Simple Summary
Lung cancer is the leading cause of cancer-related mortality among older adults, but older adults experience age-related disparities in treatment outcomes, clinical trial representation, and guideline-concordant care. This review addresses critical factors contributing to these disparities, outlining age-specific considerations to optimize management of medical and supportive care needs and cancer-directed therapy selection. Key recommendations include comprehensive geriatric assessment, individualized treatment planning, and improved resource allocation to ensure older adults receive equitable and effective care.
Keywords: older adult, geriatric oncology, comprehensive geriatric assessment, lung cancer
Abstract
Older adults with lung cancer experience inferior clinical outcomes compared to their younger counterparts. This review provides the scaffolding to address these disparities by delineating (1) the distinct and varied care needs of older adults with lung malignancies, (2) evidence-based measures for identifying subgroups within this population meriting tailored approaches to care, (3) age-specific considerations for the selection of cancer-directed therapy, and (4) opportunities for future work to enhance clinical outcomes and care delivery.
1. Introduction
Cancer disproportionately impacts older adults, a population with a heterogeneous constellation of medical, psychosocial, and supportive care needs [1,2,3,4,5,6,7,8,9,10]. As the global population ages, absolute disease burden in those over 65 is only expected to increase [1]. Yet despite these trends, age-related disparities in clinical outcomes persist. As compared to their younger counterparts, older adults with cancer experience worse survival outcomes, higher rates of treatment-related toxicity, greater variation from guideline-concordant care, and unequal clinical trial enrollment [11,12,13,14].
Paralleling findings across cancer types, age-specific differences in disease burden and outcomes endure in thoracic oncology. Though therapeutic advances over the last decade have improved prognosis for select subgroups, lung cancer remains the leading cause of cancer-related death in the United States, and older adults have up to a two–three-fold increased risk of dying from their disease [15,16]. Beyond survival, older adults with lung cancer, similar to the general geriatric oncology population, are under-represented in thoracic clinical trials and have a lower likelihood of receiving timely guideline-concordant therapy [17,18,19,20,21,22].
Parsing the reasons for these observed disparities is complex. At the patient-level, differences in medical and supportive care needs, frailty, functional status, treatment toleration, and social supports may be contributors [8,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. At a systems-level, the existing limitations in resource allocation, geriatric assessment utilization, and age-inclusive research may all be relevant [3,19,21,37,38,39,40,41,42]. Older adults with lung cancer constitute a disparate population with varied needs and care environments, and in specific circumstances, one of more of these factors may be more significant [1,2,3,4,5,6,7,8,9,10,23,24,25,26,27,28,29,30,31,32,33,34,35].
However, though future investigations are needed to untangle the interplay between these factors in different contexts, practical optimization of care for older adults with lung cancer remains possible in the present. An array of age-specific resources and guidelines already exist with demonstrated value across multiple facets of thoracic oncology care, although usage remains variable [8,17,18,24,37,38,39,40,41,42]. To support clinicians caring for older adults with lung cancer, outlining the features of this population, validated tools for care optimization, and best-practice recommendations are valuable. Further, a number of brief screening tools have been validated to assess specific domains for older patients with NSCLC (Table 1).
Table 1.
Brief assessment tools for domains related to symptom burden and experiences of older patients with non-small cell lung cancers.
| Domain | Examples of Brief Assessment Tools |
|---|---|
| Medical: Multimorbidity | Charlson Comorbidity Index (CCI) [43,44,45,46] Adult Comorbidity Evaluation-27 (ACE-27) [47] Cumulative Illness Rating Scale-Geriatric (CIRS-G) [48] Mini-COG [49] |
| Medical: Polypharmacy/PIMs | American Geriatrics Society Beers Criteria [50] STOPP/START Criteria [51,52] |
| Medical: Frailty and Geriatric Syndromes | Pictoral Fit-Frail Scale (PFFS) [53,54] Carolina Frailty Index (CFI) [55] Balducci Classification [56] SIOG 1/2 Classification [56] |
| Supportive Care: Treatment-Related Symptom Burden, QOL, and Functional Status | Patient Health Questionnaire for Anxiety and Depression (PHQ-4) [57] Geriatric Depression Scale (GDS-15) [58] Revised Edmonton Symptom Assessment System (ESAS-r) [59] MDASI-LC [60] EORTC-QLQ-LC13 [61] EORTC QLQ-C30 [62] EORTC QLQ-ELD-14 [63] VES-13 [64] |
| Supportive Care: Socioeconomic Supports & Burdens | Comprehensive Score for Financial Toxicity (COST) [65,66] Caregiver Reaction Assessment (CRA) [67] CareGiver Oncology Quality of Life Questionnaire (CarGOQoL) [68] Zarit Caregiver Burden Interview (ZBI) [69,70] 8-Item Medical Outcomes Study Social Support Survey [71] |
| Supportive Care: Advanced Care Planning | Respecting Choices [72,73,74,75,76] Prepare for Your Care [72] VIDEO-PCE [73] |
This review provides the scaffolding to support these aims by delineating the following: (1) the distinct and varied care needs of older adults, (2) evidence-based measures for identifying subgroups within this population meriting different approaches to care, (3) age-specific considerations for selection of cancer-directed therapy, and (4) opportunities for future work to enhance clinical outcomes and care delivery.
2. Medical Needs
Older adults with lung cancer have a heterogeneous array of medical needs which deserve consideration across the care continuum. Issues specific to older adults in this domain include multimorbidity, and polypharmacy, as well as frailty and other geriatric syndromes. Each of these issues is discussed further below.
2.1. Multimorbidity and Polypharmacy
Multimorbidity and polypharmacy are central considerations for providers managing older adults with lung cancer. The comorbidity burden increases with age, and older adults with lung cancer have, on average, a median of two other chronic conditions at the time of cancer diagnosis [79,80]. Multimorbidity matters because it may influence the time to diagnosis, treatment experiences, and survival [25,43,79,81,82,83,84]. Regarding treatment, both older adults and those with a greater comorbidity burden independently have a lower likelihood of receiving active therapy, suggesting potential compounding of these challenges in multimorbid geriatric populations [22,28]. Specific comorbidities may also constrain therapeutic options and increase the risk of select treatment-related toxicities (i.e., treatment-related pneumonitis in a patient with pre-existing chronic obstructive pulmonary disease). These challenges arise particularly commonly in thoracic oncology given the frequent co-occurrence of smoking-related cardiopulmonary comorbidities and lung cancer [80,82,83].
Beyond the direct effects of comorbidity burden on therapy selection and toxicity, older adults with multimorbidity may also experience difficulties related to polypharmacy, potentially inappropriate medications, and drug interactions [29,30,85]. In the general elderly population, the prevalence of polypharmacy, defined as the concurrent use of five or more drugs, remains high, ranging from 22.8 to 44% [29,86,87,88]. Similarly, in older adults with lung cancer, a recent systematic review and meta-analysis demonstrated a pooled prevalence of polypharmacy of 38% [30]. Though in some circumstances the use of numerous concurrent medications may be appropriate, presence of polypharmacy in geriatric oncology populations can be associated with an array of adverse outcomes including: functional impairment, frailty, falls, delirium, cognitive impairment, adverse drug reactions, drug–drug interactions, cancer-related treatment toxicity, hospitalization, and inferior overall survival [29,30,36,89,90,91,92,93,94,95,96,97]. Polypharmacy is also associated with other suboptimal medication-related practices which are independently associated with poor prognosis, including the usage of potentially inappropriate medications (PIMs), generally defined as medications that have an unfavorable benefit–risk ratio in older adults [85,98,99]. In older adults with lung cancer, PIM prescribing is common, impacting 28–43% of patients, and is associated with all-cause mortality [30].
Collectively, these findings underscore the importance of assessing for multimorbidity, optimizing comorbidities, collaborating with other specialists, and reducing inappropriate medication usage and polypharmacy where feasible. Ideally, these interventions would occur within the context of a larger comprehensive geriatric assessment (CGA). However, though optimal mechanisms for comprehensive assessment in resource-rich settings will be discussed in subsequent sections, it is worth noting that targeted screening for polypharmacy and PIMs can be quickly performed by any provider simply by counting medications prescribed and referencing one of several validated tools for PIM identification (Table 1) [50,51,52]. Similarly, screening for severe comorbidities and coordinating care with relevant specialists can readily be accomplished by clinicians within the confines of a clinical visit [43,44,45,46,47,48]. Consideration of even these types of narrow interventions has the potential to enhance the care of older adults with lung cancer.
2.2. Frailty and Geriatric Syndromes
As aging occurs, all individuals are at an increased risk of geriatric syndromes: clinical conditions arising from accrued impairments in multiple systems coupled with individual inability to compensate for these derangements [24,100]. Unsurprisingly, older adults with cancer experience these syndromes at higher rates than their aging counterparts in the general population due to the coupled stressors of disease burden and cancer-directed therapy [101]. Common geriatric syndromes in older adults with cancer include frailty, falls, cognitive impairment, delirium, incontinence, and pressure ulcers [24,100]. These types of syndromes pose management challenges in thoracic oncology because they defy conventional organ-based categorizations, requiring a systems-based approach. They are relevant to the care of older adults given their associations with quality of life, functional impairment, hospitalization, success of cancer-directed treatments, and risk of death [24,101,102,103].
Of the array of geriatric syndromes, the most well studied in older adults with lung cancer is frailty. Frailty definitions vary, with different definitions conceptualizing it as physical disability, limitations in basic or instrumental daily activities (ADLs/iADLs), or simply as heightened vulnerability to adverse outcomes [104]. However, two general paradigms exist, with most models following one or the other approach. The first paradigm is the phenotype model of frailty, which incorporates five criteria: grip weakness, self-reported exhaustion, slow gait speed, low physical activity, and unintentional weight loss. In this model, patients are defined as frail if they meet three or more of these criteria [105]. The second paradigm is the deficit accumulation model of frailty, which counts the total number of health deficits an individual has (symptoms, disabilities, comorbidities, lab derangements, etc.). In this model, frailty is conceptualized along a spectrum, with higher scores indicating greater vulnerability [106]. Across models, effective assessments of frailty typically encompass some measure of the patients’ symptoms, functional impairment (physical, cognitive, and social), and body composition changes [55,107,108,109]. An array of specific screening instruments exist for usage in older adults with lung cancer and other neoplasms [53,54,55,56]. Importantly, as highlighted in a recent large meta-analysis of 2359 lung cancer patients, even across screening tools, the presence of frailty by any definition may be associated with higher overall mortality and therapeutic toxicity (HR 1.20, 95% CI 1.05–1.38; OR 1.72, 95% CI 1.18–2.51, respectively) [110]. Frailty has also been reliably shown to be associated with other meaningful measures of medical status, including body composition changes, symptom burden, and functional decline [108,111,112,113]. Irrespective of the specific tool used, these findings underscore the importance of identifying factors contributing to frailty in older adults with lung cancer and using this information to guide care decisions and toxicity management.
As emphasized elsewhere, the evaluation of any single parameter of medical status, whether multimorbidity, polypharmacy, or frailty, should ideally occur within the framework of a CGA. However, for frailty, similar to other measures, it is again important to emphasize that even if a complete CGA cannot be performed on every older adult due to resource constraints, basic relevant information can still be collected. More specifically, weight changes, ability to perform iADLs/ADLs, and recent falls are datapoints related to frailty that can be readily captured in any clinical setting and should be obtained for every older adult with lung cancer. Importantly, there are also brief 8–13 question validated survey tools which can be used to identify vulnerable subgroups who would most benefit from a CGA (i.e., Vulnerable Elders Survey-13, G-8 Geriatric Screening Tool) [64,77,78]. Physicians should be emboldened to incorporate whatever metrics are feasible, within practice constraints, to capture facets of frailty in clinical care and identify high-risk individuals (Table 1).
2.3. Supportive Care Needs
Alongside medical needs, older adults with lung cancer also have a unique constellation of supportive care needs. Relevant domains include treatment-related side effects, socioeconomic supports and burdens, and advanced care planning. Each of these issues is discussed further below.
2.3.1. Treatment-Related Side Effects
Across patient groups, lung cancer is associated with substantial longitudinal symptom burden [114]. Interestingly, emerging evidence suggests that older adults with cancer may experience different patterns of physical and psychologic symptoms than their younger counterparts during treatment [5,7,111,115]. More specifically, though data quality is poor, investigations encompassing varied cancers generally suggest that older adults may experience a different intensity of specific symptoms (i.e., appetite change, psychological distress), and an overall larger and more varied array of physical and psychological concerns than their younger counterparts [6,7,111,115,116,117]. Treatment-related changes in weight, appetite, and nutrition may be of particular concern given the high rates of cachexia observed in geriatric oncology populations [118].
Older adults are also more likely to be undertreated for specific symptoms, such as pain [119,120,121]. These findings underscore the importance of monitoring for the full array of potential symptoms older adults may experience during treatment and promptly addressing concerns where present.
Interestingly, for specific symptom clusters such as mood symptoms, data also suggest greater individual heterogeneity in symptom presentation among older adults, which can complicate identification [122,123,124]. Pseudodementia, a neuropsychiatric condition manifesting as neurocognitive and functional impairment mimicking neurodegenerative disease, is a well-documented phenomenon in older adults [123]. However, cognitive symptoms such as difficulty concentrating or forgetfulness may also simply reflect aging and not an underlying mood disorder [122]. In older adults with cancer, identifying clinical depression and anxiety is particularly challenging given the range of somatic symptoms associated with underlying disease and treatment, some of which may mimic psychological disorders [122]. These findings underscore the importance of evaluating treatment-related symptoms within a broader context rather than leaping to pathologize them. Report of specific symptoms during treatment should catalyze a broader dialogue about overall mood, coping strategies, and adequacy of existing support systems. In circumstances where a clinical mood disorder or substantial psychological distress is identified, providers should consider integrated multimodal approaches incorporating evidence-based psychosocial interventions, behavioral techniques, and pharmacotherapy where appropriate [125].
Beyond divergent patterns of symptoms, older adults with lung cancer may also experience different consequences of treatment-related symptoms on their daily functioning. For example, in one large study of 903 patients with solid tumors receiving therapy, 90% of older adults reported treatment-related symptoms interfering with quality of life (QOL) or daily functioning, and older adults had greater symptom-related distress than their younger counterparts [111]. Other studies in older adults with cancer have demonstrated similar relationships, irrespective of cancer type, disease stage, or number of comorbidities [126]. Mirroring these findings, in lung cancer-specific geriatric cohorts, studies have also shown consistent relationships between symptom burden, QOL, and functional impairment [127,128]. Treatment-related functional decline is of particular concern in older adults with lung cancer, particularly those with a pre-existing disability, because it may influence their ability to live independently and complete meaningful and necessary daily activities. Function and quality of life (QOL) changes arising from symptoms are also particularly relevant given the importance that older adults place on these factors. In one large study of 226 patients with limited life expectancy due to cancer or cardiopulmonary disease, over 70% reported they would not want a treatment resulting in functional impairment even if it improved survival [129]. These findings highlight the importance of identifying function and QOL priorities to support informed decision-making throughout the treatment process. Where feasible, clinicians should assess patient-reported symptoms, QOL, and frailty using available validated tools (Table 1) [55,57,59,60,61,62,63,64,78,130,131,132,133,134,135,136,137].
2.3.2. Socioeconomic Supports and Burdens
Cancer care occurs within a patient’s socioeconomic context. Economic consequences arising from cancer diagnosis and treatment, often termed “financial toxicity” (FT), are an important but often overlooked component of older adults’ experiences with cancer [138,139,140]. Approximately one-fifth of elderly patients with cancer experience treatment-related financial distress, and those endorsing financial strain also commonly report reduced QOL and higher rates of mood symptoms [139,140]. The best approach to screening for and mitigating these stressors remains unclear, with varied approaches in current use and no national consensus guidelines. Regarding screening, several available questionnaires exist that capture elements of financial toxicity, but further validation across contexts will be valuable [65,66,138,141,142]. Similarly, regarding mitigation, emerging evidence suggests that embedded financial navigation services may reduce out-of-pocket spending for patients. These services can generally assist with health insurance optimization, health literacy education, and referral to social support services. However, whether these interventions meaningfully reduce cumulative financial burden remains unknown [143]. To better address these factors shaping patient experiences, further investigation is needed to define best practices.
Alongside the financial context, social support, defined as the constellation of individuals available to provide psychological, physical, and financial help to a patient, also plays a vital role in oncologic outcomes [144]. More specifically, existing evidence suggests support is closely associated with physical health, psychological adjustment, hospital readmission, and overall survival [145,146,147,148,149,150,151,152]. As aging occurs, social networks may be narrowed by death and illness, while concurrently, medical and supportive care needs may increase. In one recent survey of 1460 older adults with cancer, two-thirds reported at least one unmet social support need, underscoring the scope of the challenge [153].
Beyond the impact of age on the experience of patients, the caregivers of older adults may also experience physical, emotional, and financial stressors during their loved one’s cancer treatment. These caregivers are typically older adults themselves and may have similar vulnerabilities as their patient counterparts, with research suggesting at least a third have pre-existing fair to poor health and/or a serious health condition [154,155]. Seventy-five percent of caregivers report experiencing at least some degree of caregiver burden as the result of their loved one’s diagnosis, and up to one-fifth may experience a decline in QOL as the result of caregiving responsibilities [156,157]. This burden is heightened when patients are highly symptomatic and have reduced functional status, and concerningly, is associated with increased risk of mortality [158,159]. These findings emphasize the importance of a timely CGA, which will be discussed in subsequent sections, but can help identify individual patient and caregiver needs and mobilize appropriate resources. However, even in settings where comprehensive assessment is not possible for every patient, every clinician should inquire about capacity to perform basic and instrumental activities of daily living and existing social supports. An array of brief patient-and caregiver-facing validated screening tools that can be incorporated into clinic also exist, which can readily be used to identify patients at a particularly high risk for poor outcomes (Table 1) [67,68,69,70,71,160,161,162,163].
2.3.3. Advanced Care Planning
Beyond attending to treatment-related and socioeconomic needs, engaging older adults with lung cancer in advanced care planning (ACP) is also of pivotal importance. ACP is the serial process through which patients delineate their goals, values, and preferences for medical care, and may include the exploration of care goals with clinicians and chosen community, completion of an advanced directive, and/or designation of a healthcare proxy (HCP) [164]. This process is the cornerstone of high-quality care in older adults with cancer, and is associated with more goal-concordant care delivery, increased caregiver and patient satisfaction, and reduced rates of high-intensity end-of-life care [72,165,166,167,168,169]. However, despite growing evidence suggesting most older adults with cancer want to participate in ACP early and iteratively, rates of engagement remain low [170,171]. Estimates vary, but in a recent large study of cancer patients aged 55 and older and their families, 65% reported discussing end-of-life care in any context, 61.9% had designated a medical decision-maker, and only 54.1% had advanced directives [172]. Alongside observed limitations in ACP, prior work has suggested that over half of older adults with cancer and their caregivers have prognostic perceptions diverging from their providers [9,173,174,175]. These findings highlight ongoing communication challenges meriting further interrogation in lung-specific populations.
Meaningful advanced care planning is supported by a shared understanding of age-associated vulnerability, cancer prognosis, life expectancy, therapeutic tradeoffs, values, goals, and preferences [9]. Age may be a relevant consideration in these discussions as it can influence patient perspectives on tradeoffs between quality of life, function, and life-prolonging treatment [129,176,177,178,179]. Age may also influence preferences for mode of information delivery and mechanisms for coping with illness [6,177,178]. However, importantly, age is one factor of many. Older adults possess a varied suite of medical, supportive, and psychosocial needs, and identification of age group does not obviate individualized decision-making [1,2,3,4,5,6,7,8,9,10]. In older adults, as in younger patients, ACP should begin early and occur frequently. A variety of validated tools exist which may support clinicians engaging older adults with lung cancer on this important domain (Table 1) [72,73,74,75,76]. Other mechanisms for enhancing care-related communication, including timely palliative care engagement, have also been shown to increase ACP rates and enhance prognostic awareness across age groups [180,181,182,183]. As will be discussed further in subsequent sections, in older adults, these discussions are optimally informed by a preceding CGA [9].
3. Comprehensive Geriatric Assessment
The CGA begins with a multifaceted interdisciplinary evaluation which captures the medical, psychosocial, and functional needs of an older adult. The evaluation component of CGA leverages validated tools which assess function and mobility, medical comorbidities and polypharmacy, cognition, psychological state, and nutrition, as well as social functioning and supports [184,185,186]. The second component of the CGA entails implementing adjustments to the cancer management plan as needed, based on positive findings.
The CGA is broadly recommended by an array of professional organizations including the American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN), and International Society of Geriatric Oncology (SIOG) in patients aged 65 or older with cancer. Granular indications vary by organization, but CGA usage is generally recommended in those receiving chemotherapy, and individuals where concerns exist regarding treatment toleration, as well as those with abnormalities identified by a geriatric screening tool [184,185,186]. Common validated instruments include the G8 geriatric screening tool and Vulnerable Elders Survey (VES-13), each of which has been extensively used in thoracic populations and can be completed in under ten minutes.
How did the CGA become so broadly utilized? These recommendations arise from the demonstrated associations between the CGA and an array of clinically relevant outcomes, including patient and caregiver satisfaction, enhanced communication, quality of life, physical function, decreased treatment toxicity, and possibly survival, although the latter has been less consistently shown [39,40,41,187,188,189,190,191,192,193,194,195,196,197,198,199]. The CGA is also a valuable tool for optimizing non-oncologic care during cancer therapy and increasing advanced care planning [41,197]. Finally, the CGA may be valuable for identifying unmet supportive care needs in older adults, which can subsequently be addressed through the modification of services. For all these reasons, it remains the gold standard of adjunctive assessment for older adults undergoing cancer directed therapy.
4. Age-Specific Considerations for Selection of Lung Cancer-Directed Therapy
Just as age-specific considerations should inform the provision of medical and supportive care, so too should they shape the selection and delivery of cancer-directed therapy for older adults. In the treatment of lung cancer, appropriate therapy is stage-specific. Thus, for each stage, the following sections outline current treatment guidelines, the ways in which aging may shape their implementation, and recommendations for individualizing and optimizing outcomes of care.
4.1. Early-Stage Non-Small Cell Lung Cancer
For early-stage non-small cell lung cancer (NSCLC), the goal of treatment in the general population is curative, and the mainstay of definitive, local therapy is surgical resection [200]. Eligibility for surgery depends on tumor resectability and medical operability, with the latter largely determined by cardiopulmonary reserve [201]. While cardiac fitness is necessary to withstand general anesthesia, the stresses of surgery, and postoperative recovery, pulmonary reserve is needed to compensate for the loss of resected lung [201]. Thus, patients must undergo pulmonary function testing, ventilation–perfusion imaging, and at times exercise testing to assess suitability [201]. For those deemed medically inoperable or those who decline surgery, definitive radiotherapy (RT), preferably with stereotactic ablative body radiation (SABR), is recommended. Otherwise, image-guided thermal ablation (e.g., cryotherapy, microwave, radiofrequency ablation) remains an option for select patients [200].
In older adults with early-stage NSCLC, management recommendations are notably aligned with general guidelines. Surgery remains the primary treatment of choice, and retrospective studies have shown similar overall survival between elderly and younger patients following resection [202,203]. In one such study, 90-day mortality was comparable among both groups [203]. In another, higher rates of surgery for stages I and II NSCLC were associated with improved one-year survival, even when older and sicker patients underwent resection [204]. Collectively, these data underscore that eligible adults who are medically fit can derive benefits from and should be offered surgical treatment.
Still, important age-related considerations apply. With regard to surgery, normal aging leads to cardiopulmonary changes which can limit physiologic reserve, thereby reducing one’s ability to tolerate perioperative stress [205]. Age-related changes can also predispose individuals to increased comorbidities, in particular cardiovascular pathology and chronic obstructive pulmonary disease (COPD) [205]. In one study, elderly patients were found to be nearly half as likely to undergo resection compared to their younger counterparts; the most frequent reasons for not operating were poor pulmonary function (58%), heart disease (17%), and comorbid illness (17%) [206]. In shaping physiologic changes central to eligibility for therapy, aging can pose particular barriers to the receipt of lung cancer care.
Another necessary consideration involves the tolerance of treatment-related toxicity. For elderly patients with early-stage NSCLC, published results on post-operative morbidity and mortality are currently conflicting, with only some showing a positive association between increasing age and these outcomes [207]. However, for other local therapies, such as radiation, the data may be clearer. Evidence specifically suggests that severe radiation pneumonitis may occur at higher rates in elderly patients compared to younger counterparts [207]. Specific comorbidities, such as collagen vascular diseases, hypersensitivity syndromes, diabetes mellitus, and hypertension, may also negatively impact patients’ ability to tolerate radiotherapy [207]. Given the potential for greater treatment toxicity in older adults, comprehensive assessments, in addition to patient-centered discussions of care goals and values, should be used to inform management decisions.
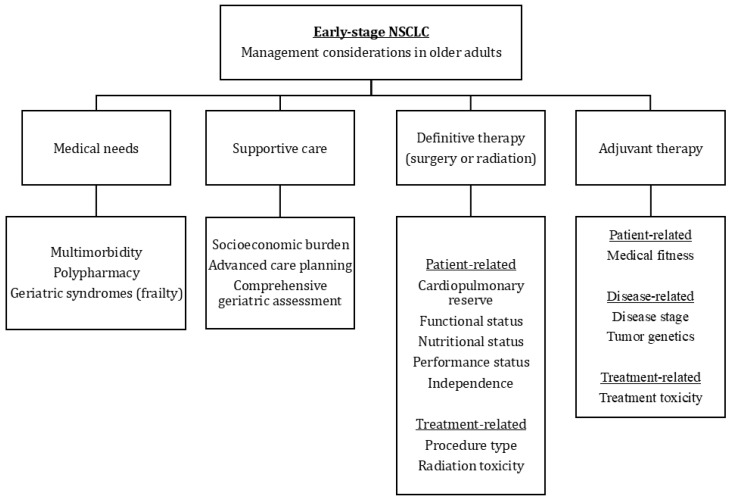
Lastly, for those able to receive definitive treatment, special care should be taken during implementation. In the pre-operative setting, in addition to standard evaluations, older adults should undergo optimization of multimorbidity, polypharmacy, functional status, and nutrition (Figure 1) [207]. Clinicians should also consider the use of a number of risk classification systems developed to predict surgical outcomes. Among them, a tool developed by SIOG uses an extended CGA to estimate operative risk, with features such as moderate/severe fatigue, any dependent IADL/ADL, and performance status scores found to be predictive of post-operative complications and extended hospital stay [208]. While planning for surgery, attention should be given to treatment timing and choice of procedure. Broadly, lobectomies are considered standard of care for elderly individuals, and newer surgical approaches such as minimally invasive techniques and video-assisted thoracic surgery may offer the promise of reducing perioperative morbidity or mortality among older adults. In contrast, increased surgery time, extended lung resection, and pneumonectomies have all been associated with poorer outcomes in elderly patients [207]. In tending to these factors, improvements in post-operative outcomes can be attained.
Figure 1.
Management considerations in older patients with early-stage non-small cell lung cancer.
Alongside definitive therapy with either surgery or radiation, a final question in the management of those with early-stage NSCLC revolves around when to offer systemic therapy. Current guidelines recommend that patients of any age with stage IB disease or higher be assessed for preoperative therapy, with strong consideration for chemo-immunotherapy for patients with tumors ≥ 4 cm or with node-positive disease [195]. Additionally, those with disease classified as high-risk stage IB or stages IIA or higher should be indicated for adjuvant therapy [209]. Options for the latter include chemotherapy and radiation, followed by newer immunotherapies and targeted agents [205]. Immune checkpoint inhibitors can be considered for those with stage II EGFR- and ALK- wild-type disease based on PDL-1 expression [210]. Targeted therapies can also be recommended for specific driver alterations (e.g., Osimertinib for EGFR-sensitizing mutations in stage IB/II disease, alectinib for ALK alterations in IB-IIIA disease) [210].
In older adults with early-stage NSCLC, treatment options for systemic therapy are similar, with newer therapies presenting exciting possibilities. As before, adjuvant chemotherapy is still supported for medically fit patients [202]. Concurrently, recent trials with moderate representation of fit older adults have demonstrated the efficacy and tolerability of novel agents, including osimertinib and pembrolizumab, suggesting these options may be viable in some patients [211,212]. Broadly, immunotherapies are considered to be better tolerated compared to chemotherapy, and a recent review on the use of immune checkpoint inhibitors across four major tumor types suggested similar toxicity between older and younger adults [213]. Still, older adults, and frail elderly individuals in particular, remain underrepresented in clinical trials [213]. Thus, decisions around adjuvant therapy should continue to be individualized, taking into account frailty, functional status, and patient preferences.
4.2. Locally Advanced NSCLC
For locally advanced NSCLC, standard of care consists of multimodal therapy potentially involving surgical resection, local radiation therapy, or systemic therapy (e.g., chemotherapy, immunotherapy, or targeted molecular therapy). If the primary tumor and involved lymph nodes are resectable, guidelines recommend neoadjuvant systemic therapy or concurrent chemoradiotherapy followed by resection; post-surgical treatment includes immunotherapy or targeted molecular therapy if appropriate (e.g., anti-PD-L1 for PD-L1 positive tumors, Osimertinib for EGFR exon 19 deletion or L858R mutation) [200,214]. Post-surgical treatment may also include chemotherapy if not previously offered or radiation in some settings. For inoperable primary tumors, standard treatment is concurrent chemotherapy and radiation therapy followed by systemic consolidation therapy [200,214].
In addition to staging and molecular and genetic testing, treatment considerations include patient performance status, comorbidity burden, cardiac and pulmonary function, systemic therapy tolerability, and socioeconomic supports [200]. In geriatric populations, physicians must also account for multimorbidity, polypharmacy, decreased functional status, and potential geriatric syndromes, particularly frailty [110]. Furthermore, there may be specific concerns related to poor toleration of surgery, immunotherapy, or chemotherapy [215]. Currently, there are limited data addressing the role and feasibility of surgery in elderly patients [215]. For immunotherapy, geriatric patients appear to have similar overall survival outcomes as the general population, but may have an increased risk of developing immune checkpoint inhibitor-related pneumonitis [215]. In terms of chemotherapy, single-agent therapy has been the standard given the risk of increased toxicities with additional agents; although carboplatin-based combinations may provide increased survival benefit, toxicities and treatment-related mortality also appear to increase [215]. Given the lack of data establishing absolute age-specific treatment recommendations for geriatric populations, best practices should include a comprehensive assessment, such as the CGA, to guide treatment decisions appropriate for each patient.
4.3. Metastatic NSCLC
For patients with advanced NSCLC with a known driver mutation, standard of care consists of targeted molecular therapy with chemotherapy; second-line and subsequent treatments may include another targeted agent or combination immunotherapy and chemotherapy [200,216]. For patients without driver mutations, anti-PD-L1 agents alone are recommended for high levels of PD-L1 expression, and combined immunotherapy with chemotherapy is recommended for low levels of PD-L1 expression [200,217].
Similar to other stages of NSCLC, treatment approaches should account for patient performance status, comorbidity burden, and supportive care needs. As described above, physicians must account for additional considerations for geriatric patients, e.g., multimorbidity, polypharmacy, and frailty. For geriatric patients with advanced NSCLC, concerns for poor immunotherapy and chemotherapy toleration are particularly salient [110,218]. Regarding immunotherapy, elderly patients have similar toxicities and overall survival when compared with the general population, however, among patients with ECOG ≥ 2, overall survival is lower [218]. For chemotherapy, patient performance status and frailty should be considered, given fit older adults may benefit from platinum-based combination therapy while their less fit counterparts may be better candidates for monotherapy due to the increased risk of toxicities [218]. Collectively, when caring for geriatric patients with metastatic NSCLC, patients should consider functional status and leverage the CGA to stratify risk and guide treatment decisions.
5. Conclusions and Future Directions
Future work should evaluate the medical and supportive care needs of older adults with NSCLC more granularly across treatment settings. Despite the growing number of elderly patients with NSCLC, there is limited evidence regarding age- and functional status-specific considerations for treatment. For each treatment modality, future studies should further characterize both potential outcome benefits and toxicity risks to better guide clinical decision-making. Furthermore, in the domains of socioeconomic burden and advanced care planning, needs and effective interventions remain under-investigated, impeding potential clinical improvement. Studies evaluating novel interventions in older adults must consider outcomes beyond overall survival, including quality-of-life, symptom burden, and functional outcomes.
Future research should also prioritize the integration of geriatric assessment tools into clinical trials for older patients with NSCLC. By comprehensively evaluating frailty, comorbidities, functional status, and cognitive impairment, these tools may be able to identify appropriate patients for treatment intensification and deintensification. Developing and validating treatment algorithms grounded in geriatric assessment will be crucial for supporting evidence-based decision-making appropriate for each patient. Coupling these algorithms with age-specific biomarkers and molecular profiling could further enhance treatment personalization, improving outcomes.
Across disease stages, older adults with NSCLC have a varied array of medical, psychosocial, and supportive care needs. Enhancing care in this population requires a multifaceted approach integrating comprehensive evaluation with personalized treatment planning. By further delineating patient needs and evaluating geriatric assessment tools across settings, future work has the potential to enhance treatment efficacy, reduce age-based disparities in care delivery, and minimize unnecessary symptom burden. Ultimately, care should be individualized, informed by evidence-based evaluation, and aligned with each patient’s overall health, functional status, and care goals.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All were involved in drafting the article or revising it critically for important intellectual content. All provided final approval of the manuscript and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yancik R. Population Aging and Cancer: A Cross-National Concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro C.L. Cancer Survivorship. N. Engl. J. Med. 2018;379:2438–2450. doi: 10.1056/NEJMra1712502. [DOI] [PubMed] [Google Scholar]
- 3.Hamaker M.E., Vos A.G., Smorenburg C.H., de Rooij S.E., van Munster B.C. The Value of Geriatric Assessments in Predicting Treatment Tolerance and All-Cause Mortality in Older Patients with Cancer. Oncologist. 2012;17:1439–1449. doi: 10.1634/theoncologist.2012-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puts M.T.E., Hardt J., Monette J., Girre V., Springall E., Alibhai S.M.H. Use of Geriatric Assessment for Older Adults in the Oncology Setting: A Systematic Review. J. Natl. Cancer Inst. 2012;104:1134–1164. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linden W., Vodermaier A., Mackenzie R., Greig D. Anxiety and Depression after Cancer Diagnosis: Prevalence Rates by Cancer Type, Gender, and Age. J. Affect. Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Mor V., Allen S., Malin M. The Psychosocial Impact of Cancer on Older versus Younger Patients and Their Families. Cancer. 1994;74:2118–2127. doi: 10.1002/1097-0142(19941001)74:7+<2118::AID-CNCR2820741720>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Cheung W.Y., Le L.W., Gagliese L., Zimmermann C. Age and Gender Differences in Symptom Intensity and Symptom Clusters among Patients with Metastatic Cancer. Support. Care Cancer. 2011;19:417–423. doi: 10.1007/s00520-010-0865-2. [DOI] [PubMed] [Google Scholar]
- 8.Nipp R.D., Subbiah I.M., Loscalzo M. Convergence of Geriatrics and Palliative Care to Deliver Personalized Supportive Care for Older Adults with Cancer. J. Clin. Oncol. 2021;39:2185–2194. doi: 10.1200/JCO.21.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuMontier C., Loh K.P., Soto-Perez-de-Celis E., Dale W. Decision Making in Older Adults with Cancer. J. Clin. Oncol. 2021;39:2164–2174. doi: 10.1200/JCO.21.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komici K., Bencivenga L., Navani N., D’Agnano V., Guerra G., Bianco A., Rengo G., Perrotta F. Frailty in Patients with Lung Cancer: A Systematic Review and Meta-Analysis. Chest. 2022;162:485–497. doi: 10.1016/j.chest.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R.L., Giaquinto A.N., Jemal A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 12.van Abbema D.L., van den Akker M., Janssen-Heijnen M.L., van den Berkmortel F., Hoeben A., de Vos-Geelen J., Buntinx F., Kleijnen J., Tjan-Heijnen V.C.G. Patient- and Tumor-Related Predictors of Chemotherapy Intolerance in Older Patients with Cancer: A Systematic Review. J. Geriatr. Oncol. 2019;10:31–41. doi: 10.1016/j.jgo.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Arnold M., Rutherford M.J., Bardot A., Ferlay J., Andersson T.M.-L., Myklebust T.Å., Tervonen H., Thursfield V., Ransom D., Shack L., et al. Progress in Cancer Survival, Mortality, and Incidence in Seven High-Income Countries 1995–2014 (ICBP SURVMARK-2): A Population-Based Study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh H., Kanapuru B., Smith C., Fashoyin-Aje L., Myers A., Kim G., Pazdur R. FDA Analysis of Enrollment of Older Adults in Clinical Trials for Cancer Drug Registration: A 10-Year Experience by the U.S. Food and Drug Administration. J. Clin. Oncol. 2017;35:10009. doi: 10.1200/JCO.2017.35.15_suppl.10009. [DOI] [Google Scholar]
- 15.Tesfaw L.M., Dessie Z.G., Fenta H.M. Lung Cancer Mortality and Associated Predictors: Systematic Review Using 32 Scientific Research Findings. Front. Oncol. 2023;13:1308897. doi: 10.3389/fonc.2023.1308897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum P., Winter H., Eichhorn M.E., Roesch R.M., Taber S., Christopoulos P., Wiegering A., Lenzi J. Trends in Age- and Sex-Specific Lung Cancer Mortality in Europe and Northern America: Analysis of Vital Registration Data from the WHO Mortality Database between 2000 and 2017. Eur. J. Cancer. 2022;171:269–279. doi: 10.1016/j.ejca.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Fang P., He W., Gomez D.R., Hoffman K.E., Smith B.D., Giordano S.H., Jagsi R., Smith G.L. Influence of Age on Guideline-Concordant Cancer Care for Elderly Patients in the United States. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:748–757. doi: 10.1016/j.ijrobp.2017.01.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadpara P., Madhavan S.S., Tworek C. Guideline-Concordant Timely Lung Cancer Care and Prognosis among Elderly Patients in the United States: A Population-Based Study. Cancer Epidemiol. 2015;39:1136–1144. doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang M., Pearson S.-A., Schaffer A.L., Lewis C.R., John T., Simes R.J., Lee C.K. Are Clinical Trial Eligibility Criteria Representative of Older Patients with Lung Cancer? A Population-Based Data Linkage Study. J. Geriatr. Oncol. 2021;12:930–936. doi: 10.1016/j.jgo.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Ganti A.K., Klein A.B., Cotarla I., Seal B., Chou E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021;7:1824–1832. doi: 10.1001/jamaoncol.2021.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang H.H., Wang X., Stinchcombe T.E., Wong M.L., Cheng P., Ganti A.K., Sargent D.J., Zhang Y., Hu C., Mandrekar S.J., et al. Enrollment Trends and Disparity Among Patients with Lung Cancer in National Clinical Trials, 1990 to 2012. J. Clin. Oncol. 2016;34:3992–3999. doi: 10.1200/JCO.2016.67.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blom E.F., Haaf K.T., Arenberg D.A., de Koning H.J. Disparities in Receiving Guideline-Concordant Treatment for Lung Cancer in the United States. Ann. Am. Thorac. Soc. 2020;17:186–194. doi: 10.1513/AnnalsATS.201901-094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher J.A., Fox S.T., Reid N., Hubbard R.E., Ladwa R. The Impact of Frailty on Health Outcomes in Older Adults with Lung Cancer: A Systematic Review. Cancer Treat. Res. Commun. 2022;33:100652. doi: 10.1016/j.ctarc.2022.100652. [DOI] [PubMed] [Google Scholar]
- 24.Presley C.J., Reynolds C.H., Langer C.J. Caring for the Older Population with Advanced Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2017;37:587–596. doi: 10.1200/EDBK_179850. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen T.L., Hallas J., Friis S., Herrstedt J. Comorbidity in Elderly Cancer Patients in Relation to Overall and Cancer-Specific Mortality. Br. J. Cancer. 2012;106:1353–1360. doi: 10.1038/bjc.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loh K.P., Lam V., Webber K., Padam S., Sedrak M.S., Musinipally V., Grogan M., Presley C.J., Grandi J., Sanapala C., et al. Characteristics Associated with Functional Changes During Systemic Cancer Treatments: A Systematic Review Focused on Older Adults. J. Natl. Compr. Cancer Netw. 2021;19:1055–1062. doi: 10.6004/jnccn.2020.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M.A., Keeney T., Papaila A., Ogarek J., Khurshid H., Wulff-Burchfield E., Olszewski A., Bélanger E., Panagiotou O.A. Functional Status and Survival in Older Nursing Home Residents with Advanced Non-Small-Cell Lung Cancer: A SEER-Medicare Analysis. JCO Oncol. Pract. 2022;18:e886–e895. doi: 10.1200/OP.21.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittberg R., Decker K., Lambert P., Bravo J., John P.S., Turner D., Czaykowski P., Dawe D.E. Impact of Age, Comorbidity, and Polypharmacy on Receipt of Systemic Therapy in Advanced Cancers: A Retrospective Population-Based Study. J. Geriatr. Oncol. 2024;15:101689. doi: 10.1016/j.jgo.2023.101689. [DOI] [PubMed] [Google Scholar]
- 29.Chen L.-J., Trares K., Laetsch D.C., Nguyen T.N.M., Brenner H., Schöttker B. Systematic Review and Meta-Analysis on the Associations of Polypharmacy and Potentially Inappropriate Medication with Adverse Outcomes in Older Cancer Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2021;76:1044–1052. doi: 10.1093/gerona/glaa128. [DOI] [PubMed] [Google Scholar]
- 30.Tian F., Chen Z., Zhou D., Mo L. Prevalence of Polypharmacy and Potentially Inappropriate Medication Use in Older Lung Cancer Patients: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022;13:1044885. doi: 10.3389/fphar.2022.1044885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes F., Wong M., Battisti N.M.L., Kordbacheh T., Kiderlen M., Greystoke A., Luciani A. Immunotherapy in Older Patients with Non-Small Cell Lung Cancer: Young International Society of Geriatric Oncology Position Paper. Br. J. Cancer. 2020;123:874–884. doi: 10.1038/s41416-020-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajra A., Zemla T.J., Jatoi A., Feliciano J.L., Wong M.L., Chen H., Maggiore R., McMurray R.P., Hurria A., Muss H.B., et al. Time-to-Treatment-Failure and Related Outcomes Among 1000+ Advanced Non–Small Cell Lung Cancer Patients: Comparisons Between Older Versus Younger Patients (Alliance A151711) J. Thorac. Oncol. 2018;13:996–1003. doi: 10.1016/j.jtho.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nipp R.D., Greer J.A., El-Jawahri A., Traeger L., Gallagher E.R., Park E.R., Jackson V.A., Pirl W.F., Temel J.S. Age and Gender Moderate the Impact of Early Palliative Care in Metastatic Non-Small Cell Lung Cancer. Oncologist. 2016;21:119–126. doi: 10.1634/theoncologist.2015-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nipp R.D., El-Jawahri A., Traeger L., Jacobs J.M., Gallagher E.R., Park E.R., Jackson V.A., Pirl W.F., Temel J.S., Greer J.A. Differential Effects of Early Palliative Care Based on the Age and Sex of Patients with Advanced Cancer from a Randomized Controlled Trial. Palliat. Med. 2018;32:757–766. doi: 10.1177/0269216317751893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambers A., Damone E., Chen Y.T., Nyrop K., Deal A., Muss H., Charlot M. Social Support and Outcomes in Older Adults with Lung Cancer. J. Geriatr. Oncol. 2022;13:214–219. doi: 10.1016/j.jgo.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamed M.R., Ramsdale E., Loh K.P., Arastu A., Xu H., Obrecht S., Castillo D., Sharma M., Holmes H.M., Nightingale G., et al. Associations of Polypharmacy and Inappropriate Medications with Adverse Outcomes in Older Adults with Cancer: A Systematic Review and Meta-Analysis. Oncologist. 2020;25:e94–e108. doi: 10.1634/theoncologist.2019-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dale W., Williams G.R., R MacKenzie A., Soto-Perez-de-Celis E., Maggiore R.J., Merrill J.K., Katta S., Smith K.T., Klepin H.D. How Is Geriatric Assessment Used in Clinical Practice for Older Adults with Cancer? A Survey of Cancer Providers by the American Society of Clinical Oncology. JCO Oncol. Pract. 2021;17:336–344. doi: 10.1200/OP.20.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corre R., Greillier L., Le Caër H., Audigier-Valette C., Baize N., Bérard H., Falchero L., Monnet I., Dansin E., Vergnenègre A., et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients with Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J. Clin. Oncol. 2016;34:1476–1483. doi: 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- 39.Mohile S.G., Mohamed M.R., Xu H., Culakova E., Loh K.P., Magnuson A., Flannery M.A., Obrecht S., Gilmore N., Ramsdale E., et al. Evaluation of Geriatric Assessment and Management on the Toxic Effects of Cancer Treatment (GAP70+): A Cluster-Randomised Study. Lancet. 2021;398:1894–1904. doi: 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D., Sun C.-L., Kim H., Soto-Perez-de-Celis E., Chung V., Koczywas M., Fakih M., Chao J., Chien L.C., Charles K., et al. Geriatric Assessment–Driven Intervention (GAIN) on Chemotherapy-Related Toxic Effects in Older Adults with Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021;7:e214158. doi: 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamaker M., Lund C., Te Molder M., Soubeyran P., Wildiers H., van Huis L., Rostoft S. Geriatric Assessment in the Management of Older Patients with Cancer—A Systematic Review (Update) J. Geriatr. Oncol. 2022;13:761–777. doi: 10.1016/j.jgo.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Hamaker M.E., Te Molder M., Thielen N., van Munster B.C., Schiphorst A.H., van Huis L.H. The Effect of a Geriatric Evaluation on Treatment Decisions and Outcome for Older Cancer Patients—A Systematic Review. J. Geriatr. Oncol. 2018;9:430–440. doi: 10.1016/j.jgo.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Asmis T.R., Ding K., Seymour L., Shepherd F.A., Leighl N.B., Winton T.L., Whitehead M., Spaans J.N., Graham B.C., Goss G.D., et al. Age and Comorbidity as Independent Prognostic Factors in the Treatment of Non Small-Cell Lung Cancer: A Review of National Cancer Institute of Canada Clinical Trials Group Trials. J. Clin. Oncol. 2008;26:54–59. doi: 10.1200/JCO.2007.12.8322. [DOI] [PubMed] [Google Scholar]
- 44.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 45.George M., Smith A., Sabesan S., Ranmuthugala G. Physical Comorbidities and Their Relationship with Cancer Treatment and Its Outcomes in Older Adult Populations: Systematic Review. JMIR Cancer. 2021;7:e26425. doi: 10.2196/26425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Firat S., Byhardt R.W., Gore E. Comorbidity and Karnofksy Performance Score Are Independent Prognostic Factors in Stage III Non-Small-Cell Lung Cancer: An Institutional Analysis of Patients Treated on Four RTOG Studies. Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2002;54:357–364. doi: 10.1016/S0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 47.Chipidza F.E., Franco I., Chen Y.H., Baldini E.H., Chen A.B., Kozono D.E., Mak R.H. Comorbidity Is a Prognostic Factor in Early Stage Non-Small Cell Lung Cancer Patients Treated with Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:e677. doi: 10.1016/j.ijrobp.2018.07.1829. [DOI] [Google Scholar]
- 48.Wedding U., Roehrig B., Klippstein A., Steiner P., Schaeffer T., Pientka L., Höffken K. Comorbidity in Patients with Cancer: Prevalence and Severity Measured by Cumulative Illness Rating Scale. Crit. Rev. Oncol. Hematol. 2007;61:269–276. doi: 10.1016/j.critrevonc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Borson S., Scanlan J., Brush M., Vitaliano P., Dokmak A. The Mini-Cog: A Cognitive “vital Signs” Measure for Dementia Screening in Multi-Lingual Elderly. Int. J. Geriatr. Psychiatry. 2000;15:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::AID-GPS234>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 50.Fick D.M., Semla T.P., Steinman M., Beizer J., Brandt N., Dombrowski R. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 51.O’Mahony D. STOPP/START Criteria for Potentially Inappropriate Medications/Potential Prescribing Omissions in Older People: Origin and Progress. Expert. Rev. Clin. Pharmacol. 2020;13:15–22. doi: 10.1080/17512433.2020.1697676. [DOI] [PubMed] [Google Scholar]
- 52.O’Mahony D., O’Sullivan D., Byrne S., O’Connor M.N., Ryan C., Gallagher P. STOPP/START Criteria for Potentially Inappropriate Prescribing in Older People: Version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theou O., Andrew M., Ahip S.S., Squires E., McGarrigle L., Blodgett J.M., Goldstein J., Hominick K., Godin J., Hougan G., et al. The Pictorial Fit-Frail Scale: Developing a Visual Scale to Assess Frailty. Can. Geriatr. J. 2019;22:64–74. doi: 10.5770/cgj.22.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper L., Deeb A., Dezube A.R., Mazzola E., Dumontier C., Bader A.M., Theou O., Jaklitsch M.T., Frain L.N. Validation of the Pictorial Fit-Frail Scale in a Thoracic Surgery Clinic. Ann. Surg. 2023;277:e1150–e1156. doi: 10.1097/SLA.0000000000005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guerard E.J., Deal A.M., Chang Y., Williams G.R., Nyrop K.A., Pergolotti M., Muss H.B., Sanoff H.K., Lund J.L. Frailty Index Developed from a Cancer-Specific Geriatric Assessment and the Association with Mortality Among Older Adults with Cancer. J. Natl. Compr. Cancer Netw. 2017;15:894–902. doi: 10.6004/jnccn.2017.0122. [DOI] [PubMed] [Google Scholar]
- 56.Ferrat E., Paillaud E., Caillet P., Laurent M., Tournigand C., Lagrange J.-L., Droz J.-P., Balducci L., Audureau E., Canouï-Poitrine F., et al. Performance of Four Frailty Classifications in Older Patients with Cancer: Prospective Elderly Cancer Patients Cohort Study. J. Clin. Oncol. 2017;35:766–777. doi: 10.1200/JCO.2016.69.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a Brief Depression Severity Measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lafont C., Wakilian A.C., Lemogne C., Gouraud C., Fossey-Diaz V., Orvoen G., Lhuillier N., Paillaud E., Bastuji-Garin S., Zebachi S., et al. Diagnostic Performance of the 4-Item Geriatric Depression Scale for Depression Screening in Older Patients with Cancer: The ELCAPA Cohort Study. Oncologist. 2021;26:e983–e991. doi: 10.1002/onco.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yogananda M.N., Muthu V., Prasad K.T., Kohli A., Behera D., Singh N. Utility of the Revised Edmonton Symptom Assessment System (ESAS-r) and the Patient-Reported Functional Status (PRFS) in Lung Cancer Patients. Support. Care Cancer. 2018;26:767–775. doi: 10.1007/s00520-017-3887-1. [DOI] [PubMed] [Google Scholar]
- 60.Mendoza T.R., Wang X.S., Lu C., Palos G.R., Liao Z., Mobley G.M., Kapoor S., Cleeland C.S. Measuring the Symptom Burden of Lung Cancer: The Validity and Utility of the Lung Cancer Module of the MD Anderson Symptom Inventory. Oncologist. 2011;16:217–227. doi: 10.1634/theoncologist.2010-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergman B., Aaronson N.K., Ahmedzai S., Kaasa S., Sullivan M., EORTC Study Group on Quality of Life The EORTC QLQ-LC13: A Modular Supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for Use in Lung Cancer Clinical Trials. Eur. J. Cancer. 1994;30:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 62.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., de Haes J.C., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 63.Wheelwright S., Darlington A.-S., Fitzsimmons D., Fayers P., Arraras J.I., Bonnetain F., Brain E., Bredart A., Chie W.-C., Giesinger J., et al. International Validation of the EORTC QLQ-ELD14 Questionnaire for Assessment of Health-Related Quality of Life Elderly Patients with Cancer. Br. J. Cancer. 2013;109:852–858. doi: 10.1038/bjc.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saliba D., Elliott M., Rubenstein L.Z., Solomon D.H., Young R.T., Kamberg C.J., Roth C., MacLean C.H., Shekelle P.G., Sloss E.M., et al. The Vulnerable Elders Survey: A Tool for Identifying Vulnerable Older People in the Community. J. Am. Geriatr. Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 65.de Souza J.A., Yap B.J., Hlubocky F.J., Wroblewski K., Ratain M.J., Cella D., Daugherty C.K. The Development of a Financial Toxicity Patient-Reported Outcome in Cancer: The COST Measure. Cancer. 2014;120:3245–3253. doi: 10.1002/cncr.28814. [DOI] [PubMed] [Google Scholar]
- 66.Ng M.S.N., Choi K.C., Chan D.N.S., Wong C.L., Xing W., Ho P.S., Au C., Chan M., Tong M., Ling W.M., et al. Identifying a Cut-off Score for the COST Measure to Indicate High Financial Toxicity and Low Quality of Life among Cancer Patients. Support. Care Cancer. 2021;29:6109–6117. doi: 10.1007/s00520-020-05962-4. [DOI] [PubMed] [Google Scholar]
- 67.Given C.W., Given B., Stommel M., Collins C., King S., Franklin S. The Caregiver Reaction Assessment (CRA) for Caregivers to Persons with Chronic Physical and Mental Impairments. Res. Nurs. Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 68.Minaya P., Baumstarck K., Berbis J., Goncalves A., Barlesi F., Michel G., Salas S., Chinot O., Grob J.-J., Seitz J.F., et al. The CareGiver Oncology Quality of Life Questionnaire (CarGOQoL): Development and Validation of an Instrument to Measure the Quality of Life of the Caregivers of Patients with Cancer. Eur. J. Cancer. 2012;48:904–911. doi: 10.1016/j.ejca.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Zarit S.H., Reever K.E., Bach-Peterson J. Relatives of the Impaired Elderly: Correlates of Feelings of Burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 70.Higginson I.J., Gao W., Jackson D., Murray J., Harding R. Short-Form Zarit Caregiver Burden Interviews Were Valid in Advanced Conditions. J. Clin. Epidemiol. 2010;63:535–542. doi: 10.1016/j.jclinepi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Moser A., Stuck A.E., Silliman R.A., Ganz P.A., Clough-Gorr K.M. The Eight-Item Modified Medical Outcomes Study Social Support Survey: Psychometric Evaluation Showed Excellent Performance. J. Clin. Epidemiol. 2012;65:1107–1116. doi: 10.1016/j.jclinepi.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nouri S.S., Barnes D.E., Shi Y., Volow A.M., Shirsat N., Kinderman A.L., Harris H.A., Sudore R.L. The PREPARE for Your Care Program Increases Advance Care Planning Engagement among Diverse Older Adults with Cancer. Cancer. 2021;127:3631–3639. doi: 10.1002/cncr.33676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volandes A.E., Zupanc S.N., Lakin J.R., Cabral H.J., Burns E.A., Carney M.T., Lopez S., Itty J., Emmert K., Martin N.J., et al. Video Intervention and Goals-of-Care Documentation in Hospitalized Older Adults: The VIDEO-PCE Randomized Clinical Trial. JAMA Netw. Open. 2023;6:e2332556. doi: 10.1001/jamanetworkopen.2023.32556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tinetti M.E., Naik A.D., Dindo L., Costello D.M., Esterson J., Geda M., Rosen J., Hernandez-Bigos K., Smith C.D., Ouellet G.M., et al. Association of Patient Priorities—Aligned Decision-Making with Patient Outcomes and Ambulatory Health Care Burden Among Older Adults with Multiple Chronic Conditions: A Nonrandomized Clinical Trial. JAMA Intern. Med. 2019;179:1688–1697. doi: 10.1001/jamainternmed.2019.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McAlpine K., Lewis K.B., Trevena L.J., Stacey D. What Is the Effectiveness of Patient Decision Aids for Cancer-Related Decisions? A Systematic Review Subanalysis. JCO Clin. Cancer Inform. 2018;2:1–13. doi: 10.1200/CCI.17.00148. [DOI] [PubMed] [Google Scholar]
- 76.Epstein R.M., Duberstein P.R., Fenton J.J., Fiscella K., Hoerger M., Tancredi D.J., Xing G., Gramling R., Mohile S., Franks P., et al. Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol. 2017;3:92–100. doi: 10.1001/jamaoncol.2016.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia M.V., Agar M.R., Soo W.-K., To T., Phillips J.L. Screening Tools for Identifying Older Adults with Cancer Who May Benefit from a Geriatric Assessment: A Systematic Review. JAMA Oncol. 2021;7:616–627. doi: 10.1001/jamaoncol.2020.6736. [DOI] [PubMed] [Google Scholar]
- 78.Bellera C.A., Rainfray M., Mathoulin-Pélissier S., Mertens C., Delva F., Fonck M., Soubeyran P.L. Screening Older Cancer Patients: First Evaluation of the G-8 Geriatric Screening Tool. Ann. Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 79.Bensken W.P., Schiltz N.K., Warner D.F., Kim D.H., Wei M.Y., Quiñones A.R., Ho V.P., Kelley A.S., Owusu C., Kent E.E., et al. Comparing the Association between Multiple Chronic Conditions, Multimorbidity, Frailty, and Survival among Older Patients with Cancer. J. Geriatr. Oncol. 2022;13:1244–1252. doi: 10.1016/j.jgo.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fowler H., Belot A., Ellis L., Maringe C., Luque-Fernandez M.A., Njagi E.N., Navani N., Sarfati D., Rachet B. Comorbidity Prevalence among Cancer Patients: A Population-Based Cohort Study of Four Cancers. BMC Cancer. 2020;20:2. doi: 10.1186/s12885-019-6472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Extermann M., Overcash J., Lyman G.H., Parr J., Balducci L. Comorbidity and Functional Status Are Independent in Older Cancer Patients. J. Clin. Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 82.Aarts M.J., Aerts J.G., van den Borne B.E., Biesma B., Lemmens V.E.P.P., Kloover J.S. Comorbidity in Patients with Small-Cell Lung Cancer: Trends and Prognostic Impact. Clin. Lung Cancer. 2015;16:282–291. doi: 10.1016/j.cllc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Islam K.M.M., Jiang X., Anggondowati T., Lin G., Ganti A.K. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2015;24:1079–1085. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]
- 84.Boakye D., Günther K., Niedermaier T., Haug U., Ahrens W., Nagrani R. Associations between Comorbidities and Advanced Stage Diagnosis of Lung, Breast, Colorectal, and Prostate Cancer: A Systematic Review and Meta-Analysis. Cancer Epidemiol. 2021;75:102054. doi: 10.1016/j.canep.2021.102054. [DOI] [PubMed] [Google Scholar]
- 85.Sharma M., Loh K.P., Nightingale G., Mohile S.G., Holmes H.M. Polypharmacy and Potentially Inappropriate Medication Use in Geriatric Oncology. J. Geriatr. Oncol. 2016;7:346–353. doi: 10.1016/j.jgo.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kantor E.D., Rehm C.D., Haas J.S., Chan A.T., Giovannucci E.L. Trends in Prescription Drug Use Among Adults in the United States from 1999–2012. JAMA. 2015;314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morin L., Johnell K., Laroche M.-L., Fastbom J., Wastesson J.W. The Epidemiology of Polypharmacy in Older Adults: Register-Based Prospective Cohort Study. Clin. Epidemiol. 2018;10:289–298. doi: 10.2147/CLEP.S153458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rawle M.J., Richards M., Davis D., Kuh D. The Prevalence and Determinants of Polypharmacy at Age 69: A British Birth Cohort Study. BMC Geriatr. 2018;18:118. doi: 10.1186/s12877-018-0795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed M.R., Mohile S.G., Juba K.M., Awad H., Wells M., Loh K.P., Flannery M., Culakova E., Tylock R.G., Ramsdale E.E. Association of Polypharmacy and Potential Drug-Drug Interactions with Adverse Treatment Outcomes in Older Adults with Advanced Cancer. Cancer. 2023;129:1096–1104. doi: 10.1002/cncr.34642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Turner J.P., Shakib S., Singhal N., Hogan-Doran J., Prowse R., Johns S., Bell J.S. Prevalence and Factors Associated with Polypharmacy in Older People with Cancer. Support. Care Cancer. 2014;22:1727–1734. doi: 10.1007/s00520-014-2171-x. [DOI] [PubMed] [Google Scholar]
- 91.Şenel G., Uysal N., Oguz G., Kaya M., Kadioullari N., Koçak N., Karaca S. Delirium Frequency and Risk Factors Among Patients with Cancer in Palliative Care Unit. Am. J. Hosp. Palliat. Care. 2017;34:282–286. doi: 10.1177/1049909115624703. [DOI] [PubMed] [Google Scholar]
- 92.Oliveira R.F., Oliveira A.I., Cruz A.S., Ribeiro O., Afreixo V., Pimentel F. Polypharmacy and Drug Interactions in Older Patients with Cancer Receiving Chemotherapy: Associated Factors. BMC Geriatr. 2024;24:557. doi: 10.1186/s12877-024-05135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lavan A.H., O’Mahony D., Buckley M., O’Mahony D., Gallagher P. Adverse Drug Reactions in an Oncological Population: Prevalence, Predictability, and Preventability. Oncologist. 2019;24:e968–e977. doi: 10.1634/theoncologist.2018-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhalwani N.N., Fahami R., Sathanapally H., Seidu S., Davies M.J., Khunti K. Association between Polypharmacy and Falls in Older Adults: A Longitudinal Study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pamoukdjian F., Aparicio T., Zelek L., Boubaya M., Caillet P., François V., de Decker L., Lévy V., Sebbane G., Paillaud E. Impaired Mobility, Depressed Mood, Cognitive Impairment and Polypharmacy Are Independently Associated with Disability in Older Cancer Outpatients: The Prospective Physical Frailty in Elderly Cancer Patients (PF-EC) Cohort Study. J. Geriatr. Oncol. 2017;8:190–195. doi: 10.1016/j.jgo.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 96.Popa M.A., Wallace K.J., Brunello A., Extermann M., Balducci L. Potential Drug Interactions and Chemotoxicity in Older Patients with Cancer Receiving Chemotherapy. J. Geriatr. Oncol. 2014;5:307–314. doi: 10.1016/j.jgo.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mohamed M.R., Ramsdale E., Loh K.P., Xu H., Patil A., Gilmore N., Obrecht S., Wells M., Nightingale G., Juba K.M., et al. Association of Polypharmacy and Potentially Inappropriate Medications with Physical Functional Impairments in Older Adults with Cancer. J. Natl. Compr. Cancer Netw. 2021;19:267–274. doi: 10.6004/jnccn.2020.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dimitrow M.S., Airaksinen M.S.A., Kivelä S.-L., Lyles A., Leikola S.N.S. Comparison of Prescribing Criteria to Evaluate the Appropriateness of Drug Treatment in Individuals Aged 65 and Older: A Systematic Review. J. Am. Geriatr. Soc. 2011;59:1521–1530. doi: 10.1111/j.1532-5415.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 99.Nightingale G., Hajjar E., Swartz K., Andrel-Sendecki J., Chapman A. Evaluation of a Pharmacist-Led Medication Assessment Used to Identify Prevalence of and Associations with Polypharmacy and Potentially Inappropriate Medication Use among Ambulatory Senior Adults with Cancer. J. Clin. Oncol. 2015;33:1453–1459. doi: 10.1200/JCO.2014.58.7550. [DOI] [PubMed] [Google Scholar]
- 100.Inouye S.K., Studenski S., Tinetti M.E., Kuchel G.A. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept. J. Am. Geriatr. Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mohile S.G., Fan L., Reeve E., Jean-Pierre P., Mustian K., Peppone L., Janelsins M., Morrow G., Hall W., Dale W. Association of Cancer with Geriatric Syndromes in Older Medicare Beneficiaries. J. Clin. Oncol. 2011;29:1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halter J.B., Ouslander J.G., Studenski S., High K.P., Asthana S., Supiano M.A., Ritchie C. Hazzard’s Geriatric Medicine and Gerontology. 7th ed. McGraw-Hill Education; New York, NY, USA: 2017. [Google Scholar]
- 103.Magnuson A., Sattar S., Nightingale G., Saracino R., Skonecki E., Trevino K.M. A Practical Guide to Geriatric Syndromes in Older Adults with Cancer: A Focus on Falls, Cognition, Polypharmacy, and Depression. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:e96–e109. doi: 10.1200/EDBK_237641. [DOI] [PubMed] [Google Scholar]
- 104.Sternberg S.A., Schwartz A.W., Karunananthan S., Bergman H., Clarfield A.M. The Identification of Frailty: A Systematic Literature Review. J. Am. Geriatr. Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 105.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 106.Rockwood K., Mitnitski A. Frailty in Relation to the Accumulation of Deficits. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 107.Nipp R.D., Thompson L.L., Temel B., Fuh C.-X., Server C., Kay P.S., Landay S., Lage D.E., Traeger L., Scott E., et al. Screening Tool Identifies Older Adults with Cancer at Risk for Poor Outcomes. J. Natl. Compr. Cancer Netw. 2020;18:305–313. doi: 10.6004/jnccn.2019.7355. [DOI] [PubMed] [Google Scholar]
- 108.Lage D.E., El-Jawahri A., Fuh C.-X., Newcomb R.A., Jackson V.A., Ryan D.P., Greer J.A., Temel J.S., Nipp R.D. Functional Impairment, Symptom Burden, and Clinical Outcomes Among Hospitalized Patients with Advanced Cancer. J. Natl. Compr. Cancer Netw. 2020;18:747–754. doi: 10.6004/jnccn.2019.7385. [DOI] [PubMed] [Google Scholar]
- 109.Nipp R.D., Fuchs G., El-Jawahri A., Mario J., Troschel F.M., Greer J.A., Gallagher E.R., Jackson V.A., Kambadakone A., Hong T.S., et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist. 2018;23:97–104. doi: 10.1634/theoncologist.2017-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dai S., Yang M., Song J., Dai S., Wu J. Impacts of Frailty on Prognosis in Lung Cancer Patients: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:715513. doi: 10.3389/fmed.2021.715513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohile S.G., Heckler C., Fan L., Mustian K., Jean-Pierre P., Usuki K., Sprod L., Janelsins M., Purnell J., Peppone L., et al. Age-Related Differences in Symptoms and Their Interference with Quality of Life in 903 Cancer Patients Undergoing Radiation Therapy. J. Geriatr. Oncol. 2011;2:225–232. doi: 10.1016/j.jgo.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kirkhus L., Benth J.S., Grønberg B.H., Hjermstad M.J., Rostoft S., Harneshaug M., Selbæk G., Wyller T.B., Jordhøy M.S. Frailty Identified by Geriatric Assessment Is Associated with Poor Functioning, High Symptom Burden and Increased Risk of Physical Decline in Older Cancer Patients: Prospective Observational Study. Palliat. Med. 2019;33:312–322. doi: 10.1177/0269216319825972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Williams G.R., Deal A.M., Muss H.B., Weinberg M.S., Sanoff H.K., Guerard E.J., Nyrop K.A., Pergolotti M., Shachar S.S. Frailty and Skeletal Muscle in Older Adults with Cancer. J. Geriatr. Oncol. 2018;9:68–73. doi: 10.1016/j.jgo.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sugimura H., Yang P. Long-Term Survivorship in Lung Cancer: A Review. Chest. 2006;129:1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 115.Cataldo J.K., Paul S., Cooper B., Skerman H., Alexander K., Aouizerat B., Blackman V., Merriman J., Dunn L., Ritchie C., et al. Differences in the Symptom Experience of Older versus Younger Oncology Outpatients: A Cross-Sectional Study. BMC Cancer. 2013;13:6. doi: 10.1186/1471-2407-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Degner L.F., Sloan J.A. Symptom Distress in Newly Diagnosed Ambulatory Cancer Patients and as a Predictor of Survival in Lung Cancer. J. Pain Symptom Manag. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 117.Oksholm T., Miaskowski C., Kongerud J.S., Cooper B., Paul S.M., Laerum L., Rustoen T. Does Age Influence the Symptom Experience of Lung Cancer Patients Prior to Surgery? Lung Cancer. 2013;82:156–161. doi: 10.1016/j.lungcan.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 118.Poisson J., Martinez-Tapia C., Heitz D., Geiss R., Albrand G., Falandry C., Gisselbrecht M., Couderc A.-L., Boulahssass R., Liuu E., et al. Prevalence and Prognostic Impact of Cachexia among Older Patients with Cancer: A Nationwide Cross-Sectional Survey (NutriAgeCancer) J. Cachexia Sarcopenia Muscle. 2021;12:1477–1488. doi: 10.1002/jcsm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bernabei R., Gambassi G., Lapane K., Landi F., Gatsonis C., Dunlop R., Lipsitz L., Steel K., Mor V., SAGE Study Group Management of Pain in Elderly Patients with Cancer. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 120.Rao A., Cohen H.J. Symptom Management in the Elderly Cancer Patient: Fatigue, Pain, and Depression. J. Natl. Cancer Inst. Monogr. 2004;2004:150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- 121.Paice J.A., Von Roenn J.H. Under- or Overtreatment of Pain in the Patient with Cancer: How to Achieve Proper Balance. J. Clin. Oncol. 2014;32:1721–1726. doi: 10.1200/JCO.2013.52.5196. [DOI] [PubMed] [Google Scholar]
- 122.Trevino K.M., Saracino R.M., Roth A.J. Symptomatology, Assessment, and Treatment of Anxiety in Older Adults with Cancer. J. Geriatr. Oncol. 2021;12:316–319. doi: 10.1016/j.jgo.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sekhon S., Marwaha R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. Depressive Cognitive Disorders. [PubMed] [Google Scholar]
- 124.Balsamo M., Cataldi F., Carlucci L., Fairfield B. Assessment of Anxiety in Older Adults: A Review of Self-Report Measures. Clin. Interv. Aging. 2018;13:573–593. doi: 10.2147/CIA.S114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Milbury K., Li Y., Durrani S., Liao Z.X., Yang C.C., Tsao A.S., Cohen L., Bruera E. Results of a Pilot Randomized Controlled Trial: A Couple-Based Meditation Intervention for Patients with Metastatic Lung Cancer and Their Partners. J. Clin. Oncol. 2019;37:135. doi: 10.1200/JCO.2019.37.31_suppl.135. [DOI] [Google Scholar]
- 126.Given C.W., Given B., Azzouz F., Kozachik S., Stommel M. Predictors of Pain and Fatigue in the Year Following Diagnosis among Elderly Cancer Patients. J. Pain Symptom Manag. 2001;21:456–466. doi: 10.1016/S0885-3924(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 127.Gift A.G., Jablonski A., Stommel M., Given C.W. Symptom Clusters in Elderly Patients with Lung Cancer. Oncol. Nurs. Forum. 2004;31:202–212. doi: 10.1188/04.ONF.203-212. [DOI] [PubMed] [Google Scholar]
- 128.Cooley M.E., Short T.H., Moriarty H.J. Symptom Prevalence, Distress, and Change over Time in Adults Receiving Treatment for Lung Cancer. Psycho-Oncol. J. Psychol. Soc. Behav. Dimens. Cancer. 2003;12:694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 129.Fried T.R., Bradley E.H., Towle V.R., Allore H. Understanding the Treatment Preferences of Seriously Ill Patients. N. Engl. J. Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 130.Cella D. The Functional Assessment of Cancer Therapy—Lung and Lung Cancer Subscale Assess Quality of Life and Meaningful Symptom Improvement in Lung Cancer. Semin. Oncol. 2004;31:11–15. doi: 10.1053/j.seminoncol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 131.Soo W.K., King M., Pope A., Steer C., Devitt B., Chua S., Parente P., Davis I.D., Dārziņš P. The Elderly Functional Index (ELFI), a Patient-Reported Outcome Measure of Functional Status in Patients with Cancer: A Multicentre, Prospective Validation Study. Lancet Healthy Longev. 2021;2:e24–e33. doi: 10.1016/S2666-7568(20)30036-2. [DOI] [PubMed] [Google Scholar]
- 132.D’Ath P., Katona P., Mullan E., Evans S., Katona C. Screening, Detection and Management of Depression in Elderly Primary Care Attenders. I: The Acceptability and Performance of the 15 Item Geriatric Depression Scale (GDS15) and the Development of Short Versions. Fam. Pract. 1994;11:260–266. doi: 10.1093/fampra/11.3.260. [DOI] [PubMed] [Google Scholar]
- 133.Sheikh J.I., Yesavage J.A. 9/Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version. Clin. Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01_09. [DOI] [Google Scholar]
- 134.Marc L.G., Raue P.J., Bruce M.L. Screening Performance of the 15-Item Geriatric Depression Scale in a Diverse Elderly Home Care Population. Am. J. Geriatr. Psychiatry. 2008;16:914–921. doi: 10.1097/JGP.0b013e318186bd67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. An Ultra-Brief Screening Scale for Anxiety and Depression: The PHQ-4. Psychosomatics. 2009;50:613–621. doi: 10.1176/appi.psy.50.6.613. [DOI] [PubMed] [Google Scholar]
- 136.Chau Y.F., Zhou H., Chen B., Ren H., Ma Z., Zhang X., Duan J. Screening for Depression and Anxiety in Lung Cancer Patients: A Real-World Study Using GAD-7 and HADS. Thorac. Cancer. 2024;15:1041–1049. doi: 10.1111/1759-7714.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Segal D.L., June A., Payne M., Coolidge F.L., Yochim B. Development and Initial Validation of a Self-Report Assessment Tool for Anxiety among Older Adults: The Geriatric Anxiety Scale. J. Anxiety Disord. 2010;24:709–714. doi: 10.1016/j.janxdis.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 138.Khan H.M., Ramsey S., Shankaran V. Financial Toxicity in Cancer Care: Implications for Clinical Care and Potential Practice Solutions. J. Clin. Oncol. 2023;41:3051–3058. doi: 10.1200/JCO.22.01799. [DOI] [PubMed] [Google Scholar]
- 139.Arastu A., Patel A., Mohile S.G., Ciminelli J., Kaushik R., Wells M., Culakova E., Lei L., Xu H., Dougherty D.W., et al. Assessment of Financial Toxicity Among Older Adults with Advanced Cancer. JAMA Netw. Open. 2020;3:e2025810. doi: 10.1001/jamanetworkopen.2020.25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Giri S., Clark D., Al-Obaidi M., Varnado W., Kumar S., Paluri R., Gbolahan O., Bhatia S., Williams G.R. Financial Distress Among Older Adults with Cancer. JCO Oncol. Pract. 2021;17:e764–e773. doi: 10.1200/OP.20.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khera N., Sugalski J., Krause D., Butterfield R., Zhang N., Stewart F.M., Carlson R.W., Griffin J.M., Zafar S.Y., Lee S.J. Current Practices for Screening and Management of Financial Distress at NCCN Member Institutions. J. Natl. Compr. Cancer Netw. 2020;18:825–831. doi: 10.6004/jnccn.2020.7538. [DOI] [PubMed] [Google Scholar]
- 142.Riba M.B., Donovan K.A., Ahmed K., Andersen B., Braun I., Breitbart W.S., Brewer B.W., Corbett C., Fann J., Fleishman S., et al. NCCN Guidelines Insights: Distress Management, Version 2.2023: Featured updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2023;21:450–457. doi: 10.6004/jnccn.2023.0026. [DOI] [PubMed] [Google Scholar]
- 143.Yezefski T., Steelquist J., Watabayashi K., Sherman D., Shankaran V. Impact of Trained Oncology Financial Navigators on Patient Out-of-Pocket Spending. Am. J. Manag. Care. 2018;24:S74–S79. [PubMed] [Google Scholar]
- 144.Definition of Social Support—NCI Dictionary of Cancer Terms—NCI. [(accessed on 14 September 2024)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/social-support.
- 145.Kadambi S., Soto-Perez-de-Celis E., Garg T., Loh K.P., Krok-Schoen J.L., Battisti N.M.L., Moffat G.T., Gil L.A., Jr., Mohile S., Hsu T. Social Support for Older Adults with Cancer: Young International Society of Geriatric Oncology Review Paper. J. Geriatr. Oncol. 2020;11:217–224. doi: 10.1016/j.jgo.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Usta Y.Y. Importance of Social Support in Cancer Patients. Asian Pac. J. Cancer Prev. 2012;13:3569–3572. doi: 10.7314/APJCP.2012.13.8.3569. [DOI] [PubMed] [Google Scholar]
- 147.Nausheen B., Gidron Y., Peveler R., Moss-Morris R. Social Support and Cancer Progression: A Systematic Review. J. Psychosom. Res. 2009;67:403–415. doi: 10.1016/j.jpsychores.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 148.Yang Y.C., Li T., Ji Y. Impact of Social Integration on Metabolic Functions: Evidence from a Nationally Representative Longitudinal Study of US Older Adults. BMC Public Health. 2013;13:1210. doi: 10.1186/1471-2458-13-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pinquart M., Duberstein P.R. Associations of Social Networks with Cancer Mortality: A Meta-Analysis. Crit. Rev. Oncol. Hematol. 2010;75:122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boen C., Barrow D., Bensen J.T., Farnan L., Gerstel A., Hendrix L., Yang Y.C. Social Relationships, Inflammation, and Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2018;27:541–549. doi: 10.1158/1055-9965.EPI-17-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adashek J.J., Subbiah I.M. Caring for the Caregiver: A Systematic Review Characterising the Experience of Caregivers of Older Adults with Advanced Cancers. ESMO Open. 2020;5:e000862. doi: 10.1136/esmoopen-2020-000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hofman A., Zajdel N., Klekowski J., Chabowski M. Improving Social Support to Increase QoL in Lung Cancer Patients. Cancer Manag. Res. 2021;13:2319–2327. doi: 10.2147/CMAR.S278087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Williams G.R., Pisu M., Rocque G.B., Williams C.P., Taylor R.A., Kvale E.A., Partridge E.E., Bhatia S., Kenzik K.M. Unmet Social Support Needs among Older Adults with Cancer. Cancer. 2019;125:473–481. doi: 10.1002/cncr.31809. [DOI] [PubMed] [Google Scholar]
- 154.Navaie-Waliser M., Feldman P.H., Gould D.A., Levine C., Kuerbis A.N., Donelan K. When the Caregiver Needs Care: The Plight of Vulnerable Caregivers. Am. J. Public Health. 2002;92:409–413. doi: 10.2105/AJPH.92.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hunt G.G., Longacre M.L., Kent E.E., Weber-Raley L. Cancer Caregiving in the US: An Intense, Episodic, and Challenging Care Experience. National Alliance for Caregiving; Washington, DC, USA: Cancer Support Community; Washington, DC, USA: 2017. [Google Scholar]
- 156.Hsu T., Loscalzo M., Ramani R., Forman S., Popplewell L., Clark K., Katheria V., Feng T., Strowbridge R., Rinehart R., et al. Factors Associated with High Burden in Caregivers of Older Adults with Cancer. Cancer. 2014;120:2927–2935. doi: 10.1002/cncr.28765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hsu T., Nathwani N., Loscalzo M., Chung V., Chao J., Karanes C., Koczywas M., Forman S., Lim D., Siddiqi T., et al. Understanding Caregiver Quality of Life in Caregivers of Hospitalized Older Adults with Cancer. J. Am. Geriatr. Soc. 2019;67:978–986. doi: 10.1111/jgs.15841. [DOI] [PubMed] [Google Scholar]
- 158.Jayani R., Hurria A. Caregivers of Older Adults with Cancer. Semin. Oncol. Nurs. 2012;28:221–225. doi: 10.1016/j.soncn.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schulz R., Beach S.R. Caregiving as a Risk Factor for Mortality: The Caregiver Health Effects Study. JAMA. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 160.Broadhead W.E., Gehlbach S.H., De Gruy F.V., Kaplan B.H. The Duke–UNC Functional Social Support Questionnaire: Measurement of Social Support in Family Medicine Patients. Med. Care. 1988;26:709. doi: 10.1097/00005650-198807000-00006. [DOI] [PubMed] [Google Scholar]
- 161.Zimet G.D., Powell S.S., Farley G.K., Werkman S., Berkoff K.A. Psychometric Characteristics of the Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 1990;55:610–617. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 162.Tarlov A.R., Ware J.E., Greenfield S., Nelson E.C., Perrin E., Zubkoff M. The Medical Outcomes Study. An Application of Methods for Monitoring the Results of Medical Care. JAMA. 1989;262:925–930. doi: 10.1001/jama.1989.03430070073033. [DOI] [PubMed] [Google Scholar]
- 163.Bédard M., Molloy D.W., Squire L., Dubois S., Lever J.A., O’Donnell M. The Zarit Burden Interview: A New Short Version and Screening Version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 164.Sudore R.L., Lum H.D., You J.J., Hanson L.C., Meier D.E., Pantilat S.Z., Matlock D.D., Rietjens J.A.C., Korfage I.J., Ritchie C.S., et al. Defining Advance Care Planning for Adults: A Consensus Definition from a Multidisciplinary Delphi Panel. J. Pain Symptom Manag. 2017;53:821–832.e1. doi: 10.1016/j.jpainsymman.2016.12.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bischoff K.E., Sudore R., Miao Y., Boscardin W.J., Smith A.K. Advance Care Planning and the Quality of End-of-Life Care in Older Adults. J. Am. Geriatr. Soc. 2013;61:209–214. doi: 10.1111/jgs.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Levoy K., Buck H., Behar-Zusman V. The Impact of Varying Levels of Advance Care Planning Engagement on Perceptions of the End-of-Life Experience Among Caregivers of Deceased Patients with Cancer. Am. J. Hosp. Palliat. Care. 2020;37:1045–1052. doi: 10.1177/1049909120917899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McDermott C.L., Engelberg R.A., Sibley J., Sorror M.L., Curtis J.R. The Association between Chronic Conditions, End-of-Life Health Care Use, and Documentation of Advance Care Planning among Patients with Cancer. J. Palliat. Med. 2020;23:1335–1341. doi: 10.1089/jpm.2019.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Silveira M.J., Kim S.Y.H., Langa K.M. Advance Directives and Outcomes of Surrogate Decision Making before Death. N. Engl. J. Med. 2010;362:1211–1218. doi: 10.1056/NEJMsa0907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Detering K.M., Hancock A.D., Reade M.C., Silvester W. The Impact of Advance Care Planning on End of Life Care in Elderly Patients: Randomised Controlled Trial. BMJ. 2010;340:c1345. doi: 10.1136/bmj.c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kubi B., Istl A.C., Lee K.T., Conca-Cheng A., Johnston F.M. Advance Care Planning in Cancer: Patient Preferences for Personnel and Timing. JCO Oncol. Pract. 2020;16:e875–e883. doi: 10.1200/JOP.19.00367. [DOI] [PubMed] [Google Scholar]
- 171.Waller A., Turon H., Bryant J., Zucca A., Evans T.-J., Sanson-Fisher R. Medical Oncology Outpatients’ Preferences and Experiences with Advanced Care Planning: A Cross-Sectional Study. BMC Cancer. 2019;19:63. doi: 10.1186/s12885-019-5272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parajuli J., Walsh A., Hicks A., Grant K.A., Crane P., Chen Z.J., Williams G.R., Sun V., Myers J.S., Bakitas M. Factors Affecting Advance Care Planning in Older Adults with Cancer. J. Geriatr. Oncol. 2024;15:101839. doi: 10.1016/j.jgo.2024.101839. [DOI] [PubMed] [Google Scholar]
- 173.Thompson L.L., Temel B., Fuh C.-X., Server C., Kay P., Landay S., Lage D.E., Traeger L., Scott E., Jackson V.A., et al. Perceptions of Medical Status and Treatment Goal among Older Adults with Advanced Cancer. J. Geriatr. Oncol. 2020;11:937–943. doi: 10.1016/j.jgo.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 174.Loh K.P., Mohile S.G., Lund J.L., Epstein R., Lei L., Culakova E., McHugh C., Wells M., Gilmore N., Mohamed M.R., et al. Beliefs About Advanced Cancer Curability in Older Patients, Their Caregivers, and Oncologists. Oncologist. 2019;24:e292–e302. doi: 10.1634/theoncologist.2018-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loh K.P., Soto-Perez-de-Celis E., Duberstein P.R., Culakova E., Epstein R.M., Xu H., Kadambi S., Flannery M., Magnuson A., McHugh C., et al. Patient and Caregiver Agreement on Prognosis Estimates for Older Adults with Advanced Cancer. Cancer. 2020;127:149–159. doi: 10.1002/cncr.33259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nipp R.D., Greer J.A., El-Jawahri A., Moran S.M., Traeger L., Jacobs J.M., Jacobsen J.C., Gallagher E.R., Park E.R., Ryan D.P., et al. Coping and Prognostic Awareness in Patients with Advanced Cancer. J. Clin. Oncol. 2017;35:2551–2557. doi: 10.1200/JCO.2016.71.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nipp R.D., El-Jawahri A., Fishbein J.N., Eusebio J., Stagl J.M., Gallagher E.R., Park E.R., Jackson V.A., Pirl W.F., Greer J.A., et al. The Relationship between Coping Strategies, Quality of Life, and Mood in Patients with Incurable Cancer. Cancer. 2016;122:2110–2116. doi: 10.1002/cncr.30025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chouliara Z., Kearney N., Stott D., Molassiotis A., Miller M. Perceptions of Older People with Cancer of Information, Decision Making and Treatment: A Systematic Review of Selected Literature. Ann. Oncol. 2004;15:1596–1602. doi: 10.1093/annonc/mdh423. [DOI] [PubMed] [Google Scholar]
- 179.Fried T.R., Tinetti M.E., Iannone L., O’Leary J.R., Towle V., Van Ness P.H. Health Outcome Prioritization as a Tool for Decision Making among Older Persons with Multiple Chronic Conditions. Arch. Intern. Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hoerger M., Greer J.A., Jackson V.A., Park E.R., Pirl W.F., El-Jawahri A., Gallagher E.R., Hagan T., Jacobsen J., Perry L.M., et al. Defining the Elements of Early Palliative Care That Are Associated with Patient-Reported Outcomes and the Delivery of End-of-Life Care. J. Clin. Oncol. 2018;36:1096–1102. doi: 10.1200/JCO.2017.75.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cohen M.G., Althouse A.D., Arnold R.M., White D., Chu E., Rosenzweig M., Smith K.J., Schenker Y. Primary Palliative Care Improves Uptake of Advance Care Planning Among Patients with Advanced Cancers. J. Natl. Compr. Cancer Netw. 2023;21:383–390. doi: 10.6004/jnccn.2023.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Koffler S., Mintzker Y., Shai A. Association between Palliative Care and the Rate of Advanced Care Planning: A Systematic Review. Palliat. Support. Care. 2020;18:589–601. doi: 10.1017/S1478951519001068. [DOI] [PubMed] [Google Scholar]
- 183.Temel J.S., Greer J.A., Admane S., Gallagher E.R., Jackson V.A., Lynch T.J., Lennes I.T., Dahlin C.M., Pirl W.F. Longitudinal Perceptions of Prognosis and Goals of Therapy in Patients with Metastatic Non-Small-Cell Lung Cancer: Results of a Randomized Study of Early Palliative Care. J. Clin. Oncol. 2011;29:2319–2326. doi: 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 184.Wildiers H., Heeren P., Puts M., Topinkova E., Janssen-Heijnen M.L.G., Extermann M., Falandry C., Artz A., Brain E., Colloca G., et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients with Cancer. J. Clin. Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stuck A.E., Siu A.L., Wieland G.D., Adams J., Rubenstein L.Z. Comprehensive Geriatric Assessment: A Meta-Analysis of Controlled Trials. Lancet. 1993;342:1032–1036. doi: 10.1016/0140-6736(93)92884-V. [DOI] [PubMed] [Google Scholar]
- 186.Devons C.A.J. Comprehensive Geriatric Assessment: Making the Most of the Aging Years. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:19–24. doi: 10.1097/00075197-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 187.Mohile S.G., Epstein R.M., Hurria A., Heckler C.E., Canin B., Culakova E., Duberstein P., Gilmore N., Xu H., Plumb S., et al. Communication with Older Patients with Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020;6:196–204. doi: 10.1001/jamaoncol.2019.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Soda S., Mirokuji K., Saito R., Takamura K., Morita R., Furuya N., Kuyama S., Shiono A., Arai D., Hosokawa S., et al. Satisfaction in Older Patients by Geriatric Assessment Using a Novel Tablet-Based Questionnaire System: A Cluster-Randomized, Phase III Trial of Patients with Non–Small-Cell Lung Cancer (ENSURE-GA Study; NEJ041/CS-Lung001) J. Clin. Oncol. 2023;41:12041. doi: 10.1200/JCO.2023.41.16_suppl.12041. [DOI] [Google Scholar]
- 189.Furuya N., Kanaji N., Yokoo K., Yanagawa T., Yamamoto H., Takayama K., Kawase S., Matsunaga K., Takeda K., Nagashima H., et al. Geriatric Assessment in Older Patients with Non-Small Cell Lung Cancer: Insights from a Cluster-Randomized, Phase III Trial—ENSURE-GA Study (NEJ041/CS-Lung001) J. Clin. Oncol. 2024;42:1502. doi: 10.1200/JCO.2024.42.16_suppl.1502. [DOI] [Google Scholar]
- 190.DuMontier C., Uno H., Hshieh T., Zhou G., Chen R., Magnavita E.S., Mozessohn L., Javedan H., Stone R.M., Soiffer R.J., et al. Randomized Controlled Trial of Geriatric Consultation versus Standard Care in Older Adults with Hematologic Malignancies. Haematologica. 2022;107:1172–1180. doi: 10.3324/haematol.2021.278802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jeppesen S.S., Matzen L.-E., Brink C., Bliucukiene R., Kasch S., Schytte T., Kristiansen C., Hansen O. Impact of Comprehensive Geriatric Assessment on Quality of Life, Overall Survival, and Unplanned Admission in Patients with Non-Small Cell Lung Cancer Treated with Stereotactic Body Radiotherapy. J. Geriatr. Oncol. 2018;9:575–582. doi: 10.1016/j.jgo.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 192.Eriksen G.F., Benth J.Š., Grønberg B.H., Rostoft S., Kirkhus L., Kirkevold Ø., Oldervoll L.M., Bye A., Hjelstuen A., Slaaen M. Geriatric Impairments Are Associated with Reduced Quality of Life and Physical Function in Older Patients with Cancer Receiving Radiotherapy—A Prospective Observational Study. J. Geriatr. Oncol. 2023;14:101379. doi: 10.1016/j.jgo.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 193.Anwar M.R., Yeretzian S.T., Ayala A.P., Matosyan E., Breunis H., Bote K., Puts M., Habib M.H., Li Q., Sahakyan Y., et al. Effectiveness of Geriatric Assessment and Management in Older Cancer Patients: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2023;115:1483–1496. doi: 10.1093/jnci/djad200. [DOI] [PubMed] [Google Scholar]
- 194.Chuang M.-H., Chen J.-Y., Tsai W.-W., Lee C.-W., Lee M.-C., Tseng W.-H., Hung K.-C. Impact of Comprehensive Geriatric Assessment on the Risk of Adverse Events in the Older Patients Receiving Anti-Cancer Therapy: A Systematic Review and Meta-Analysis. Age Ageing. 2022;51:afac145. doi: 10.1093/ageing/afac145. [DOI] [PubMed] [Google Scholar]
- 195.Hurria A., Togawa K., Mohile S.G., Owusu C., Klepin H.D., Gross C.P., Lichtman S.M., Gajra A., Bhatia S., Katheria V., et al. Predicting Chemotherapy Toxicity in Older Adults with Cancer: A Prospective Multicenter Study. J. Clin. Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Extermann M., Boler I., Reich R.R., Lyman G.H., Brown R.H., DeFelice J., Levine R.M., Lubiner E.T., Reyes P., Schreiber F.J., et al. Predicting the Risk of Chemotherapy Toxicity in Older Patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) Score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 197.Loh K.P., Mohile S.G. Geriatric Assessment and Management: Is Decreasing Treatment Toxicity Good Enough? J. Natl. Cancer Inst. 2023;115:1445–1447. doi: 10.1093/jnci/djad207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wildes T.M., Ruwe A.P., Fournier C., Gao F., Carson K.R., Piccirillo J.F., Tan B., Colditz G.A. Geriatric Assessment Is Associated with Completion of Chemotherapy, Toxicity, and Survival in Older Adults with Cancer. J. Geriatr. Oncol. 2013;4:227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jonna S., Chiang L., Liu J., Carroll M.B., Flood K., Wildes T.M. Geriatric Assessment Factors Are Associated with Mortality after Hospitalization in Older Adults with Cancer. Support. Care Cancer. 2016;24:4807–4813. doi: 10.1007/s00520-016-3334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Riely G.J., Wood D.E., Ettinger D.S., Aisner D.L., Akerley W., Bauman J.R., Bharat A., Bruno D.S., Chang J.Y., Chirieac L.R., et al. Non–Small Cell Lung Cancer, Version 4.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024;22:249–274. doi: 10.6004/jnccn.2204.0023. [DOI] [PubMed] [Google Scholar]
- 201.Chaft J.E., Rimner A., Weder W., Azzoli C.G., Kris M.G., Cascone T. Evolution of Systemic Therapy for Stages I–III Non-Metastatic Non-Small-Cell Lung Cancer. Nat. Rev. Clin. Oncol. 2021;18:547–557. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sterlacci W., Stockinger R., Schmid T., Bodner J., Hilbe W., Waldthaler C., Oberaigner W., Tzankov A., Fiegl M. The Elderly Patient with Surgically Resected Non-Small Cell Lung Cancer—A Distinct Situation? Exp. Gerontol. 2012;47:237–242. doi: 10.1016/j.exger.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 203.Rivera C., Falcoz P.-E., Bernard A., Thomas P.A., Dahan M. Surgical Management and Outcomes of Elderly Patients with Early Stage Non-Small Cell Lung Cancer: A Nested Case-Control Study. Chest. 2011;140:874–880. doi: 10.1378/chest.10-2841. [DOI] [PubMed] [Google Scholar]
- 204.Gray S.W., Landrum M.B., Lamont E.B., McNeil B.J., Jaklitsch M.T., Keating N.L. Improved Outcomes Associated with Higher Surgery Rates for Older Patients with Early Stage Nonsmall Cell Lung Cancer. Cancer. 2012;118:1404–1411. doi: 10.1002/cncr.26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Castillo M.D., Heerdt P.M. Pulmonary Resection in the Elderly. Curr. Opin. Anaesthesiol. 2007;20:4–9. doi: 10.1097/ACO.0b013e32801271fa. [DOI] [PubMed] [Google Scholar]
- 206.Baldvinsson K., Oskarsdottir G.N., Orrason A.W., Halldorsson H., Thorsteinsson H., Sigurdsson M.I., Jonsson S., Gudbjartsson T. Resection Rate and Operability of Elderly Patients with Non-Small Cell Lung Cancer: Nationwide Study from 1991 to 2014. Interact. Cardiovasc. Thorac. Surg. 2017;24:733–739. doi: 10.1093/icvts/ivw415. [DOI] [PubMed] [Google Scholar]
- 207.Blanco R., Maestu I., de la Torre M.G., Cassinello A., Nuñez I. A Review of the Management of Elderly Patients with Non-Small-Cell Lung Cancer. Ann. Oncol. 2015;26:451–463. doi: 10.1093/annonc/mdu268. [DOI] [PubMed] [Google Scholar]
- 208.PACE Participants. Audisio R.A., Pope D., Ramesh H.S.J., Gennari R., van Leeuwen B.L., West C., Corsini G., Maffezzini M., Hoekstra H.J., et al. Shall We Operate? Preoperative Assessment in Elderly Cancer Patients (PACE) Can Help: A SIOG Surgical Task Force Prospective Study. Crit. Rev. Oncol. Hematol. 2008;65:156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 209.Kim M.-H., Kim S.H., Lee M.K., Eom J.S. Recent Advances in Adjuvant Therapy for Non–Small-Cell Lung Cancer. Tuberc. Respir. Dis. 2024;87:31–39. doi: 10.4046/trd.2023.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Spicer J.D., Cascone T., Wynes M.W., Ahn M.J., Dacic S., Felip E., Forde P.M., Higgins K.A., Kris M.G., Mitsudomi T., et al. Neoadjuvant and Adjuvant Treatments for Early Stage Resectable NSCLC: Consensus Recommendations from the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2024;19:1373–1414. doi: 10.1016/j.jtho.2024.06.010. [DOI] [PubMed] [Google Scholar]
- 211.Wu Y.-L., Tsuboi M., He J., John T., Grohe C., Majem M., Goldman J.W., Laktionov K., Kim S.-W., Kato T., et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 212.O’Brien M., Paz-Ares L., Marreaud S., Dafni U., Oselin K., Havel L., Esteban E., Isla D., Martinez-Marti A., Faehling M., et al. Pembrolizumab versus Placebo as Adjuvant Therapy for Completely Resected Stage IB–IIIA Non-Small-Cell Lung Cancer (PEARLS/KEYNOTE-091): An Interim Analysis of a Randomised, Triple-Blind, Phase 3 Trial. Lancet Oncol. 2022;23:1274–1286. doi: 10.1016/S1470-2045(22)00518-6. [DOI] [PubMed] [Google Scholar]
- 213.Presley C.J., Gomes F., Burd C.E., Kanesvaran R., Wong M.L. Immunotherapy in Older Adults with Cancer. J. Clin. Oncol. 2021;39:2115–2127. doi: 10.1200/JCO.21.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Daly M.E., Singh N., Ismaila N., Antonoff M.B., Arenberg D.A., Bradley J., David E., Detterbeck F., Früh M., Gubens M.A., et al. Management of Stage III Non–Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022;40:1356–1384. doi: 10.1200/JCO.21.02528. [DOI] [PubMed] [Google Scholar]
- 215.Bonanno L., Attili I., Pavan A., Sepulcri M., Pasello G., Rea F., Guarneri V., Conte P. Treatment Strategies for Locally Advanced Non-Small Cell Lung Cancer in Elderly Patients: Translating Scientific Evidence into Clinical Practice. Crit. Rev. Oncol. Hematol. 2021;163:103378. doi: 10.1016/j.critrevonc.2021.103378. [DOI] [PubMed] [Google Scholar]
- 216.Owen D.H., Ismaila N., Freeman-Daily J., Roof L., Singh N., Velazquez A.I., Leighl N.B. Therapy for Stage IV Non–Small Cell Lung Cancer with Driver Alterations: ASCO Living Guideline, Version 2024.1. J. Clin. Oncol. 2024;42:e44–e59. doi: 10.1200/JCO.24.00762. [DOI] [PubMed] [Google Scholar]
- 217.Jaiyesimi I.A., Leighl N.B., Ismaila N., Alluri K., Florez N., Gadgeel S., Masters G., Schenk E.L., Schneider B.J., Sequist L., et al. Therapy for Stage IV Non-Small Cell Lung Cancer without Driver Alterations: ASCO Living Guideline, Version 2023.3. J. Clin. Oncol. 2024;42:e23–e43. doi: 10.1200/JCO.23.02746. [DOI] [PubMed] [Google Scholar]
- 218.Decoster L., Schallier D. Treatment of Older Patients with Advanced Non-Small Cell Lung Cancer: A Challenge. J. Geriatr. Oncol. 2019;10:528–533. doi: 10.1016/j.jgo.2018.09.008. [DOI] [PubMed] [Google Scholar]