Simple Summary
As one of the most common genetic alterations, KRAS mutations play a pivotal role in the growth of lung cancer, particularly non-small cell lung cancer. After 40 years of research, patients with KRAS G12C-mutated lung cancer can now benefit from novel KRAS-targeting drugs. However, the efficacy is far from optimal, but newer experimental drugs showed promising results. It is also likely that other KRAS mutations will likely be druggable in the future.
Keywords: KRAS, G12C, lung cancer, non-small cell lung cancer, targeted therapy
Abstract
KRAS mutation is one of the most common oncogenic drivers in non-small cell lung cancer. Since its discovery about four decades ago, drug development targeting KRAS has been met with countless failures. Recently, KRAS G12C, a subvariant of KRAS, became the first druggable KRAS mutation. The efficacy of the first-generation KRAS inhibitor is modest, but with scientific advancement, KRAS G12C inhibitors with higher potency are on the horizon. Additionally, novel therapeutic approaches targeting other KRAS subvariants are also being explored in clinical trials with encouraging early data. We will review the clinical advances and challenges for patients with KRAS-mutated non-small cell lung cancer, with a focus on small molecule inhibitors.
1. Introduction
As one of the most common driver oncogenes in cancers, including non-small cell lung cancers (NSCLCs) [1], KRAS mutation has long been considered as undruggable [2]. Numerous attempts have been made to target it directly or through up- or downstream pathways, but success has not been widely seen. However, scientific and technological advances have moved the needle in recent years. A subvariant, G12C, became the first targetable KRAS mutation with approved drugs. Nevertheless, the efficacy of first-generation KRAS G12C inhibitors is relatively modest compared to the deep and durable response seen in other targeted therapies [3,4] inhibiting EGFR, ALK, ROS-1, etc. [5,6]. In this review article, we discuss the advances and challenges in targeting KRAS mutations in NSCLC with a focus on strategies that are currently in clinical development. Clinical trials were identified on clinicaltrials.gov using the following search criteria: (1) condition/disease: lung cancer; (2) other terms: KRAS; (3) study status: recruiting or active but not recruiting; (4) age: adult (18–64 years old) or older adult (65+); (5) study type: interventional.
2. KRAS Mutation in NSCLC
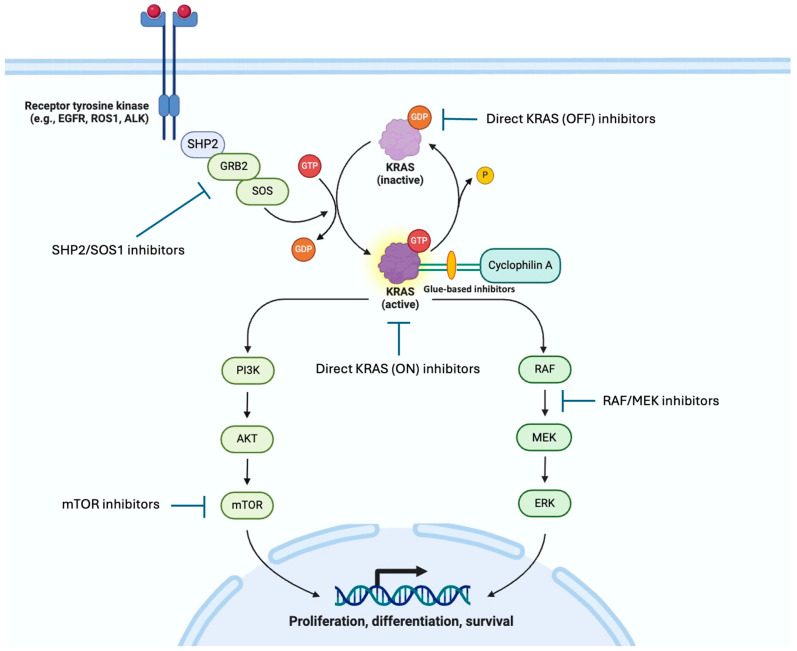
KRAS is the most common oncogenic driver in lung adenocarcinoma, accounting for about a quarter of patients [7]. It belongs to a large member of plasma-bound protein known as GTPase. When it is bound to GTP, it is in the active state (ON). Then, GTP is hydrolyzed to GDP, an inactive state (OFF). KRAS cycles through ON and OFF states, controlling the downstream effectors, including the MAPK, PI3K, and RALGDS pathways and many others, promoting the growth and survival of cancer cells [8]. The mutated KRAS oncoprotein can keep KRAS in its GTP-bound ON state, driving cancer growth (Figure 1) [9]. Due to a lack of targeted therapy options, KRAS mutation is associated with worse survival compared to other driver oncogenes.
Figure 1.
KRAS pathway and targets for treatment.
Unlike driver oncogenes like EGFR, ALK, and ROS-1, KRAS-driven NSCLC is more likely to be associated with smoking and sensitivity to immune checkpoint inhibitors [10,11,12,13]. It is also accompanied by co-occurring mutations, which are associated with differing sensitivity not only to immune checkpoint inhibitors and chemotherapy, but also to novel KRAS inhibitors. KRAS-mutated NSCLC with STK11 and/or KEAP1 mutations have been reported to have poorer prognosis in various reports [14]. This may create opportunities for KRAS inhibitors to improve outcomes for those subsets of patients.
KRAS G12C is the most common subvariant in NSCLC, accounting for 30–40% of the KRAS-mutated cases, followed by G12V, G12D, G12A, and G13X [15,16]. In the era of chemotherapy, the KRAS subvariant appears to be similar in terms of prognosis [17]. However, the biological implications of these subvariants may vary. For example, KRAS G12A is preferentially associated with the activated PI3K and MAPK pathways, while G12C/V had activated Ral signaling and decreased Akt activation [18]. KRAS G12D may be associated with enhanced glutathione-mediated detoxification [19].
3. Previous Attempts in Targeting KRAS in NSCLC
Before the advent of direct KRAS inhibitors, various strategies were explored to target KRAS-driven cancers. RAS protein needs to be lipophilic to be attached to the cellular membrane to function. Therefore, farnesyltransferase, initially considered as the key enzyme in this process, was explored as a target but was unsuccessful [20,21,22]. It was discovered that geranylgeranyl transferase I alternatively allows the membrane location and signal transduction of KRAS. The results of targeting the downstream pathway of KRAS including RAF, MEK, PI3K, and mTOR were also disappointing [9]. SHP2 and SOS1 play a vital role in upstream RTK-mediated KRAS activation [23,24,25,26]. Therefore, the inhibitors of these two molecules were also explored in clinical trials. However, SHP2 agents are now being discontinued by several major pharmaceutical companies, possibly due to unfavorable toxicity profiles [27].
4. Direct KRAS G12c Targeting in NSCLC
4.1. Approved KRAS G12C Inhibitors
Being the most common KRAS subvariant in NSCLC, KRAS G12C became the first targetable mutation with two US FDA-approved drugs—sotorasib and adagrasib. KRAS G12C cycles through active and inactive stages. In 2013, a new pocket beneath the effector binding switch-II region was reported [28]. A covalent inhibitor binding to this pocket locks the KRAS G12C in the inactive GDP-bound state, thus disrupting downstream Raf signaling. Thus, these first-generation inhibitors are considered as KRAS G12C (OFF) inhibitors, because only the OFF (inactive) state is targeted.
Sotorasib is the first-in-class KRAS G12C-specific covalent (OFF) inhibitor that received regulatory approval [29]. CodeBreak 100 is a single-arm, phase II trial evaluating sotorasib in 126 patients with NSCLC [30]. Eligible patients must have received prior platinum-based chemotherapy and/or anti-PD(L)1 immunotherapy. The objective response rate (ORR) was 37.1% with a median progression-free survival (PFS) of 6.8 months. The median overall survival (OS) was 12.5 months. The treatment discontinuation rate due to adverse events was 7%. FDA made the historic regulatory decision by granting accelerated approval in May 2021, eight years after the initial report of the novel binding pocket identified in 2013. To confirm sotorasib’s efficacy, it was compared head-to-head with docetaxel. The study confirmed sotorasib’s superiority over docetaxel in its primary endpoint, with a statistically significant improvement of median PFS from 4.5 to 5.6 months (HR 0.66). Sotorasib also improved ORR with fewer serious treatment-related adverse events (TRAEs). There was no difference in OS, but the trial was not powered to test OS difference.
Adagrasib is another first-generation KRAS G12C-specific (OFF) inhibitor that received the FDA’s accelerated approval [31]. KRYSTAL-1 enrolled 116 previously treated patients with an ORR of 42.9% and median PFS at 6.5 months. Most recently, its confirmatory KRYSTAL-12 trial, a head-to-head comparison against docetaxel, was read out [32]. The primary endpoint, PFS, was met with an improvement from 3.8 to 5.5 months (HR 0.58).
Fulzerasib (GFH925/IBI351) is a KRAS G12C (OFF) inhibitor that has received approval in China. The phase 2 single-arm trial of previously treated patients showed a confirmed ORR of 49% and a median PFS of 9.7 months [33].
However, the incremental efficacy over docetaxel appears to be modest and is overshadowed by the durable response when targeting other oncogenic drivers. The FDA called an oncologic drugs advisor committee (ODAC) meeting to discuss CodeBreak 200 data [34]. The FDA held the stance that the small sample size, marginal PFS difference, and concerns about trial conduct integrity may not be interpretable. The ODAC upheld the FDA’s stance overwhelmingly by voting 10-2. There are numerous novel compounds that are in active clinical investigation (Table 1).
Table 1.
Ongoing clinical trials of KRAS G12C small molecule inhibitor monotherapies.
| Compound | Mechanism of Action | Clinical Trial Indication | Phase of Study |
|---|---|---|---|
| Sotorasib (AMG510) |
KRAS G12C OFF inhibitor |
|
3 2 2 2 2 2 2 2 2 |
| Adagrasib (MRTX849) |
KRAS G12C OFF inhibitor |
|
3 2 2 1/2 |
| Garsorasib (D-1553) |
KRAS G12C OFF inhibitor |
|
3 1/2 |
| Divarasib (GDC-6036) |
KRAS G12C OFF inhibitor |
|
3 1 |
| Opnurasib (JDQ-443) |
KRAS G12C OFF inhibitor |
|
3 1/2 |
| JDQ443 | KRAS G12C OFF inhibitor |
|
3 |
| Glecirasib (JAB-21822) |
KRAS G12C OFF inhibitor |
|
2 1/2 |
| HBI-2438 | KRAS G12C OFF |
|
1 |
| Olomorasib (LY3537982) |
KRAS G12C OFF |
|
1/2 |
| FMC-376 | KRAS G12C ON and OFF inhibitor |
|
1/2 |
| RMC-6291 | Tri-complex (glue-based) mechanism of KRAS G12C targeting |
|
1 |
4.2. Resistance to Direct KRAS G12C (OFF) Inhibitors
Similarly to other targeted therapies, resistance inevitably develops to KRAS G12C (OFF) inhibitors. Intrinsic resistance mechanisms may involve co-occurring mutations, including KEAP1 [4]. The acquired resistance mechanism largely remains unclear. However, preliminary clinical results suggest that putative resistance mechanisms may be diverse and heterogeneous [35]. Other acquired KRAS subvariants, including KRAS G12X, G13D, Q61H, etc., were reported [36]. Putative bypass resistance mechanisms included MET amplification, a mutation in NRAS, BRAF, and oncogenic fusions, including ALK and RET. Histological transformation to squamous-cell carcinoma was also reported. These putative resistance alterations also co-exist in 41% of the patients, making it challenging to develop a one-size-fits-all strategy to target. Preclinical studies also showed that the newly synthesized KRAS G12C protein upon treatment maintains its ON state, leading to resistance to KRAS G12C OFF inhibitors [37]. Recent studies have demonstrated that Hippo (YAP/TAZ) signaling is a significant contributor to resistance against KRAS G12C inhibitors in vitro and in vivo [38,39]. Combining therapies targeting this pathway could enhance the efficacy of G12C inhibitors in cancer patients.
4.3. Frontline Efforts
The less-than-expected efficiency of monotherapy does not appear to compare favorably to frontline options—immunotherapy and/or platinum doublet [40,41,42]. To move the currently approved KRAS G12C inhibitors to the frontline, a combination would be the natural strategy (Table 2).
Table 2.
Ongoing clinical trials of combination therapies with KRAS inhibitors.
| Combination Strategy | Compound | Clinical Trial Indication |
|---|---|---|
| Combination with Chemo, RT and/or ICI | Adagrasib |
|
| Olomorasib (LY3537982) |
|
|
| Sotorasib |
|
|
| BBO-8520 |
|
|
| MK-1084 |
|
|
| Fulzerasib(IBI351) |
|
|
| Garsorasib (D-1553)- |
|
|
| GFH925 |
|
|
| Divarasib (GDC-6036) |
|
|
| RMC-6291 or RMC-6236 |
|
|
| Combination with SHP2/SOS1 | Sotorasib |
|
| Adagrasib |
|
|
| Glecirasib and JAB 3312 |
|
|
| Other combinations | Sotorasib with Ras-Raf-Mek-ERK inhibitor |
|
| Sotorasib with proteosome inhibitor |
|
|
| Sotorasib in combination with IL-8 receptor inhibitor |
|
|
| Adagrasib with pan-KRAS inhibitor |
|
|
| Adagrasib with mTOR inhibitor |
|
|
| Adagrasib with PARP inhibitor |
|
|
| Adagrasib with RAF/MEK clamp |
|
|
| KRAS:KRAS G12C ON inhibitor |
|
Sotorasib combination with immune checkpoint inhibitors (ICIs, pembrolizumab, or atezolizumab) was examined in the CodeBreak 100/101 trial. The concurrent administration of sotorasib and ICI was associated with grade 3 or higher TRAE in 60–79% of the patients, driven by hepatotoxicity (~50%). Although sotorasib lead-in is associated with lower TRAE, the rate remains to be high at 30–53% [43]. This is consistent with a retrospective report that sotorasib after ICI is associated with a higher risk of immune-related toxicity, particularly when ICI exposure was 12 weeks prior to sotorasib [44]. Sotorasib plus platinum doublet chemotherapy was examined in the CodeBreak 101 trial, with an ORR of 65% and median PFS at 10.8 months, in the first-line setting [43]. Currently, CodeBreak 202 is a phase 3 trial to directly combine sotorasib vs. pembrolizumab in combination with platinum doublet chemotherapy in PD-L1 negative patients [45,46].
Adagrasib appears to have more favorable toxicity data when in combination with ICIs. KRYSTAL-7 is a phase 2 clinical trial which showed that treatment-related hepatic events occurred in less than 10% of the patients [47]. The trial also reported an ORR of 49% and 63% among all PD-L1 subgroups and PD-L1 TPS ≥ 50%, respectively. The phase 3 portion of a head-to-head comparison of adagrasib + pembrolizumab vs. pembrolizumab monotherapy is ongoing in patients with PD-L1 TPS ≥ 50%. Of note, the dosage of adagrasib explored in this combination was 400 mg twice daily, lower than the approved monotherapy dosage of 600 mg twice daily.
Fulzerasib and cetuximab combination was explored in previously untreated patients, showing an encouraging ORR of 82%. In patients with low or negative PD-L1 expression, the response was also above 60%, which is not dependent on baseline EGFR expression [48].
4.4. Emerging KRAS G12C Inhibitors
More potent KRAS G12C inhibitors are urgently needed for better efficacy. Divarasib is a novel KRAS G12C (OFF) inhibitor. A phase 1 study showed confirmed ORR of 53%, comparing favorably to currently approved inhibitors [49]. Olomorasib is another promising KRAS G12C (OFF) inhibitor with an ORR of 39% in patients who have experienced prior KRAS G12C inhibitors [50]. Its combination with pembrolizumab also showed an ORR of 77% and 40% in first-line and previously treated cohorts, respectively. Grade 3 or higher TRAEs were reported in 27% of the patients. The permanent discontinuation of olomorasib and pembrolizumab was reported in 3% and 11% of the patients, respectively. Its first-line phase 3 study—SUNRAY-01—is ongoing to directly compare the addition of olomorasib to the standard of care vs. standard of care alone [51].
KRAS G12C (OFF) inhibitors are inherently limited in that only one conformation is targeted; thus, (ON) inhibitors would be ideal to overcome this shortcoming. RMC-6291 is unique in its novel tri-complex mechanism of KRAS G12C targeting. Instead of binding to a pocket opening at OFF conformation, it serves as a molecular glue with cyclophilin A to bind the KRAS protein to block downstream RAS effector signaling. Therefore, it can inhibit both ON and OFF conformation. In a phase 1 study, 57% of the NSCLC patients had a response [52]. The most common Grade 3 TREAE was QTc prolongation, but all patients were able to stay on treatment after dose reduction.
BBO-8520 and FMC-376 are direct KRAS G12C (ON) and (OFF) inhibitors that have the potential to overcome the inherent shortcoming of OFF state-only targeting. Preclinical data showed that BBO-8520 and FMC-376 are more potent than first-generation (OFF) inhibitors in vitro and in vivo [53,54]. Phase 1 clinical trials are ongoing (NCT06244771, NCT06343402).
5. Beyond KRAS G12c Only
Although G12C is the most common KRAS subvariant, the majority (~60%) of KRAS mutations are non-G12C, including G12D/V/R, G13X, etc. These subvariants can also emerge as a secondary resistance mechanism. Thus, to fully drug KRAS mutations, strategies are urgently needed to expand to non-G12C [55]. Table 3, Table 4 and Table 5 summarize the current ongoing clinical trials investigating KRAS inhibitors beyond the G12C subvariant.
Table 3.
Ongoing clinical trials of other KRAS single subvariant small molecule inhibitor monotherapies.
| Compound | Mechanism of Action | Clinical Trial Indication | Phase of Study |
|---|---|---|---|
| MRTX1133 | KRAS G12D inhibitor |
|
1/2 1/2 |
| TSN1611 | KRAS G12D inhibitor |
|
1/2 |
| RMC-9805 (RM-036) |
KRAS G12D inhibitor |
|
1 |
| ASP3082 | KRAS G12D inhibitor |
|
1 |
| QLC1101 | KRAS G12D inhibitor |
|
1 |
| INCB161734 | KRAS G12D inhibitor |
|
1 |
Table 4.
Ongoing clinical trials of pan-KRAS direct small molecule inhibitor monotherapies.
| Compound | Mechanism of Action | Clinical Trial Indication | Phase of Study |
|---|---|---|---|
| RMC-6236 | Pan-RAS inhibitor |
|
1 |
| PF-07934040 | Pan-KRAS inhibitor |
|
1 |
| YL-17231 | Pan-KRAS inhibitor |
|
1 |
Table 5.
Other KRAS-targeting strategies.
| Compound | Mechanism of Action | Indication |
|---|---|---|
| KRAS peptide vaccine | Vaccine |
|
| Targovax TG-01/Stimulon QS-21 | Vaccine |
|
| KRAS TCR-Transduced PBL and GRT-C903/GRT-R904 | KRAS TCR-transduced PBL in combination with KRAS G12D and G12V vaccine |
|
| FH-A11KRASG12V-TCR | Autologous transgenic T cells expressing high-affinity KRASG12V mutation-specific T cell receptors |
|
| AFNT-211 | Autologous CD8+ and CD4+ engineered T cell receptor T Cell |
|
| NT-112 | KRAS G12D autologous T cell therapy |
|
ASP3082 is a protein degrader, specifically targeting KRAS G12D. Early phase I pan-tumor data showed an ORR of 33.3% in 12 patients at 300 mg, including prostate cancer, non-small cell lung cancer, and colorectal cancer [56].
Utilizing the same tri-complex mechanism described above, RMC-6236 is a RASMULTI(ON) inhibitor that has activity across all KRAS mutations. In a phase 1 study of KRAS G12X, RMC-6236 resulted in an ORR of 38% in patients with KRAS G12X in previously treated patients, with activity seen across various subvariants [57]. Only 1% of the patients discontinued treatment due to TRAE in the full NSCLC and pancreatic cohorts. RMC-6236 is also being hypothesized to overcome resistance to a single subvariant-directed inhibitor in a phase 1 trial (NCT06040541). Larger phase 3 data of RMC-6236 is eagerly awaited.
There are also other approaches targeting non-G12C KRAS subvariants, including RAS-RAF clamp (NCT05786924), RAF/MEK clamp [58], protein degradation [59], KRAS-SOS1 inhibition [25].
6. Beyond KRAS Small Molecule Inhibitors
In addition to small molecule inhibitors, other therapeutic strategies are also being explored. Notably, immunotherapy including vaccine and cellular therapy trials are ongoing (NCT05254184, NCT06253520, NCT06043713, NCT06105021). The efficacy of those strategies is eagerly awaited.
RAS plays a role in metabolic pathways, such as tumor metabolism, glutaminolysis, redox homeostasis, lipid metabolism, and nutrient scavenging. Targeting oncogenic RAS-related metabolism is a potential area for further investigation [60]. This presents a promising avenue for future research, potentially leading to novel translational therapies for KRAS-mutant NSCLC.
7. Conclusions and Future Directions
Forty years since the initial discovery of KRAS mutation, numerous publications have highlighted the pivotal role of KRAS mutation in various cancers, including NSCLC. Today, we are finally in an era when a subset of KRAS can be targeted. This success is just the beginning, with more exciting progress and opportunities awaiting ahead.
Academic and industry partners will continue to take on the challenges of (1) making all KRAS subvariants targetable with balanced tolerability and efficacy; (2) frontline KRAS therapeutics strategy; (3) intrinsic and acquired resistance mechanisms; (4) tolerable and rational combination approach.
Author Contributions
Writing—original draft preparation, L.D. and S.E.D.; writing—review and editing, L.D. and S.E.D.; supervision, L.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
L.D. declares the following conflicts of interest: Honoraria from MJH Life Sciences, Precisca; Consulting fees from Bristol-Myers Squibb, Regeneron; Travel from Merck, MJH Life Sciences; Institutional research funding from Bridgebio Oncology Therapeutics.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore A.R., Rosenberg S.C., McCormick F., Malek S. RAS-Targeted Therapies: Is the Undruggable Drugged? Nat. Rev. Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Langen A.J., Johnson M.L., Mazieres J., Dingemans A.-M.C., Mountzios G., Pless M., Wolf J., Schuler M., Lena H., Skoulidis F., et al. Sotorasib versus Docetaxel for Previously Treated Non-Small-Cell Lung Cancer with KRASG12C Mutation: A Randomised, Open-Label, Phase 3 Trial. Lancet. 2023;401:733–746. doi: 10.1016/S0140-6736(23)00221-0. [DOI] [PubMed] [Google Scholar]
- 4.Jänne P.A., Riely G.J., Gadgeel S.M., Heist R.S., Ou S.-H.I., Pacheco J.M., Johnson M.L., Sabari J.K., Leventakos K., Yau E., et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 5.Solomon B.J., Liu G., Felip E., Mok T.S.K., Soo R.A., Mazieres J., Shaw A.T., de Marinis F., Goto Y., Wu Y.-L., et al. Lorlatinib Versus Crizotinib in Patients With Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024;42:JCO2400581. doi: 10.1200/JCO.24.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drilon A., Camidge D.R., Lin J.J., Kim S.-W., Solomon B.J., Dziadziuszko R., Besse B., Goto K., de Langen A.J., Wolf J., et al. Repotrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024;390:118–131. doi: 10.1056/NEJMoa2302299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kris M.G., Johnson B.E., Berry L.D., Kwiatkowski D.J., Iafrate A.J., Wistuba I.I., Varella-Garcia M., Franklin W.A., Aronson S.L., Su P.-F., et al. Using Multiplexed Assays of Oncogenic Drivers in Lung Cancers to Select Targeted Drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L., Guo Z., Wang F., Fu L. KRAS Mutation: From Undruggable to Druggable in Cancer. Signal Transduct. Target. Ther. 2021;6:386. doi: 10.1038/s41392-021-00780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrer I., Zugazagoitia J., Herbertz S., John W., Paz-Ares L., Schmid-Bindert G. KRAS-Mutant Non-Small Cell Lung Cancer: From Biology to Therapy. Lung Cancer. 2018;124:53–64. doi: 10.1016/j.lungcan.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Dogan S., Shen R., Ang D.C., Johnson M.L., D’Angelo S.P., Paik P.K., Brzostowski E.B., Riely G.J., Kris M.G., Zakowski M.F., et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3,026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riely G.J., Kris M.G., Rosenbaum D., Marks J., Li A., Chitale D.A., Nafa K., Riedel E.R., Hsu M., Pao W., et al. Frequency and Distinctive Spectrum of KRAS Mutations in Never Smokers with Lung Adenocarcinoma. Clin. Cancer Res. 2008;14:5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodenhuis S., van de Wetering M.L., Mooi W.J., Evers S.G., van Zandwijk N., Bos J.L. Mutational Activation of the K-Ras Oncogene. A Possible Pathogenetic Factor in Adenocarcinoma of the Lung. N. Engl. J. Med. 1987;317:929–935. doi: 10.1056/NEJM198710083171504. [DOI] [PubMed] [Google Scholar]
- 13.Mazieres J., Drilon A., Lusque A., Mhanna L., Cortot A.B., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skoulidis F., Heymach J.V. Co-Occurring Genomic Alterations in Non-Small-Cell Lung Cancer Biology and Therapy. Nat. Rev. Cancer. 2019;19:495–509. doi: 10.1038/s41568-019-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judd J., Abdel Karim N., Khan H., Naqash A.R., Baca Y., Xiu J., VanderWalde A.M., Mamdani H., Raez L.E., Nagasaka M., et al. Characterization of KRAS Mutation Subtypes in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2021;20:2577–2584. doi: 10.1158/1535-7163.MCT-21-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim T.K.H., Skoulidis F., Kerr K.M., Ahn M.-J., Kapp J.R., Soares F.A., Yatabe Y. KRAS G12C in Advanced NSCLC: Prevalence, Co-Mutations, and Testing. Lung Cancer. 2023;184:107293. doi: 10.1016/j.lungcan.2023.107293. [DOI] [PubMed] [Google Scholar]
- 17.Yu H.A., Sima C.S., Shen R., Kass S., Gainor J., Shaw A., Hames M., Iams W., Aston J., Lovly C.M., et al. Prognostic Impact of KRAS Mutation Subtypes in 677 Patients with Metastatic Lung Adenocarcinomas. J. Thorac. Oncol. 2015;10:431–437. doi: 10.1097/JTO.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ihle N.T., Byers L.A., Kim E.S., Saintigny P., Lee J.J., Blumenschein G.R., Tsao A., Liu S., Larsen J.E., Wang J., et al. Effect of KRAS Oncogene Substitutions on Protein Behavior: Implications for Signaling and Clinical Outcome. J. Natl. Cancer Inst. 2012;104:228–239. doi: 10.1093/jnci/djr523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerr E.M., Gaude E., Turrell F.K., Frezza C., Martins C.P. Mutant Kras Copy Number Defines Metabolic Reprogramming and Therapeutic Susceptibilities. Nature. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adjei A.A., Mauer A., Bruzek L., Marks R.S., Hillman S., Geyer S., Hanson L.J., Wright J.J., Erlichman C., Kaufmann S.H., et al. Phase II Study of the Farnesyl Transferase Inhibitor R115777 in Patients with Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2003;21:1760–1766. doi: 10.1200/JCO.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 21.Kim E.S., Kies M.S., Fossella F.V., Glisson B.S., Zaknoen S., Statkevich P., Munden R.F., Summey C., Pisters K.M.W., Papadimitrakopoulou V., et al. Phase II Study of the Farnesyltransferase Inhibitor Lonafarnib with Paclitaxel in Patients with Taxane-Refractory/Resistant Nonsmall Cell Lung Carcinoma. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 22.Riely G.J., Johnson M.L., Medina C., Rizvi N.A., Miller V.A., Kris M.G., Pietanza M.C., Azzoli C.G., Krug L.M., Pao W., et al. A Phase II Trial of Salirasib in Patients with Lung Adenocarcinomas with KRAS Mutations. J. Thorac. Oncol. 2011;6:1435–1437. doi: 10.1097/JTO.0b013e318223c099. [DOI] [PubMed] [Google Scholar]
- 23.Ryan M.B., Fece de la Cruz F., Phat S., Myers D.T., Wong E., Shahzade H.A., Hong C.B., Corcoran R.B. Vertical Pathway Inhibition Overcomes Adaptive Feedback Resistance to KRASG12C Inhibition. Clin. Cancer Res. 2020;26:1633–1643. doi: 10.1158/1078-0432.CCR-19-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li T., Kikuchi O., Zhou J., Wang Y., Pokharel B., Bastl K., Gokhale P., Knott A., Zhang Y., Doench J.G., et al. Developing SHP2-Based Combination Therapy for KRAS-Amplified Cancer. JCI Insight. 2023;8:e152714. doi: 10.1172/jci.insight.152714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann M.H., Gmachl M., Ramharter J., Savarese F., Gerlach D., Marszalek J.R., Sanderson M.P., Kessler D., Trapani F., Arnhof H., et al. BI-3406, a Potent and Selective SOS1-KRAS Interaction Inhibitor, Is Effective in KRAS-Driven Cancers through Combined MEK Inhibition. Cancer Discov. 2021;11:142–157. doi: 10.1158/2159-8290.CD-20-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltanás F.C., García-Navas R., Rodríguez-Ramos P., Calzada N., Cuesta C., Borrajo J., Fuentes-Mateos R., Olarte-San Juan A., Vidaña N., Castellano E., et al. Critical Requirement of SOS1 for Tumor Development and Microenvironment Modulation in KRASG12D-Driven Lung Adenocarcinoma. Nat. Commun. 2023;14:5856. doi: 10.1038/s41467-023-41583-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor N.P. Genentech Sinks SHP2 Pact, Leaving Relay to Race Thinning Field. [(accessed on 8 November 2024)]. Available online: https://www.fiercebiotech.com/biotech/genentech-sinks-shp2-pact-leaving-relay-race-thinning-field.
- 28.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) Inhibitors Allosterically Control GTP Affinity and Effector Interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Center for Drug Evaluation and Research . FDA Grants Accelerated Approval to Sotorasib for KRAS G12C Mutated NSCLC. FDA; Silver Spring, MD, USA: 2024. [Google Scholar]
- 30.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA Grants Accelerated Approval to Adagrasib with Cetuximab for KRAS G12C-Mutated Colorectal Cancer. FDA; Silver Spring, MD, USA: 2024. [Google Scholar]
- 32.Mok T.S.K., Yao W., Duruisseaux M., Doucet L., Azkárate Martínez A., Gregorc V., Juan-Vidal O., Lu S., De Bondt C., de Marinis F., et al. KRYSTAL-12: Phase 3 Study of Adagrasib versus Docetaxel in Patients with Previously Treated Advanced/Metastatic Non-Small Cell Lung Cancer (NSCLC) Harboring a KRASG12C Mutation. JCO. 2024;42:LBA8509. doi: 10.1200/JCO.2024.42.17_suppl.LBA8509. [DOI] [Google Scholar]
- 33.Zhou Q., Meng X., Sun L., Huang D., Yang N., Yu Y., Zhao M., Zhuang W., Guo R., Hu Y., et al. Efficacy and Safety of KRASG12C Inhibitor IBI351 Monotherapy in Patients With Advanced NSCLC: Results From a Phase 2 Pivotal Study. J. Thorac. Oncol. 2024 doi: 10.1016/j.jtho.2024.08.005. in press . [DOI] [PubMed] [Google Scholar]
- 34.October 5, 2023: Meeting of the Oncologic Drugs Advisory Committee Meeting Announcement—10/05/2023. [(accessed on 26 September 2024)]; Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/october-5-2023-meeting-oncologic-drugs-advisory-committee-meeting-announcement-10052023.
- 35.Zhao Y., Murciano-Goroff Y.R., Xue J.Y., Ang A., Lucas J., Mai T.T., Da Cruz Paula A.F., Saiki A.Y., Mohn D., Achanta P., et al. Diverse Alterations Associated with Resistance to KRAS(G12C) Inhibition. Nature. 2021;599:679–683. doi: 10.1038/s41586-021-04065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awad M.M., Liu S., Rybkin I.I., Arbour K.C., Dilly J., Zhu V.W., Johnson M.L., Heist R.S., Patil T., Riely G.J., et al. Acquired Resistance to KRASG12C Inhibition in Cancer. N. Engl. J. Med. 2021;384:2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue J.Y., Zhao Y., Aronowitz J., Mai T.T., Vides A., Qeriqi B., Kim D., Li C., de Stanchina E., Mazutis L., et al. Rapid Non-Uniform Adaptation to Conformation-Specific KRAS(G12C) Inhibition. Nature. 2020;577:421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhopadhyay S., Huang H.-Y., Lin Z., Ranieri M., Li S., Sahu S., Liu Y., Ban Y., Guidry K., Hu H., et al. Genome-Wide CRISPR Screens Identify Multiple Synthetic Lethal Targets That Enhance KRASG12C Inhibitor Efficacy. Cancer Res. 2023;83:4095. doi: 10.1158/0008-5472.CAN-23-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mira A., Ambrogio C. YAP and TAZ Orchestrate Adaptive Resistance to KRAS Inhibitors. Nat. Cancer. 2023;4:784–786. doi: 10.1038/s43018-023-00580-5. [DOI] [PubMed] [Google Scholar]
- 40.Garassino M.C., Gadgeel S., Speranza G., Felip E., Esteban E., Dómine M., Hochmair M.J., Powell S.F., Bischoff H.G., Peled N., et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023;41:1992–1998. doi: 10.1200/JCO.22.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novello S., Kowalski D.M., Luft A., Gümüş M., Vicente D., Mazières J., Rodríguez-Cid J., Tafreshi A., Cheng Y., Lee K.H., et al. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023;41:1999–2006. doi: 10.1200/JCO.22.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 43.Li B.T., Falchook G.S., Durm G.A., Burns T.F., Skoulidis F., Ramalingam S.S., Spira A., Bestvina C.M., Goldberg S.B., Veluswamy R., et al. OA03.06 CodeBreaK 100/101: First Report of Safety/Efficacy of Sotorasib in Combination with Pembrolizumab or Atezolizumab in Advanced KRAS p.G12C NSCLC. J. Thorac. Oncol. 2022;17:S10–S11. doi: 10.1016/j.jtho.2022.07.025. [DOI] [Google Scholar]
- 44.Thummalapalli R., Bernstein E., Herzberg B., Li B.T., Iqbal A., Preeshagul I., Santini F.C., Eng J., Ladanyi M., Yang S.-R., et al. Clinical and Genomic Features of Response and Toxicity to Sotorasib in a Real-World Cohort of Patients With Advanced KRAS G12C-Mutant Non-Small Cell Lung Cancer. JCO Precis. Oncol. 2023;7:e2300030. doi: 10.1200/PO.23.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barlesi F., Felip E., Popat S., Solomon B.J., Wolf J., Li B.T., Wu Y.-L., Kerr K., Akamatsu H., Camidge D.R., et al. Sotorasib versus Pembrolizumab in Combination with Platinum Doublet Chemotherapy as First-Line Treatment for Metastatic or Locally Advanced, PD-L1 Negative, KRAS G12C-Mutated NSCLC (CodeBreaK 202) JCO. 2024;42:TPS8653. doi: 10.1200/JCO.2024.42.16_suppl.TPS8653. [DOI] [Google Scholar]
- 46.A Study Evaluating Sotorasib Platinum Doublet Combination Versus Pembrolizumab Platinum Doublet Combination as a Front-Line Therapy in Participants With Stage IV or Advanced Stage IIIB/C Nonsquamous Non-Small Cell Lung Cancers (CodeBreaK 202) | ClinicalTrials.Gov. [(accessed on 26 September 2024)]; Available online: https://www.clinicaltrials.gov/study/NCT05920356.
- 47.Garassino M.C., Theelan W.S.M.E., Jotte R. KRYSTAL-7: Efficacy and Safety of Adagrasib with Pembrolizumab in Patients with Treatment-Naïve, Advanced Non-Small Cell Lung Cancer (NSCLC) Harboring a KRASG12C Mutation; Proceedings of the European Society for Medical Oncology Congress 2023; Madrid, Spain. 20–24 October 2023; Abstract LBA65. [Google Scholar]
- 48.Gregorc V., González-Cao M., Salvagni S., Koumarianou A., Gil-Bazo I., Maio M., Viteri S., Majem M., Gutiérrez V., Bernabe Caro R., et al. KROCUS: A Phase II Study Investigating the Efficacy and Safety of Fulzerasib (GFH925) in Combination with Cetuximab in Patients with Previously Untreated Advanced KRAS G12C Mutated NSCLC. JCO. 2024;42:LBA8511. doi: 10.1200/JCO.2024.42.17_suppl.LBA8511. [DOI] [Google Scholar]
- 49.Sacher A., LoRusso P., Patel M.R., Miller W.H., Garralda E., Forster M.D., Santoro A., Falcon A., Kim T.W., Paz-Ares L., et al. Single-Agent Divarasib (GDC-6036) in Solid Tumors with a KRAS G12C Mutation. N. Engl. J. Med. 2023;389:710–721. doi: 10.1056/NEJMoa2303810. [DOI] [PubMed] [Google Scholar]
- 50.Heist R.S., Koyama T., Murciano-Goroff Y.R., Hollebecque A., Cassier P.A., Han J.-Y., Tosi D., Sacher A.G., Burns T.F., Spira A.I., et al. Pan-Tumor Activity of Olomorasib (LY3537982), a Second-Generation KRAS G12C Inhibitor (G12Ci), in Patients with KRAS G12C-Mutant Advanced Solid Tumors. JCO. 2024;42:3007. doi: 10.1200/JCO.2024.42.16_suppl.3007. [DOI] [Google Scholar]
- 51.A Study of LY3537982 Plus Immunotherapy With or Without Chemotherapy in Participants With Non-Small Cell Lung Cancer (NSCLC) With a Change in a Gene Called KRAS G12C | ClinicalTrials.Gov. [(accessed on 26 September 2024)]; Available online: https://clinicaltrials.gov/study/NCT06119581.
- 52.Jänne P.A., Bigot F., Papadopoulos K., Eberst L., Sommerhalder D., Lebellec L., Voon P.J., Pellini B., Kalinka E., Arbour K., et al. Abstract PR014: Preliminary Safety and Anti-Tumor Activity of RMC-6291, a First-in-Class, Tri-Complex KRASG12C(ON) Inhibitor, in Patients with or without Prior KRASG12C(OFF) Inhibitor Treatment. Mol. Cancer Ther. 2023;22:PR014. doi: 10.1158/1535-7163.TARG-23-PR014. [DOI] [Google Scholar]
- 53.Maciag A.E., Stice J., Wang B., Sharma A., Chan A., Lin K., Singh D., Dyba M., Yang Y., Setoodeh S., et al. Abstract ND07: BBO-8520, a First-in-Class, Direct Inhibitor of KRASG12C (ON), Locks GTP-Bound KRASG12C in the State 1 Conformation Resulting in Rapid and Complete Blockade of Effector Binding. Cancer Res. 2024;84:ND07. doi: 10.1158/1538-7445.AM2024-ND07. [DOI] [Google Scholar]
- 54.Patel S., Bhhatarai B., Calses P., Erlanson D., Everley R., Fong S., Gerken P., Hermann J.C., Le T., Liu L., et al. Abstract 1142: Discovery of FMC-376 a Novel Orally Bioavailable Inhibitor of Activated KRASG12C. Cancer Res. 2023;83:1142. doi: 10.1158/1538-7445.AM2023-1142. [DOI] [Google Scholar]
- 55.Kim D., Herdeis L., Rudolph D., Zhao Y., Böttcher J., Vides A., Ayala-Santos C.I., Pourfarjam Y., Cuevas-Navarro A., Xue J.Y., et al. Pan-KRAS Inhibitor Disables Oncogenic Signalling and Tumour Growth. Nature. 2023;619:160–166. doi: 10.1038/s41586-023-06123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park W., Kasi A., Spira A.I., Berlin J.D., Wang J.S., Herzberg B., Kuboki Y., Kitano S., Pelster M., Goldman J.W., et al. 608O Preliminary Safety and Clinical Activity of ASP3082, a First-in-Class, KRAS G12D Selective Protein Degrader in Adults with Advanced Pancreatic (PC), Colorectal (CRC), and Non-Small Cell Lung Cancer (NSCLC) Ann. Oncol. 2024;35:S486–S487. doi: 10.1016/j.annonc.2024.08.675. [DOI] [Google Scholar]
- 57.Arbour K.C., Punekar S., Garrido-Laguna I., Hong D.S., Wolpin B., Pelster M.S., Barve M., Starodub A., Sommerhalder D., Chang S., et al. 652O Preliminary Clinical Activity of RMC-6236, a First-in-Class, RAS-Selective, Tri-Complex RAS-MULTI(ON) Inhibitor in Patients with KRAS Mutant Pancreatic Ductal Adenocarcinoma (PDAC) and Non-Small Cell Lung Cancer (NSCLC) Ann. Oncol. 2023;34:S458. doi: 10.1016/j.annonc.2023.09.1838. [DOI] [Google Scholar]
- 58.Camidge D.R., Reuss J.E., Spira A.I., Janne P.A., Rehman M., Pachter J.A., Patrick G., Denis L.J., Spigel D.R. A Phase 2 Study of VS-6766 (RAF/MEK Clamp) RAMP 202, as a Single Agent and in Combination with Defactinib (FAK Inhibitor) in Recurrent KRAS Mutant (Mt) and BRAF Mt Non–Small Cell Lung Cancer (NSCLC) JCO. 2022;40:TPS9147. doi: 10.1200/JCO.2022.40.16_suppl.TPS9147. [DOI] [Google Scholar]
- 59.Bery N., Miller A., Rabbitts T. A Potent KRAS Macromolecule Degrader Specifically Targeting Tumours with Mutant KRAS. Nat. Commun. 2020;11:3233. doi: 10.1038/s41467-020-17022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukhopadhyay S., Vander Heiden M.G., McCormick F. The Metabolic Landscape of RAS-Driven Cancers from Biology to Therapy. Nat. Cancer. 2021;2:271–283. doi: 10.1038/s43018-021-00184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]