Abstract
Background: Pediatric pain significantly affects children’s lives, leading to school absenteeism, impaired social interactions, and psychological distress. The perception of sensory signals as pain is influenced by the brain’s noradrenergic system, and recent evidence suggests that chronic pain may impact cognitive functioning and emotional regulation. Attention-Deficit/Hyperactivity Disorder (ADHD) is associated with alterations in the dopaminergic/noradrenergic systems, which could affect pain perception. Pain-associated conditions and frequent analgesic use in childhood may be linked to ADHD development and could serve as early indicators, yet data on this potential association remain limited. Study Aim: This population-based case-control study in Israel aimed to assess the prevalence of pain-related diagnoses prior to ADHD diagnosis in children aged 5 to 18. The study included children registered with Leumit Health Services (LHS) between 1 January 2006, and 30 June 2021. Children diagnosed with ADHD were compared to matched controls, selected based on age, gender, socioeconomic status, and other sociodemographic factors, who were never diagnosed with ADHD during the study period. Results: Children with ADHD (N = 18,756) and controls (N = 37,512) were precisely matched for sociodemographic characteristics. Individuals with ADHD exhibited significantly higher frequencies of diverse pain conditions, including those associated with illness [headache, earaches, and throat pain (odds ratios [OR] = 1.156 [95%CI 1.085, 1.232], 1.295 [95%CI 1.217, 1.377], and 1.080 [95%CI 1.019, 1.145], respectively; p < 0.01)] and injury [sprains and strains (OR = 1.233 [95% CI 1.104,1.376)]. Analgesics were more frequently purchased by individuals with ADHD, particularly paracetamol (OR = 1.194 [95%CI 1.152, 1.237], p < 0.001) and ibuprofen (OR = 1.366 [95%CI 1.318, 1.416], p = 0.001). Conclusions: This study highlights a potential connection between ADHD and pediatric pain. The elevated rates of pain diagnoses and analgesic usage among children with ADHD underscore the need for further research.
Keywords: pediatric pain-associated diagnoses, ADHD, inflammation
1. Introduction
1.1. Pain-Associated Diagnoses in Children
Pain is a primary reason for seeking medical attention across all age groups, a phenomenon not exempting children [1]. A pediatric population-based survey revealed that 54% of the respondents had experienced pain within the previous three months [2]. Common pediatric pain-associated diagnoses encompass musculoskeletal pain, headaches, abdominal pain, and disease-related pain such as otalgia and throat pain [1,3,4,5,6,7]. Pain-associated diagnoses present a considerable burden on the healthcare system [8].
Musculoskeletal pain in children and adolescents is a common complaint. Its etiology was addressed in a study of over 400 pediatric patients [7]. Non-inflammatory and mechanical musculoskeletal pain accounted for 42% of the cases, with rheumatic, infectious, and malignant causes accounting for 31%, 21.6%, and 2.4%, respectively. The prevalence of musculoskeletal pain in the pediatric population can reach up to 40% in some studies [4] with considerable consequences [8].
Various types of headaches are notably prevalent in pediatric populations and may impact children differently based on age and individual characteristics. A recent meta-analysis found a pooled prevalence of 11% for migraines, 17% for tension-type headaches, and a pooled prevalence of 62% for overall primary headaches in children and adolescents (38% and 27% in females and males, respectively [9,10].
Otalgia may be caused by infectious entities, mainly acute otitis media and otitis externa, which accounts for most ear pain [11], or caused by dental pathologies, temporomandibular joint disorders, trauma, and neuralgic causes [9,11,12]. Likewise, throat pain is most often linked to infectious causes, mainly viral upper respiratory tract infections and bacterial tonsillopharyngitis. Abdominal pain is another common diagnosis in pediatric care. In a community-based study, 75% of middle school and high school students reported abdominal pain, whereas 21% found it severe enough to affect activities, and 8% visited a physician [13,14,15,16].
1.2. Attention-Deficit/Hyperactivity Disorder
Recent epidemiological studies revealed that several pain-associated conditions had been found to occur at higher rates among children with Attention-Deficit/Hyperactivity Disorder [10,12,14].
Attention-Deficit/Hyperactivity Disorder (ADHD) is a neurodevelopmental disorder characterized by impairment and core symptoms of inattention, hyperactivity, and impulsivity. It is the most common neurodevelopmental disorder affecting children, with a reported global prevalence around 5–7% in school-aged populations [17]. The prevalence of ADHD in Western countries varies between 9% and 15%, depending on the specific diagnostic criteria used and the population being studied [18,19]. The etiology of ADHD is multifactorial, encompassing genetic, environmental, and neurobiological factors. Several studies have indicated that ADHD might be associated with infections during childhood [20,21,22,23,24]. Early exposure to infections, particularly those affecting the central nervous system, has been suggested as a potential risk factor for the development of ADHD [21,23,25]. Young individuals diagnosed with ADHD exhibit elevated levels of inflammatory markers like interleukin (IL) 1-ß, IL-6, and Tumor Necrosis Factor-alpha (TNF-α); this elevation is a possible indicator of an association between ADHD and inflammation [26,27]. This was corroborated by a recent systematic review that showed a higher predilection for cytokine gene polymorphism (IL-6 and TNF alpha genes) and elevated serum cytokines in patients with ADHD versus controls [28].
1.3. Pain and Neurodevelopmental Disorders
There is an association between ADHD and noradrenergic functioning. The locus coeruleus, with its extensive network of noradrenergic neurons projecting upwards to many structures, such as the prefrontal cortex, acts as a central hub and is thought to regulate attention and impulse control. Likewise, these A6 adrenergic nuclei have extensive axonal projections to the spinal cord and are considered to play a major role in modulating chronic and persistent pain [29].
Emerging evidence further highlights the complex interaction between pain and neurodevelopmental disorders, notably ADHD. Mundal et al. have sampled adolescents and young adults at three time points during a 9-year longitudinal study and have shown a higher prevalence of chronic and multisite pain in adolescents and young adults with ADHD as compared to controls [30]. Kaplan and colleagues have recently identified several temporal risk factors for the development of multisite pain in children, amongst which were attention difficulties. However, no formal diagnosis of ADHD was established [31].
Multiple pain experiences might influence cognitive functioning, attention, and emotional regulation, thereby potentially contributing to the manifestation of ADHD symptoms. Conversely, the sensory sensitivities often seen in individuals with ADHD could amplify pain perception and the experience of pain-associated distress. Exploring this association could uncover shared risk factors and common neurobiological pathways and guide potential intervention points. We suggest that multiple pain conditions in childhood may serve as early indicators of ADHD.
1.4. Aims of Study
We aimed to explore comprehensively the prevalence of various pain-associated diagnoses (regardless of whether they are acute or chronic) in children who were later diagnosed as having ADHD diagnosis, with a comparison to a control group.
2. Methods
2.1. Study Design
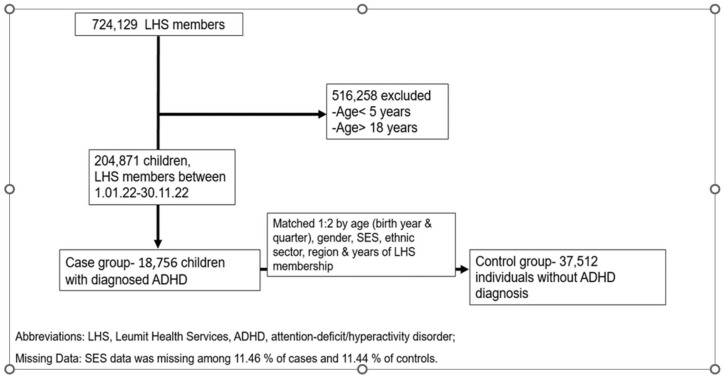
We conducted a population-based case-control study of ADHD-diagnosed children in a large Health Maintenance Organization (HMO) in Israel, Leumit Health Services (LHS), which served 724,129 persons during the study period. The information was collected from the HMO database. LHS maintains a comprehensive computerized database that is regularly updated with demographic information, medical visits, laboratory tests, hospitalizations, and medication prescriptions dating back to 1998. The database includes records of refilled and purchased prescriptions per patient. Diagnoses are recorded or updated during each physician visit according to the ICD-9 for somatic diagnoses and the ICD-10 for psychiatric diagnoses. This process ascertains the reliability of the diagnoses entered into the registry. The study design is shown in Figure 1.
Figure 1.
Study flowchart.
2.2. Study Population
We included participants between 5 and 18 years old who were registered in LHS from 1 January 2006 to 30 June 2021. We excluded those with oncological disorders or immune deficiencies, as these conditions are rare and typically associated with unusually high rates of pain and infections. Cases were patients with an established diagnosis of ADHD. Controls were selected in a 2:1 ratio, randomly chosen from the study population. Control subjects were required to be free of any ADHD diagnosis prior to the date when their matched case received an ADHD diagnosis. Each subject in the control group was precisely matched to a case subject based on age, gender, ethnic sector (general population, Ultra-Orthodox Jews, and Israeli Arabs), socioeconomic status (SES) category, and year of initial LHS membership, to minimize potential confounding effects. Missing SES data (12.4%) were categorized separately for matching purposes. For each case, the 2 control subjects with the closest birth dates to the case’s birth date were selected. A post-hoc comparison of the main demographic variables was performed to verify similar distributions between the two groups (Table 1).
Table 1.
Sociodemographic characteristics of the study population.
| Characteristics | ADHD Cases N. (%) |
MatchedControls N. (%) |
OR | p-Value |
|---|---|---|---|---|
| No. of children | 18,756 | 37,512 | ||
| Sex | ||||
| male | 11,810 (63.0%) | 23,619 (63.0%) | 1.00 | 1 |
| female | 6946 (37.0%) | 13,893 (37.0%) | 1.00 | 1 |
| Mean age of ADHD diagnosis, years, (SD) | 8.3 (2.6) | 8.3 (2.7) | - | 0.99 |
| Mean age of any pain diagnosis, years, (SD) | 4.2 ± 1.5 | 4.1 ± 1.3 | - | 0.99 |
| Sector | ||||
| Secular Jews | 8402 (44.8%) | 16,802 (44.8%) | 1.00 | 0.99 |
| Ultra-orthodox Jews | 7737 (41.3%) | 15,476 (41.3%) | 1.00 | 0.99 |
| Religious Jews | 348 (1.9%) | 696 (1.9%) | 1.00 | 1 |
| Arabs | 2269 (12.1%) | 4538 (12.1%) | 1.00 | 1 |
| SES, mean (SD) | 15.3 (25.5) | 14.5 (24.6) | 0.09 | |
| Very low | 5438 (14.5%) | 2716 (14.5%) | 1 | 1 |
| Low | 7980 (21.3%) | 3990 (21.3%) | 1 | 1 |
| Medium | 6456 (17.2%) | 3221 (17.2%) | 1 | 1 |
| High | 12,988 (34.6%) | 6494 (34.6%) | 1 | 1 |
| Missing | 4650 (12.4%) | 2335 (12.4%) | 1 | 1 |
2.3. Definitions
ADHD cases were identified based on diagnoses that met the Israeli Ministry of Health criteria, which follow international diagnostic standards. The diagnosis had to be confirmed by a qualified senior physician—either a psychiatrist or neurologist (specializing in child or adult care), or a pediatrician or family physician with specialized ADHD certification. The diagnosis is established using the criteria outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM-4 or 5, depending on the year of diagnosis). The validity of ADHD diagnoses was confirmed via a retrospective review of randomly selected electronic charts, with medical records thoroughly examined to ensure adherence to the Israeli Ministry of Health criteria and consistent documentation by certified ADHD specialists. SES was determined according to the classification of the Israeli Central Bureau of Statistics, which includes 20 subgroups. Classifications one to three are considered very low SES, four to six are low SES, seven to nine are medium SES, ten to fourteen are medium SES, and above fourteen is high SES.
2.4. Classification of Exposure
We defined two exposure categories: (1) any pain-associated diagnosis; and (2) using analgesic medications. The pain-associated diagnoses and analgesic medications use were considered as exposure only if these events occurred before the ADHD diagnosis.
2.5. Statistical Analysis
Statistical analysis was conducted using R-statistic software R 4.0.2 (R Foundation, Vienna, Austria), with two-sided tests and a significance level of 0.05. Sociodemographic characteristics between the ADHD and non-ADHD control groups were compared using the t-test and Fisher exact χ2 test for continuous and categorical variables based on the normal distribution and variable characteristics. The probabilities of having had pain-associated diagnoses among children with ADHD compared to the controls were compared using logistic regression analysis. The odds ratio (OR) and 95% confidence interval (CI) were calculated to demonstrate the effect sizes in this study. We used the Benjamini–Hochberg procedure to control the false discovery rate (FDR) for multiple tests.
2.6. Ethical Consideration
This study followed the Code of Ethics of the World Medical Association. The study was approved by institutional review committees of the LHS (approval number: LEU-0005-22). Due to this study’s retrospective and database nature, the need for informed consent was waived.
3. Results
3.1. Characteristics of the Study Groups
The ADHD case group included 18,756 subjects aged 5–18 years (mean 8.3 years, SD 2.6 years), and the 2:1 matched control group included 37,512 subjects. The sociodemographic attributes of the study cohorts are presented in Table 1. Across all variables, encompassing age distribution, age categories, gender, associated sector, and SES, a remarkable similarity was observed with no statistically significant distinctions. Many parameters were identical in the ADHD and control groups. This outcome underscores the effectiveness of the matching process.
3.2. Pain-Associated Diagnoses
The types and rates of pain-associated diagnoses in both the ADHD and control groups are detailed in Table 2. The frequency of pain diagnoses was notably higher in children with ADHD compared to the meticulously matched control group. The more prevalent pain diagnoses were not confined to a singular bodily system; instead, they encompassed diverse general pain conditions such as headache, otalgia, and throat pain (odds ratios (OR) and 95% confidence interval (95% CI): 1.156 [95% CI, 1.085, 1.232], 1.295 [95% CI, 1.217, 1.377] and 1.080 [95% CI, 1.019, 1.145], respectively, p < 0.01) as depicted in Table 2. Markedly elevated rates were found for all types of abdominal pain diagnoses, with an average effect size of 14% (p < 0.01 for all); for all types of limb pain diagnoses, with an average effect size of 35% (p < 0.01 for all), all types of arthralgias, with an average effect size of 40% (p < 0.001 for all), and diagnoses of sprains and strains of joints and adjacent muscles (OR 1.233, [95% CI 1.104, 1.376], p < 0.01).
Table 2.
Diagnoses of pain syndromes in children with Attention-Deficit/Hyperactivity Disorder (ADHD) and controls.
| ICD-9 Code—Diagnosis, Number (%) | ADHD Cases N = 1,875,699 |
Matched Controls N = 37,512 |
OR [CI] | p-Value | FDR BH |
|---|---|---|---|---|---|
| 784.0—Headache | 1653 (8.80%) | 2894 (7.71%) | 1.156 [1.085, 1.232] | 0.0001 | 0.0001 |
| 346—Migraine | 61 (0.32%) | 80 (0.21%) | 1.527 [1.075, 2.158] | 0.0154 | 0.1523 |
| 379.91—Eye Or Eye Region Pain | 75 (8.80%) | 115 (0.31%) | 1.306 [0.962, 1.763] | 0.0763 | 0.4753 |
| 388.7—Otalgia [Ear Pain] | 1853 (9.88%) | 2928 (7.81%) | 1.295 [1.217, 1.377] | 0.0001 | 0.0001 |
| 388.70—Otalgia, Unspecified [Ear Pain] | 367 (1.96%) | 643 (1.71%) | 1.144 [1.003, 1.305] | 0.0433 | 0.3313 |
| 388.71—Otogenic Pain | 98 (0.52%) | 147 (0.39%) | 1.335 [1.023, 1.737] | 0.0296 | 0.2577 |
| 784.1—Throat Pain | 1975 (10.53%) | 3685 (9.82%) | 1.080 [1.019, 1.145] | 0.0088 | 0.1008 |
| 789.00—Abdominal Pain, Unspecified Site | 2528 (13.48%) | 4371 (11.65%) | 1.181 [1.120, 1.245] | 0.0001 | 0.0001 |
| 789.07—Abdominal Pain, Generalized | 1175 (6.26%) | 2117 (5.64%) | 1.117 [1.037, 1.203] | 0.0033 | 0.0458 |
| 789.05—Abdominal Pain, Periumbilical | 696 (3.70%) | 1266 (3.37%) | 1.103 [1.003, 1.213] | 0.0430 | 0.3298 |
| 789.03—Abdominal Pain, Lower Quadrant | 442 (2.36%) | 766 (2,04%) | 1.158 [1.026, 1.305] | 0.0162 | 0.1582 |
| 729.58—Pain In Leg | 653 (3.48%) | 1063 (2.83%) | 1.237 [1.118, 1.367] | 0.0001 | 0.0006 |
| 729.56—Pain In Knee | 305 (1.63%) | 468 (1.25%) | 1.308 [1.128, 1.516] | 0.0004 | 0.0063 |
| 729.54—Pain In Hand | 174 (0.93%) | 253 (0.67%) | 1.379 [1.129, 1.681] | 0.0014 | 0.0209 |
| 729.57—Pain In Ankle | 105 (0.56%) | 143 (0.38%) | 1.471 [1.132, 1.907] | 0.0030 | 0.0410 |
| 719.460—Pain In Joint [Arthralgia] Involving Knee | 179 (0.95%) | 279 (0.74%) | 1.286 [1.059, 1.558] | 0.0096 | 0.0108 |
| 719.47—Pain In Joint [Arthralgia] Involving Ankle And Foot | 389 (2.07%) | 664 (1.77%) | 1.175 [1.033, 1.336] | 0.0133 | 0.1366 |
| 719.43—Pain In Joint [Arthralgia] Involving Forearm | 28 (0.15%) | 32 (0.08%) | 1.751 [1.016, 3.004] | 0.0388 | 0.0302 |
| 840–848—Sprains And Strains Of Joints And Adjacent Muscles | 539 (2.87%) | 879 (2.34%) | 1.233 [1.104, 1.376] | 0.0002 | 0.0035 |
3.3. Use of Analgesics
Table 3 provides insights into the specific categories of analgesic agents the study group utilized. The rates of analgesics purchased for individuals with ADHD were significantly higher compared to those prescribed to individuals without ADHD. The data for paracetamol use were OR = 1.194 (95% CI 1.152, 1.237), p < 0.001, and for ibuprofen use were OR = 1.366 (95% CI, 1.318, 1.416), p = 0.001, (details in Table 3).
Table 3.
Analgesics use in children with Attention-Deficit/Hyperactivity Disorder (ADHD) and controls.
| Number (%) | ADHD Cases N = 18,756 |
Matched Controls N = 37,512 |
OR (CI) | p-Value | FDR BH |
|---|---|---|---|---|---|
| N02—Analgesics | 9050 (48.25%) | 16162 (43.08%) | 1.232 [1.189, 1.276] | 0.0001 | 0.0001 |
| N02B—Other Analgesics and Antipyretics | 9037 (48.18%) | 16145 (43.04%) | 1.231 [1.188, 1.275] | 0.0001 | 0.0001 |
| N02BE01—Paracetamol | 8325 (44.39%) | 15031 (40.07%) | 1.194 [1.152, 1.237] | 0.0001 | 0.0001 |
| M01AE01—Ibuprofen | 8282 (44.16%) | 13752 (36.66%) | 1.366 [1.318, 1.416] | 0.0001 | 0.0001 |
| S02DA—Analgesics and Anesthetics | 3102 (16.54%) | 5129 (13.67%) | 1.251 [1.191, 1.314] | 0.0001 | 0.0001 |
| N02BE51—Paracetamol, Combinations Excl. Psycholeptics | 947 (5.05%) | 1539 (4.10%) | 1.243 [1.143, 1.351] | 0.0001 | 0.0001 |
| M02AA15—Diclofenac | 421 (2.24%) | 702 (1.87%) | 1.204 [1.063, 1.362] | 0.0032 | 0.0172 |
| M01AE02—Naproxen | 254 (1.35%) | 356 (0.95%) | 1.433 [1.214, 1.690] | 0.0001 | 0.0002 |
| S02DA30—Anesthetic Ear Drops | 1369 (7.30%) | 2206 (5.88%) | 1.260 [1.174, 1.352] | 0.0001 | 0.0001 |
| D04AB—Anesthetics For Topical Use | 257 (1.37%) | 436 (1.16%) | 1.181 [1.008, 1.383] | 0.0385 | 0.1105 |
| M02AA—Anti-inflammatory Preparations, Non-steroids For Topical Use | 469 (2.50%) | 790 (2.11%) | 1.192 [1.060, 1.340] | 0.0030 | 0.0013 |
| M02—Topical Products For Joint and Muscular Pain | 670 (3.57%) | 1127 (3.00%) | 1.196 [1.083, 1.319] | 0.0004 | 0.0007 |
| 28612—Elastic Bandage 10 cm | 68 (0.36%) | 81 (0.22%) | 1.682 [1.199, 2.351] | 0.0022 | 0.0303 |
4. Discussion
4.1. New Findings
In the present study, we investigated the prevalence of pain diagnoses in children who were later diagnosed with ADHD, relative to children who were never diagnosed with ADHD during the study timeframe.
Our findings demonstrate not only statistically significant associations between ADHD and a whole range of pain diagnoses, but also clinically meaningful effect sizes. Children diagnosed with ADHD had a 14% higher occurrence of prior abdominal pain diagnoses, a 35% higher occurrence of limb pain diagnoses, and a 40% higher occurrence of arthralgia compared to children without an ADHD diagnosis during the study period. These findings underscore the complex nature of the association between ADHD and physical health, with pain diagnoses spanning various physiological systems.
Our findings align with emerging evidence suggesting pain as an associated factor for ADHD [25].
Several theories have been proposed to clarify the interplay between ADHD and the elevated prevalence of pain conditions. As early as 2000, Anand KJ et al. postulated that neurodevelopmental changes to the immature perinatal brain could promote behavioral changes later in life. The proposed mechanism suggests that excitotoxic damage to developing neurons, mediated by excess NMDA/excitatory amino acid, e.g., as seen in exposure to repetitive pain, could lead to distinct behavior changes that include, among other attributes, altered pain sensitivity and ADHD, thus lending support to the neurodevelopmental shared basis theory of ADHD and pain [32].
Another forefront theory suggests neuroinflammation as a common denominator for ADHD and altered pain perception. Ample evidence suggests that maternal immune activation during pregnancy could affect the fetal brain and predispose toward neonatal neurodevelopmental disorders, and a putative mechanism was postulated [33,34]. In mice, maternal immune activation was shown to be associated with altered pain sensitivity [35].
Song et al. postulate a mechanism by which mast cell activation, which is considered fundamental in some pain-associated diagnoses, could be linked through neuromodulation and neuroinflammation to the development of ADHD [35]. While our study did not assess the bidirectional relationships between ADHD and pain conditions, evidence suggests that ADHD may increase susceptibility to pain. For instance, Jain et al. identified molecular interactions between nociceptors and immune cells that could heighten pain sensitivity in individuals with ADHD [36]. Kerekes et al. suggested that the altered pain perception in ADHD may be related to common neurobiological mechanisms, which involved dopaminergic systems underlying both ADHD and pain processing [26,37].
4.2. Strengths and Limitations
This study offers several strengths. Notably, it diligently matched cases and controls, ensuring a robust comparison. The design of a nationwide population-based survey enhances the generalizability of the findings. Including a large sample size provided ample statistical power for meaningful analyses.
Certain limitations warrant consideration. While our study relied on robust clinical records for ADHD diagnoses, based on the strict criteria set by the Israeli Ministry of Health, we acknowledge that a more in-depth evaluation of ADHD symptoms and their severity would require a detailed analysis of the text-based information contained within the electronic medical records. Additionally, the classification of controls solely based on diagnostic status leaves room for the possibility that some controls may have had undiagnosed ADHD, potentially influencing the study outcomes (albeit in a manner contrary to the reported findings). Furthermore, unmeasured confounding factors might contribute to the observed associations. For instance, parents of children with ADHD may have undiagnosed ADHD by themselves and seek medical care for their children due to general anxiety related to ADHD. One of the key limitations of this study is the absence of detailed information on whether the pain diagnoses were primary or secondary for each healthcare encounter, and the data do not allow for the determination of whether the pain conditions were acute or chronic. This could influence the observed associations, as chronic pain may have a more significant impact on child development. However, the mean age of study participants was approximately 8.3 years. At this age, the prevalence of chronic pain is typically lower than in older children and adolescents, making the distinction between acute and chronic pain less critical in the context of our findings. Future research incorporating detailed pain assessments and diagnostic criteria for chronic pain is needed to further explore this relationship.
Another important limitation is the lack of assessment of stimulant and non-stimulant medication use (which could potentially influence pain perception or reporting) among children with an ADHD diagnosis. Future targeted prospective studies could help to address these limitations and provide a more comprehensive understanding of the potential relationships between ADHD and pain.
5. Conclusions
The present study contributes valuable insights into the potential connection between ADHD and pain syndromes in childhood. The elevated rate of ADHD diagnosis among children having prior pain-associated diagnoses and analgesic prescriptions underscores the need for further research to elucidate the mechanisms driving this association and inform strategies for more holistic care for individuals facing both neurodevelopmental and somatic health challenges.
The current study focused on pain diagnoses preceding ADHD diagnosis, but the link between ADHD and pain disorders warrants further investigation. Prospective research is essential to comprehensively understand whether ADHD might also precede to chronic pain conditions.
Author Contributions
All authors designed the study, analyzed the data, interpreted the results, and wrote the manuscript. E.M. (Eugene Merzon)—conceptualization, methodology, data validation, writing original draft, review and editing, supervision; Y.L.—conceptualization, methodology, review and editing; E.M. (Eli Magen), S.A., A.W. and I.M.—writing original draft, review and editing, I.G., A.G.-C., S.V., B.K. and S.V.F.—review and editing; A.I.—conceptualization, methodology, data extraction, data analysis, data validation, review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of LHS (LEU 0005-22, accessed on 16 February 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the use of anonymized data extracted from a health maintenance organization database. Since no direct patient contact or intervention was involved, and data privacy was strictly maintained, the Institutional Review Board approved the waiver of consent in accordance with relevant ethical guidelines.
Data Availability Statement
The data presented in this study are available upon request and with restrictions from Leumit Health Services due to privacy and confidentiality concerns. Access to the data requires authorization in compliance with institutional policies and relevant data protection regulations to ensure patient privacy.
Conflicts of Interest
Over the past 3 years, Eugene Merzon received support for continuing medical education programs or advisory board meetings sponsored by SK-Pharma, Medison, Teva, AstraZeneca, and Merk. Beth Krone received financial compensation as a scientific consultant to HIPPO T & C and MaxisHealth. Stephen V. Faraone received support for continuing medical education programs or advisory boards meetings sponsored by: Aardvark, Aardwolf, Akili, Atentiv, Corium, Genomind, Ironshore, Medice, Noven, Otsuka, Sandoz, Sky Therapeutics, Supernus, Tris, and Vallon. Abraham Weizman declares honoraria from the following pharmaceutical companies: Pfizer, Novartis, Janssen, Lundbeck, Teva, Unipharm, Dexcel, and Medison. Iris Manor received support for continuing medical education programs or advisory boards meetings sponsored by: Madison, Takeda Peri and Vizo. None of these are relevant to the submitted manuscript. Over the past 3 years, Eli Magen, Yaniv Levi, Shai Ashkenazi, Shlomo Vinker, Ilan Green, Ariel Israel, and Avivit Golan-Cohen have no disclosures relevant to this manuscript.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Perquin C.W., Hazebroek-Kampschreur A.A.J.M., Unfold J.A.M., Bohnen A.M., van Suijlekom-Smit L.W.A., Passchier J., van der Wouden J.C. Pain in children and adolescents: A common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- 2.Miró J., Roman-Juan J., Sánchez-Rodríguez E., Solé E., Castarlenas E., Jensen M.P. Chronic Pain and High Impact Chronic Pain in Children and Adolescents: A Cross-Sectional Study. J. Pain. 2023;24:812–823. doi: 10.1016/j.jpain.2022.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Palermo T.M. Impact of recurrent and chronic pain on child and family daily functioning: A critical review of the literature. J. Dev. Behav. Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 4.King S., Chambers C.T., Huguet A., MacNevin R.C., McGrath P.J., Parker L., MacDonald A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Groenewald C.B., Law E.F., Fisher E., Beals-Erickson S.E., Palermo T.M. Associations Between Adolescent Chronic Pain and Prescription Opioid Misuse in Adulthood. J. Pain. 2019;20:28–37. doi: 10.1016/j.jpain.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cid A., Ng A., Ip V. Addressing the Opioid Crisis—The Need for a Pain Management Intervention in Community Pharmacies in Canada: A Narrative Review. Pharmacy. 2023;11:71. doi: 10.3390/pharmacy11020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld S.B., Schroeder K., Watkins-Castillo S.I. The Economic Burden of Musculoskeletal Disease in Children and Adolescents in the United States. J. Pediatr. Orthop. 2018;38:e230–e236. doi: 10.1097/BPO.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 8.Onofri A., Pensato U., Rosignoli C., Wells-Gatnik W., Stanyer E., Ornello R., Chen H.Z., De Santis F., Torrente A., Mikulenka P., et al. Primary headache epidemiology in children and adolescents: A systematic review and meta-analysis. J. Headache Pain. 2023;24:8. doi: 10.1186/s10194-023-01541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan P.Y., Jonsson U., Şahpazoğlu Çakmak S.S., Häge A., Hohmann S., Nobel Norrman H., Buitelaar J.K., Banaschewski T., Cortese S., Coghill D., et al. Headache in ADHD as comorbidity and a side effect of medications: A systematic review and meta-analysis. Psychol. Med. 2022;52:14–25. doi: 10.1017/S0033291721004141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwa T.P., Brant J.A. Evaluation and Management of Otalgia. Med. Clin. N. Am. 2021;105:813–826. doi: 10.1016/j.mcna.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Krüger K., Töpfner N., Berner R., Windfuhr J., Oltrogge J.H., Guideline Group Clinical Practice Guideline: Sore Throat. Dtsch. Arztebl. Int. 2021;118:188–194. doi: 10.3238/arztebl.m2021.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyams J.S., Burke G., Davis P.M., Rzepski B., Andrulonis P.A. Abdominal pain and irritable bowel syndrome in adolescents: A community-based study. J. Pediatr. 1996;129:220–226. doi: 10.1016/S0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 13.Kedem S., Yust-Katz S., Carter D., Levi Z., Kedem R., Dickstein A., Daher S., Katz L.H. Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults. World J. Gastroenterol. 2020;26:6626–6637. doi: 10.3748/wjg.v26.i42.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjoukes A., Venekamp R.P., van de Pol A.C., Hay A.D., Little P., Schilder A.G., Damoiseaux R.A. Paracetamol (acetaminophen) or non-steroidal anti-inflammatory drugs, alone or combined, for pain relief in acute otitis media in children. Cochrane Database Syst. Rev. 2016;12:CD011534. doi: 10.1002/14651858.CD011534.pub2. Erratum in Cochrane Database Syst. Rev. 2023, 8, CD011534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richer L., Billinghurst L., Linsdell M.A., Russell K., Vandermeer B., Crumley E.T., Durec T., Klassen T.P., Hartling L. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database Syst. Rev. 2016;4:CD005220. doi: 10.1002/14651858.CD005220.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraone S.V., Banaschewski T., Coghill D., Zheng Y., Biederman J., Bellgrove M.A., Newcorn J.H., Gignac M., Al Saud N.M., Manor I., et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021;128:789–818. doi: 10.1016/j.neubiorev.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merzon E., Israel A., Ashkenazi S., Rotem A., Schneider T., Faraone S.V., Biederman J., Green I., Golan-Cohen A., Vinker S., et al. Attention-Deficit/Hyperactivity Disorder Is Associated with Increased Rates of Childhood Infectious Diseases: A Population-Based Case-Control Study. J. Am. Acad. Child Adolesc. Psychiatry. 2023;62:253–260.e1. doi: 10.1016/j.jaac.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Danielson M.L., Claussen A.H., Bitsko R.H., Katz S.M., Newsome K., Blumberg S.J., Kogan M.D., Ghandour RADHDPrevalence Among U.S. Children and Adolescents in 2022: Diagnosis, Severity, Co-Occurring Disorders, and Treatment. J. Clin. Child Adolesc. Psychol. 2024;53:343–360. doi: 10.1080/15374416.2024.2335625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shehadeh-Sheeny A., Baron-Epel O. Prevalence, diagnosis and treatment of ADHD in Arab and Jewish children in Israel, where are the gaps? BMC Psychiatry. 2023;23:586. doi: 10.1186/s12888-023-05090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadaka Y., Freedman J., Ashkenazi S., Vinker S., Golan-Cohen A., Green I., Israel A., Eran A., Merzon E. The Effect of Antibiotic Treatment of Early Childhood Shigellosis on Long-Term Prevalence of Attention Deficit/Hyperactivity Disorder. Children. 2021;8:880. doi: 10.3390/children8100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merzon E., Weiss M.D., Cortese S., Rotem A., Schneider T., Craig S.G., Vinker S., Golan Cohen A., Green I., Ashkenazi S., et al. The Association between ADHD and the Severity of COVID-19 Infection. J. Atten. Disord. 2022;26:491–501. doi: 10.1177/10870547211003659. [DOI] [PubMed] [Google Scholar]
- 22.Merzon E., Gutbir Y., Vinker S., Golan Cohen A., Horwitz D., Ashkenazi S., Sadaka Y. Early Childhood Shigellosis and Attention Deficit Hyperactivity Disorder: A Population-Based Cohort Study with a Prolonged Follow-up. J. Atten. Disord. 2021;25:1791–1800. doi: 10.1177/1087054720940392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merzon E., Manor I., Rotem A., Schneider T., Vinker S., Golan Cohen A., Lauden A., Weizman A., Green I. ADHD as a Risk Factor for Infection with COVID-19. J. Atten. Disord. 2021;25:1783–1790. doi: 10.1177/1087054720943271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So M., Dziuban E.J., Pedati C.S., Holbrook J.R., Claussen A.H., O’Masta B., Maher B., Cerles A.A., Mahmooth Z., MacMillan L., et al. Childhood Physical Health and Attention Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Modifiable Factors. Prev. Sci. 2022;25:316–336. doi: 10.1007/s11121-022-01398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koç S., Güler E.M., Derin S., Gültekin F., Aktaş S. Oxidative and Inflammatory Parameters in Children and Adolescents with ADHD. J. Atten. Disord. 2023;27:880–886. doi: 10.1177/10870547231159907. [DOI] [PubMed] [Google Scholar]
- 26.Kerekes N., Sanchéz-Pérez A.M., Landry M. Neuroinflammation as a possible link between attention-deficit/hyperactivity disorder (ADHD) and pain. Med. Hypotheses. 2021;157:110717. doi: 10.1016/j.mehy.2021.110717. [DOI] [PubMed] [Google Scholar]
- 27.Anand D., Colpo G.D., Zeni G., Zeni C.P., Teixeira A.L. Attention-Deficit/Hyperactivity Disorder and Inflammation: What Does Current Knowledge Tell Us? A Systematic Review. Front. Psychiatry. 2017;8:228. doi: 10.3389/fpsyt.2017.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor B.K., Westlund K.N. The noradrenergic locus coeruleus as a chronic pain generator. J. Neurosci. Res. 2017;95:1336–1346. doi: 10.1002/jnr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundal I., Schei J., Lydersen S., Thomsen P.H., Nøvik T.S., Kvitland L.R. Prevalence of chronic and multisite pain in adolescents and young adults with ADHD: A comparative study between clinical and general population samples (the HUNT study) Eur. Child. Adolesc. Psychiatry. 2023;33:1433–1442. doi: 10.1007/s00787-023-02249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan C.M., Schrepf A., Boehnke K.F., He Y., Smith T., Williams D.A., Bergmans R., Voepel-Lewis T., Hassett A.L., Harris R.E., et al. Risk Factors for the Development of Multisite Pain in Children. Clin. J. Pain. 2023;39:588–594. doi: 10.1097/AJP.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anand K.J., Scalzo F.M. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol. Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 32.Han V.X., Patel S., Jones H.F., Dale R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021;17:564–579. doi: 10.1038/s41582-021-00530-8. [DOI] [PubMed] [Google Scholar]
- 33.Han V.X., Jones H.F., Patel S., Mohammad S.S., Hofer M.J., Alshammery S., Maple-Brown E., Gold W., Brilot F., Dale R.C. Emerging evidence of Toll-like receptors as a putative pathway linking maternal inflammation and neurodevelopmental disorders in human offspring: A systematic review. Brain Behav. Immun. 2022;99:91–105. doi: 10.1016/j.bbi.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X., Erickson M., Mohammed R., Kentner A.C. Maternal immune activation accelerates puberty initiation and alters mechanical allodynia in male and female C57BL6/J mice. Dev. Psychobiol. 2022;64:e22278. doi: 10.1002/dev.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song Y., Lu M., Yuan H., Chen T., Han X. Mast cell-mediated neuroinflammation may have a role in attention deficit hyperactivity disorder (Review) Exp. Ther. Med. 2020;20:714–726. doi: 10.3892/etm.2020.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain A., Gyori B.M., Hakim S., Jain A., Sun L., Petrova V., Bhuiyan S.A., Zhen S., Wang Q., Kawaguchi R., et al. Nociceptor-immune interactomes reveal insult-specific immune signatures of pain. Nat. Immunol. 2024;25:1296–1305. doi: 10.1038/s41590-024-01857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerekes N., Lundqvist S., Schubert Hjalmarsson E., Torinsson Naluai Å., Kantzer A.K., Knez R. The associations between ADHD, pain, inflammation, and quality of life in children and adolescents—A clinical study protocol. PLoS ONE. 2022;17:e0273653. doi: 10.1371/journal.pone.0273653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request and with restrictions from Leumit Health Services due to privacy and confidentiality concerns. Access to the data requires authorization in compliance with institutional policies and relevant data protection regulations to ensure patient privacy.