection results in binding of IRF-3 and IRF-7, but not IRF-1 to the IFNB promoter (26). Cooperative interaction between all transcription factors bound to VRE of IFNB promoter is facilitated by their interaction with the transcription cofactor CBP/p300, and this transcription complex has been referred to as an enhanceosome (17, 25).
The multiple components of a transcription complex (enhanceosome) that regulates the activation of IFNA genes have not yet been fully defined. The VRE of IFNA genes does not contain NFκB and c-Jun binding sites, but it contains several copies of IRF-E binding sites. It was shown that at least two IRFs, IRF-3 and IRF-7, bind to IFNA VRE and play a key role in the activation of IFNA gene transcription in infected cells (1, 8, 11, 14, 16, 22, 23, 28; W. C. Au, W.-S. Yeow, and P. M. Pitha, submitted for publication). The IRF family presently consists of nine cellular IRFs and several viral IRFs (15, 20). Constitutively expressed IRF-3 is modified at the posttranscriptional level in response to viral infection by phosphorylation on specific serine residues in the C terminus (10). In uninfected cells, IRF-3 resides mostly in the cytoplasm, while in infected cells, phosphorylated IRF-3 binds to histone acetyltransferases, CBP/p300, and accumulates in the nucleus (9, 13, 27, 29). Constitutive expression of human IRF-7 is restricted to cells of lymphoid origin (1), but in a variety of cell lines, the expression of IRF-7 can be significantly enhanced by treating with IFN-α/β (1, 16, 22, 30). Suppression of IRF-7 expression as a consequence of the methylation of IRF-7 promoter was also observed in several tumor cell lines (14). Similarly to IRF-3, IRF-7 is also phosphorylated in infected cells, which results in an accumulation of IRF-7 in the nucleus (1, 12). Although low levels of IRF-7 were found to be continuously present in the nucleus (28), the critical role of phosphorylation in the nuclear accumulation of IRF-7 and its transactivation activity was demonstrated (12, 16, 22; Au et al., submitted).
Overexpression of IRF-3 in a human fibroblast cell line results in induction of an antiviral state (8). In the transient-expression assay, IRF-3 enhances transcription activity of IFNB promoter, murine IFNA4 and human IFNA1 promoters, but not the other IFNA promoters examined (13, 16, 22, 23, 28). In murine cells, the presence of IRF-3 was sufficient for virus-stimulated expression of IFNA4 genes, but expression of the other IFNA subtypes was dependent on the presence of IRF-7 (16, 22). However, in human cells, IRF-7 was shown to be the limiting factor for virus-mediated induction of IFNA genes, and IRF-3 alone was not sufficient for the virus-mediated expression of any of the IFNA subtypes (28). These results suggest that although the promoter regions of mouse and human IFNA genes are well conserved, virus-mediated regulation may not be identical. It is notable that differences in the response of mice and humans to IFN-α/β were also recently observed (5, 19).
The aim of this study was to analyze the role of IRF-3 in the expression of individual IFNA subtypes. In the transient-expression assay, human IRF-3 was able to activate only IFNA1 promoter but none of the other IFNA promoters (A2, A4, or A14) tested (28). Also in a DNA pulldown assay using magnetic beads with immobilized IFNA1 VRE or IFNA2 VRE and cellular lysates from infected 2fTGH or 2fTGH cells expressing IRF-7 (2fTGH/IRF-7), we detected binding of IRF-3 to IFNA1 VRE but not to IFNA2 VRE. In contrast, IRF-7 bound to both IFNA1 and IFNA2 VRE. Under the same conditions, IRF-2 binds to neither of these VRE, indicating that the observed binding is specific (Fig. 1). We were therefore interested to see whether the elimination or downregulation of IRF-3 will affect primary expression of IFNA1 or the other subtypes as well. Since all human cells and cell lines we have examined expressed IRF-3 constitutively and an IRF-3-negative cell line has not been identified, we have suppressed IRF-3 expression in 2fTGH cells using a hammerhead ribozyme.
FIG. 1.
DNA binding of in vivo-phosphorylated IRF-3. Whole-cell extracts were prepared from 2fTGH and 2fTGH/IRF-7 (clone 7/9) cells infected with Sendai virus 4 h after infection and incubated (200 μg) with magnetic beads containing either IFNA1 or IFNA2 VRE for 2 h at 4°C as described previously (Au et al., submitted). After extensive washing, proteins retained on the beads were separated on SDS–10% polyacrylamide gels and transferred to membranes, and IRF-2, IRF-3, and IRF-7 were identified by immunoblotting with specific antibodies. The relative levels of IRF-2, IRF-3, and IRF-7 in total cellular lysates were determined by Western blotting.
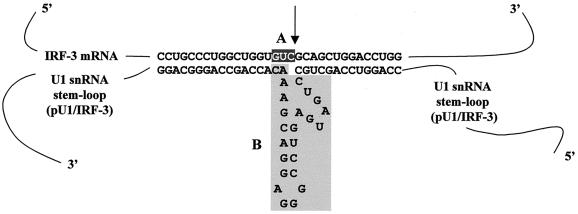
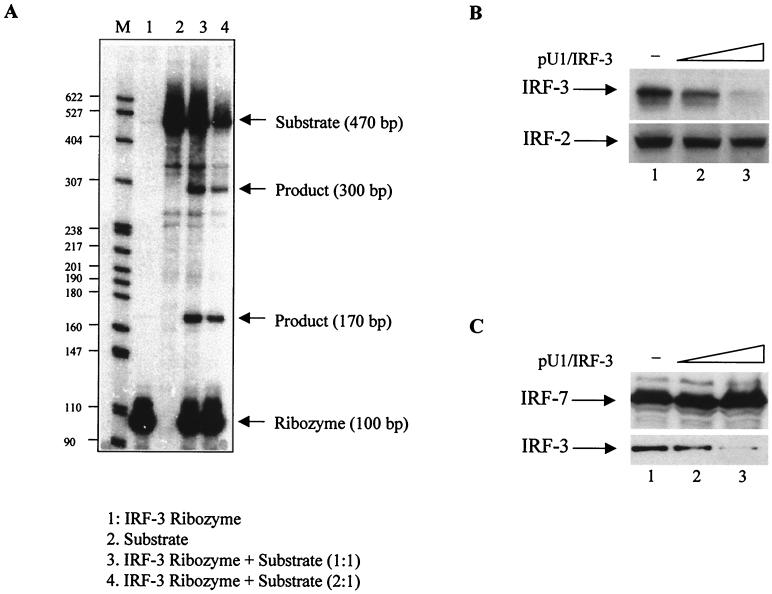
The U1 snRNA-derived vector that contains a strong, constitutively active U1 snRNA promoter (18) was used to insert the ribozyme-containing targeted core, schematically shown in Fig. 2. The consensus sequence for ribozyme cleavage, 5′GUC3′, was selected from part of IRF-3 mRNA with no secondary structure. The ribozyme recognition site is flanked by sequences complementary to the coding region from nucleotide 24 to 55 of IRF-3 mRNA, that are separated by a 24-bp hammerhead ribozyme catalytic core (3, 7). This ribozyme was designated pU1/IRF-3. The ability of pU1/IRF-3 to cleave IRF-3 transcripts was analyzed in vitro (Fig. 3A). A 470-bp fragment of IRF-3 substrate RNA (sRNA), containing the GUC ribozyme recognition site, was generated by in vitro transcription with radioactive ribonucleotide as a tracer. Neither pU1/IRF-3 nor sRNA showed significant degradation when incubated alone (Fig. 3A, lanes 1 and 2). When sRNA was incubated with increasing amounts of pU1/IRF-3 (lanes 3 and 4), two fragments of 300 and 170 bp were produced. The sizes of the cleaved products were consistent with the cleavage predicted to target immediately 3′ of the recognition site. These data indicate that IRF-3 mRNA is a substrate for ribonucleolytic cleavage by the pU1/IRF-3. To determine whether pU1/IRF-3 can downregulate expression of IRF-3 in vivo, 2fTGH cells were cotransfected with IRF-3 and pU1/IRF-3, and the relative levels of IRF-3 were determined by immunoblotting. The results in Fig. 3B showed that the levels of IRF-3 were significantly reduced in cells that were transfected with pU1/IRF-3 and that twofold excess of pU1/IRF-3 inhibited expression of IRF-3 by more then 90% (compare lanes 1 and 3). Furthermore, the inhibition was specific, since pU1/IRF-3 did not downmodulate levels of IRF-2. To further demonstrate the specificity of the ribozyme, we cotransfected the 2fTGH cells with IRF-7 and pU1/IRF-3 and compared the levels of IRF-7 and IRF-3 in cell lysates. The results showed (Fig. 3C) that the levels of IRF-7 protein were not downmodulated by pU1/IRF-3, while the relative levels of endogenous IRF-3 were decreased. These data indicate that pU1/IRF-3 downregulated the levels of both transfected and endogenous IRF-3 protein, but it does not target IRF-7 or IRF-2 transcripts.
FIG. 2.
Design of ribozyme targeted against IRF-3 mRNA. Following the strategy used for the construction of pU1/FB1 vector (18), ribozyme termed pU1/IRF-3 was targeted against the 13th codon of IRF-3 mRNA, which contains the catalytic domain GUC and no essential secondary structure. Two structural domains are marked. Box A contains the recognition sequence in IRF-3 mRNA preceding the cleavage site (marked by an arrow), and box B consists of highly conserved sequences maintained in hammerhead ribozyme (7) that are flanked by the antisense sequences that allow base pairing between IRF-3 mRNA and ribozyme RNA.
FIG. 3.
Cleavage of IRF-3 transcripts in vitro and downregulation of IRF-3 protein levels in vivo. (A) The in vitro-transcribed 32P-labeled 470-nucleotide (nt) IRF-3 transcript containing the GUC catalytic site was incubated alone (lane 2) or with an equal amount (lane 3) or molar excess of pU1/IRF-3 ribozyme (lane 4). The incubation conditions were described previously (3). Under these conditions, no degradation of template transcripts or ribozyme RNA (lanes 2 and 1, respectively) was observed. When IRF-3 template was incubated with pU1/IRF-3 ribozyme, two RNA fragments were produced, and their sizes (300 and 170 nt) were consistent with the predicted site for cleavage. (B) The relative levels of IRF-3 proteins were reduced in 2fTGH cells cotransfected with IRF-3 expression plasmid and pU1/IRF-3 ribozyme. Fifteen micrograms of whole-cell lysate of 2fTGH cells cotransfected with IRF-3 (3 μg) and pU1/IRF-3 (3 and 6 μg; lanes 2 and 3, respectively) were analyzed on an SDS-7.5% PAGE gel, and the relative levels of IRF-3 were identified by immunobloting with IRF-3 antibodies. The levels of IRF-2 were determined as a control of ribozyme specificity. IRF-3 antibodies were previously described (8), and IRF-2 antibodies were from Santa Cruz. (C) The relative levels of ectopic IRF-7 proteins were not affected in 2fTGH cells cotransfected with IRF-7 expression plasmid and pU1/IRF-3 ribozyme. Twenty micrograms of whole-cell lysate of 2fTGH cells cotransfected with IRF-7 (3 μg) and pU1/IRF-3 (3 and 6 μg; lanes 2 and 3, respectively) were analyzed on an SDS–10% PAGE gel, and the relative levels of IRF-7 and endogenous IRF-3 were determined by immunoblotting. IRF-7-specific antibody was from Santa Cruz. Relative levels of endogenous IRF-3 in these cell lysates are shown for comparison
Next we determined the effect of pU1/IRF-3 ribozyme on the virus-mediated activation of reporter plasmids in which human IFNA1 or IFNB promoters drove the expression of secreted alkaline phosphatase (SAP). In transfected 2fTGH cells, infection with Sendai virus resulted in only marginal activation of IFNB promoter (Fig. 4B). As was shown previously, these cells express IRF-3 constitutively, but the expression of IRF-7 is silenced by methylation (14, 28). Constitutive and virus-induced SAP production were both decreased to baseline levels in cells transfected with pU1/IRF-3 at a 1:2 substrate-to-ribozyme ratio. Cotransfection of IFNB SAP with IRF-3 significantly increased virus-mediated induction of SAP (eightfold increase), but a no stimulation was observed in cells which were also cotransfected with pU1/IRF-3. These data show that by reducing IRF-3 mRNA levels, pU1/IRF-3 ribozyme also reduced IRF-3 expression and consequently activation of the IFNB promoter similar to control levels.
FIG. 4.
Virus-mediated activation of IFNA1 and IFNB VRE was inhibited in cells expressing pU1/IRF-3 ribozyme. 2fTGH cells were cotransfected with 2.5 μg of SAP reporter plasmid (2, 28) containing either IFNA1 promoter (A) or IFNB promoter (B) and the IRF-3- or IRF-7- expressing plasmid (2.5 μg) in the presence and absence of pU1/IRF-3 (5 μg). The pSP64 plasmid (2.5 μg) was used as a control plasmid. All transfections were done in the presence of β-galactosidase-expressing plasmid (0.5 μg), used as a standard for transfection efficiency. Transfected cells were infected with Sendai virus 24 h posttransfection, and the levels of SAP activity in the medium were determined 16 h later as described previously (28) and normalized to the constant levels of β-galactosidase.
The interaction between IRF-3 and IRF-7 was determined by a glutathione S-transferase (GST) pull-down assay and the presence of IRF-3 and IRF-7 heterodimers was demonstrated in infected cells (12; Au et al., submitted). We were therefore interested in determining whether pU1/IRF-3 downmodulates the IRF-7-mediated activation of IFNA1 promoter that, as we show in Fig. 1, can bind both IRF-3 and IRF-7. In correlation with our previous findings, no significant stimulation of IFNA1 promoter was observed in infected 2fTGH cells, and the expression of IFNA1 SAP plasmid was decreased to basal levels in cell expressing pU1/IRF-3. Cotransfection of the IFNA1 SAP plasmid with IRF-7-expressing plasmid increased the transcription activity of IFNA1 promoter in both uninfected and infected cells. However, when cells were transfected with IRF-7 and pU1/IRF-3, both constitutive and virus-stimulated expression of SAP were reduced to basal levels. These data indicate that reduction in IRF-3 levels significantly downmodulates IRF-7-mediated activation of IFN-α/β genes, implying that IRF-3 is required for the virus-mediated activation of IFNA1 promoter.
In a transient-transfection assay, the virus-mediated transcription activity of all IFNA promoters (A1, A2, A4, and A14) was enhanced by IRF-7, but only IFNA1 was activated by IRF-3 (28). Furthermore, in a DNA pull-down assay, binding of IRF-3 to IFNA1 VRE but not to IFNA2 VRE was detected from the lysates of infected cells (Fig. 1). We therefore examined whether the decrease in IRF-3 levels in 2fTGH cells will modulate the expression of endogenous IFN-α/β genes and whether it will specifically modulate the expression of IFNA1 and not the other IFNA subtypes. To this end, the relative levels of IFNA and IFNB transcripts were analyzed in 2fTGH cells transfected with IRF-7 in the presence and absence of pU1/IRF-3.
The IFNA and IFNB mRNAs were detected by the semiquantitative reverse transcription-PCR (RT-PCR) using IFNA consensus primers designed to recognize all IFNA subtypes and IFNB-specific primers (28; Au et al., submitted). (Fig. 5A). In accordance with the previous finding, no expression of IFNA genes was detected in 2fTGH cells, but IFNA genes were induced efficiently by virus in 2fTGH cells expressing ectopic IRF-7. However, the virus-mediated induction of IFNA genes in IRF-7-expressing cells was significantly inhibited when these cells were transfected with pU1/IRF-3, indicating that downregulation of IRF-3 levels by pU1/IRF-3 had a negative effect on the induction of IFNA genes. In contrast to IFNA genes, the IFNB gene is expressed in infected 2fTGH cells, and ectopic expression of IRF-7 increases the relative levels of IFNB mRNA only by about twofold. Interestingly, low levels of expression of IFNB gene were also observed in uninfected cells overexpressing IRF-7. We had observed previously that overexpressed IRF-3 also serves as a primary inducer of IFNB gene (8). Transfection of pU1/IRF-3 resulted in a dramatic inhibition of the virus-mediated induction of IFNB gene in 2fTGH transfected with IRF-7 cells, and the relative levels of IFNB mRNA were lower than in infected 2fTGH cells. Under the experimental conditions employed, we have determined that about 70 to 75% cells are transfected. The effect of the IRF-3 ribozyme is not a consequence of the inhibition of IRF-7 expression due to the inhibition of IFNB synthesis. We have shown that IRF-7 is not expressed in 2fTGH cells (28) and that its expression is not stimulated by IFNα/β, since the IRF-7 promoter is silenced by methylation in these cells (14). Furthermore, a cytomegalovirus minimal promoter drives the ectopic expression of IRF-7. To further confirm our results, we have cotransfected a clone of 2fTGH cells that constitutively express IRF-7 (clone 7) with pU1/IRF-3 and a CD4-expressing plasmid (pCG.CD4) and then selected CD4-expressing cells with the CD4 positive-isolation kit (Dynal). The results in Fig. 5B. showed that in the CD4-negative population of cells, the relative levels of IFNA and IFNB transcripts were unmodified even in the samples that were initially transfected with pU1/IRF-3 (Fig. 5B, lanes 1 to 6). In contrast, the CD4-positive population of cells expressed CD4 transcripts, and the relative levels of IFNA and IFNB transcripts were lower in cells cotransfected with pU1/IRF-3. These data indicate that both IFNA and IFNB genes are expressed, although less effectively, even when the levels of IRF-3 are significantly decreased. Based on these data, two conclusions can be made. First, pU1/IRF-3 effectively cleaves the endogenous IRF-3 mRNA and consequently downmodulates synthesis of IRF-3 protein. Second, the levels of expression of both IFNA and IFNB genes in infected cells are affected by changes in the relative levels of IRF-3.
FIG. 5.
Activation of endogenous IFNA genes in infected cells expressing pU1/IRF-3. (A) RT-PCR analysis of IFNA and IFNB mRNA in uninfected and infected 2fTGH cells transfected with IRF-7 in the absence (lanes 3 and 4) and presence (lanes 5 and 6) of pU1/IRF-3. Total RNA were prepared from transfected cells infected with Sendai virus (SeV) at 6 h postinfection. The presence of IFNA, IFNB, and β-actin mRNA was determined by RT-PCR as previously described (28). (B) Comparative RT-PCR analysis of IFNA/B mRNA in permanently transfected 2fTGH/IRF-7 cells (clone 7/9) cotransfected with pCG.CD4 (CD4-expressing plasmid) and pU1/IRF-3. The cells were either transfected with pCG.CD4 (3 μg; lanes 1, 2, 7, and 8) or cotransfected with pCG.CD4 (3 μg) and pU1/IRF-3 (3 μg; lanes 3, 4, 9, and 10; 6 μg, lanes 5, 6, 11, and 12). Sixteen hours after transfection, cells expressing CD4 were selected, and the CD4-positive and -negative cells were grown overnight and then infected with Sendai virus for 6 h. The presence of CD4, IFNA, IFNB, and β-actin mRNA was determined by RT-PCR (28). CD4-specific primers used allow the amplification of a 347-bp fragment (20a). (C) Effect of pU1/IRF-3 ribozyme on the expression of IFNA subtypes in infected 2fTGH/IRF-7 cells. The IFNA cDNA fragments from 2fTGH cells cotransfected with IRF-7 and pU1/IRF-3 ribozyme were amplified by PCR with primers corresponding to the regions of IFNA genes that are conserved in all IFNA genes (28). The PCR-amplified fragments were cloned into pBluescript KS II plasmids, and cDNA insert isolated from 32 randomly selected clones was sequenced. DNA sequences obtained were compared with sequences of individual IFNA genes present in GenBank. The frequency of IFNA gene subtypes induced by Sendai virus in 2fTGH transiently expressing ectopic IRF-7, and two stable clones (7 and 9) of 2fTGH expressing IRF-7 have been determined previously (28). One-way ANOVA (independent samples; http://faculty.vassar.edu/∼lowry/VassarStats.html) was used to analyze the significance of IFNA subtype expression in 2fTGH cells expressing IRF-7 in the absence and presence of pU1/IRF-3 ribozyme. The F values from these ANOVA analyses are presented. The null hypothesis (indicating no significant difference between the two groups) is true if the F value is less than or equal to 1. The expression of only IFNA1, -A7, -A10, -A14, and -A17 subtypes could be compared. Zero indicates that that particular IFNA subtype was absent in the sample population analyzed.
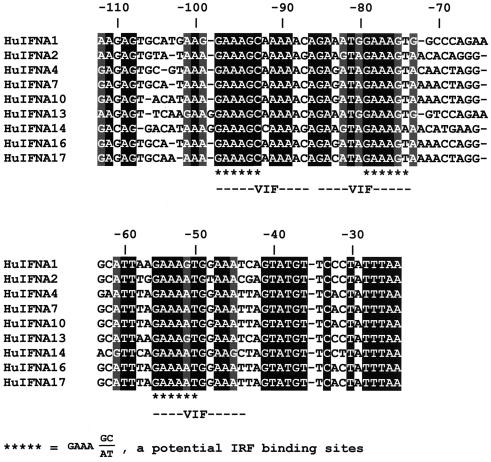
Reconstitution of IRF-7 synthesis in 2fTGH cells resulted, upon viral infection, in the activation of seven endogenous IFNA genes, of which IFNA1 was expressed at the highest level (28). In order to determine how the decrease in the IRF-3 levels in these cells affects the expression profile of IFNA subtypes, we amplified the IFNA cDNA from 2fTGH cells cotransfected with pU1/IRF-3 and IRF-7 by using primers selected to recognize all human IFNA subtypes. The amplified cDNA fragments were cloned into pBluescript KS II (Stratagene), and randomly selected clones were sequenced and identified (Fig. 5C). Six IFNA subtypes were expressed in 2fTGH cells cotransfected with pU1/IRF-3 and IRF-7, and the difference in their relative frequency of expression was large. The IFNA1, -A10, and -A17 subtypes were most abundant (28, 22, and 22%, respectively), and IFNA7 was expressed at a higher level (16%) than either A14 or A21 (6%), while no expression of A2 and A4 was detected. We used one-way analysis of variance (ANOVA) to determine whether the decrease in the relative levels of IRF-3 significantly affected IFNA subtype expression profile from that observed in infected 2fTGH-expressing IRF-7 cells. From the estimated F values, we found that the decrease in the relative levels of IRF-3 in the cells significantly changes the expression of IFNA1, -A10, and -A14 genes. The significant upregulation of IFNA10 and -A14 is of interest and suggests that this promoter is activated primarily either by IRF-7 or by IRF-7 in combination with another IRF. We have found increased levels of IFNA21 in the cells that have low levels of IRF-3, but we could not calculate whether this is significant. There is no obvious difference in the IRF-E binding motifs among these three IFNA (A10, A14, and A21) promoters and A1, A4, A8, and A16, which are downregulated when the levels of IRF-3 are decreased (Fig. 6). Additional analyses are required to clarify the differential effect of IRF-3 on the activity of these IFNA promoters.
FIG. 6.
Comparision of the promoter regions of IFNA genes that were modulated by a decrease in relative levels of IRF-3. Note that there is no information in GenBank on the promoter of IFNA8 or -A21.
These data indicate that the ratio between IRF-3 and IRF-7 determines the type of IFNA subtype induced in infected cells. Whether any other cellular IRFs may contribute to the virus-mediated induction of specific IFNA subtypes remain to be determined. Since pU1/IRF-3 substantially decreased but did not completely eliminate IRF-3 in 2fTGH cells (Fig. 3), these data do not allow us to conclude whether any of the expressed IFNA subtypes can be induced by IRF-7 in the absence of IRF-3. As was shown in recently generated IRF-3−/− mice (21), both IFNA and IFNB could be expressed at low levels in these mice. The analyses of IFNA subtypes expressed in these mice showed a significant decrease in IFNA4 gene expression. However, when an interpolation is made to the human system, it should be kept in mind that the regulation of IFNA gene expression in murine and human cells shows some differences.
IRF-3 is expressed in the majority of cell lines and primary cells examined and therefore may generate some of its effects by interaction with other members of the IRF family along with cellular transcription factors and cofactors. IRF-3 homodimers can bind to the PRDI-PRDIII element in the IFNB promoter region, but IRF-3 does not bind to PRDI (11, 23). The casting experiment further indicated that the binding of IRF-3 from infected cells requires two copies of GAAAG/C repeats (unpublished results). The IRF-3 homodimers are functionally active, since IRF-3 can induce IFNB gene in the absence of IRF-7 (8). The interaction of IRF-3 with IRF-7 was also demonstrated, and the interacting domain was narrowed to amino acids 306 to 427, which overlaps the IRF-3 IAD (amino acids 199 to 375). The formation of IRF-3/IRF-7 heterodimers can also be detected in cell lysates from infected cells (12, 26; Au et al., submitted), and it was shown that the virus-specific ternary complex VAF detected in infected cells contains IRF-3, IRF-7, and CBP/p3000. Although we detected binding of IRF-3 only to IFNA1 VRE and not to A2, A4, and A14 (28) (Fig. 1), the decrease in IRF-3 levels modulated virus-mediated expression not only of IFNA1 but also of IFNA10 and A14 genes. Whether IRF-3 is tethered to IFNA promoters by complexing with IRF-7 is currently under investigation. Thus, these results provide the first direct information on how the virus-mediated activation of specific IFNA subtypes is determined. What other factors that may participate in the differential activation of IFNA genes remains to be established.
Acknowledgments
We thank R. A. Montgomery and H. C. Dietz for the pU1 ribozyme vector, J. Skowronski for pCG.CD4 plasmid, W. Popik for the primers to amplify CD4, J. Sickler for the in vitro analysis of pU1/IRF-3 degradation, and M. Kellum for establishing the 2fTGH/IRF-7 (clones 7 and 9) cell lines.
This work was supported by NIH grant A1119737 NIAID to P.M.P. W.J.L. was supported by predoctoral training grant program in human genetics 5GM07814.
REFERENCES
- 1.Au W C, Moore P A, LaFleur D W, Tombal B, Pitha P M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273:29210–29217. doi: 10.1074/jbc.273.44.29210. [DOI] [PubMed] [Google Scholar]
- 2.Dent C L, Gewert D R. A regulatory domain within the virus-response element of the interferon alpha 1 gene acts as a transcriptional repressor sequence and determinant of cell-specific gene expression. Eur J Biochem. 1996;236:895–903. doi: 10.1111/j.1432-1033.1996.t01-1-00895.x. [DOI] [PubMed] [Google Scholar]
- 3.Dropulic B, Hermankova M, Pitha P M. A conditionally replicating HIV-1 vector interferes with wild-type HIV-1 replication and spread. Proc Natl Acad Sci USA. 1996;93:11103–11108. doi: 10.1073/pnas.93.20.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du W, Thanos D, Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 5.Farrar J D, Smith J D, Murphy T L, Leung S, Stark G R, Murphy K M. Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol. 2000;1:65–69. doi: 10.1038/76932. [DOI] [PubMed] [Google Scholar]
- 6.Fujita T, Kimura Y, Miyamoto M, Barsoumian E L, Taniguchi T. Induction of endogenous IFN-alpha and IFN-beta genes by a regulatory transcription factor, IRF-1. Nature. 1989;337:270–272. doi: 10.1038/337270a0. [DOI] [PubMed] [Google Scholar]
- 7.Haseloff J, Gerlach W L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988;334:585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- 8.Juang Y, Lowther W, Kellum M, Au W C, Lin R, Hiscott J, Pitha P M. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc Natl Acad Sci USA. 1998;95:9837–9842. doi: 10.1073/pnas.95.17.9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar K P, McBride K M, Weaver B K, Dingwall C, Reich N C. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol Cell Biol. 2000;20:4159–4168. doi: 10.1128/mcb.20.11.4159-4168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin R, Heylbroeck C, Pitha P M, Hiscott J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasomemediated degradation. Mol Cell Biol. 1998;18:2986–2996. doi: 10.1128/mcb.18.5.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin R, Genin P, Mamane Y, Hiscott J. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol Cell Biol. 2000;20:6342–6353. doi: 10.1128/mcb.20.17.6342-6353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, R., Y. Mamane, and J. Hiscott. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem., in press. [DOI] [PubMed]
- 13.Lin R, Mamane Y, Hiscott J. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol. 1999;19:2465–2474. doi: 10.1128/mcb.19.4.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu R, Au W C, Yeow W-S, Hageman N, Pitha P M. Regulation of the promoter activity of interferon regulatory factor-7 gene: activation by interferon and silencing by hypermethylation. J Biol Chem. 2000;275:31805–31812. doi: 10.1074/jbc.M005288200. [DOI] [PubMed] [Google Scholar]
- 15.Lubyova B, Pitha P M. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J Virol. 2000;74:8194–8201. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marie I, Durbin J E, Levy D E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery R A, Dietz H C. Inhibition of fibrillin 1 expression using U1 snRNA as a vehicle for the presentation of antisense targeting sequence. Hum Mol Genet. 1997;6:519–525. doi: 10.1093/hmg/6.4.519. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen K B, Cousens L P, Doughty L A, Pien G C, Durbin J E, Biron C A. Interferon α/β-mediated inhibition and promotion of interferon γ: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 20.Pitha P M, Au W C, Lowther W, Juang Y T, Schafer S L, Burysek L, Hiscott J, Moore P A. Role of the interferon regulatory factors (IRFs) in virus-mediated signaling and regulation of cell growth. Biochimie. 1998;80:651–658. doi: 10.1016/s0300-9084(99)80018-2. [DOI] [PubMed] [Google Scholar]
- 20a.Popik W, Pitha P M. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology. 1998;252:210–217. doi: 10.1006/viro.1998.9466. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 22.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 23.Schafer S L, Lin R, Moore P A, Hiscott J, Pitha P M. Regulation of type I interferon gene expression by interferon regulatory factor-3. J Biol Chem. 1998;273:2714–2720. doi: 10.1074/jbc.273.5.2714. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka N, Kawakami T, Taniguchi T. Recognition DNA sequences of interferon regulatory factor 1 (IRF-1) and IRF-2, regulators of cell growth and the interferon system. Mol Cell Biol. 1993;13:4531–4538. doi: 10.1128/mcb.13.8.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thanos D, Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 26.Wathelet M G, Lin C H, Parekh B S, Ronco L V, Howley P M, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 27.Weaver B K, Kumar K P, Reich N C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol Cell Biol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeow W-S, Au W C, Juang Y T, Fields C D, Dent C L, Gewert D R, Pitha P M. Reconstitution of virus-mediated expression of interferon alpha genes in human fibroblast cells by ectopic interferon regulatory factor-7. J Biol Chem. 2000;275:6313–6320. doi: 10.1074/jbc.275.9.6313. [DOI] [PubMed] [Google Scholar]
- 29.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]