Abstract
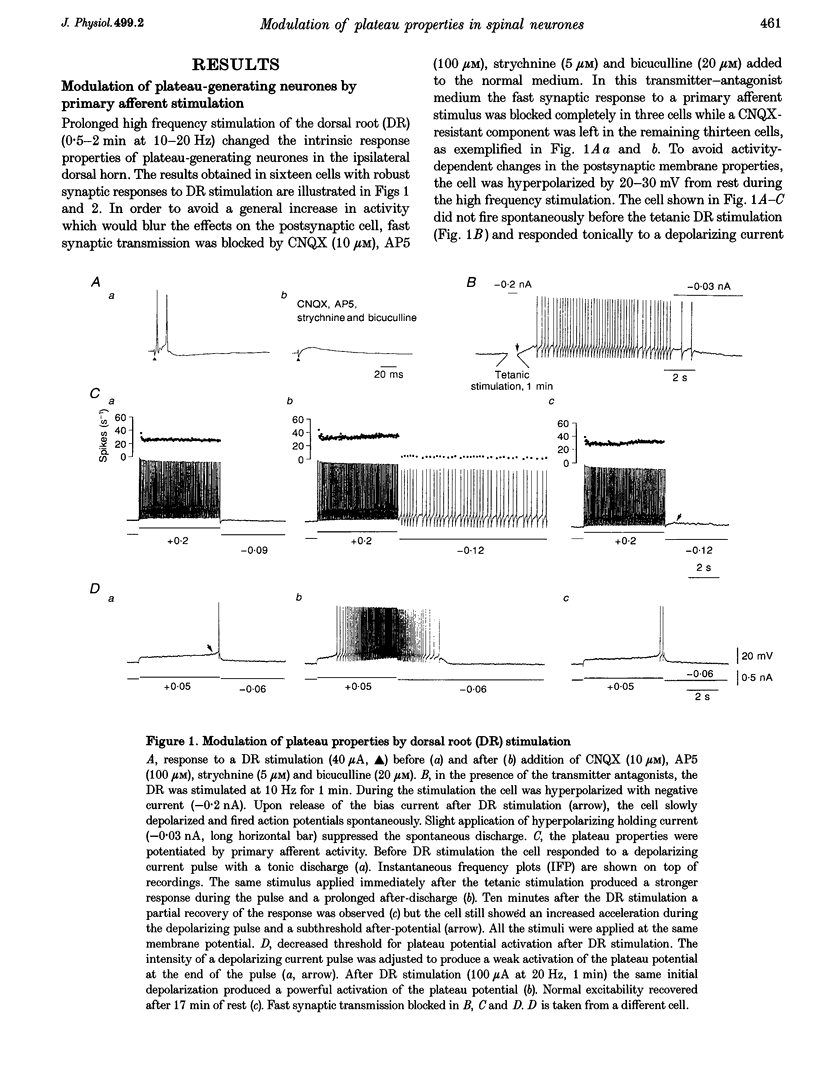
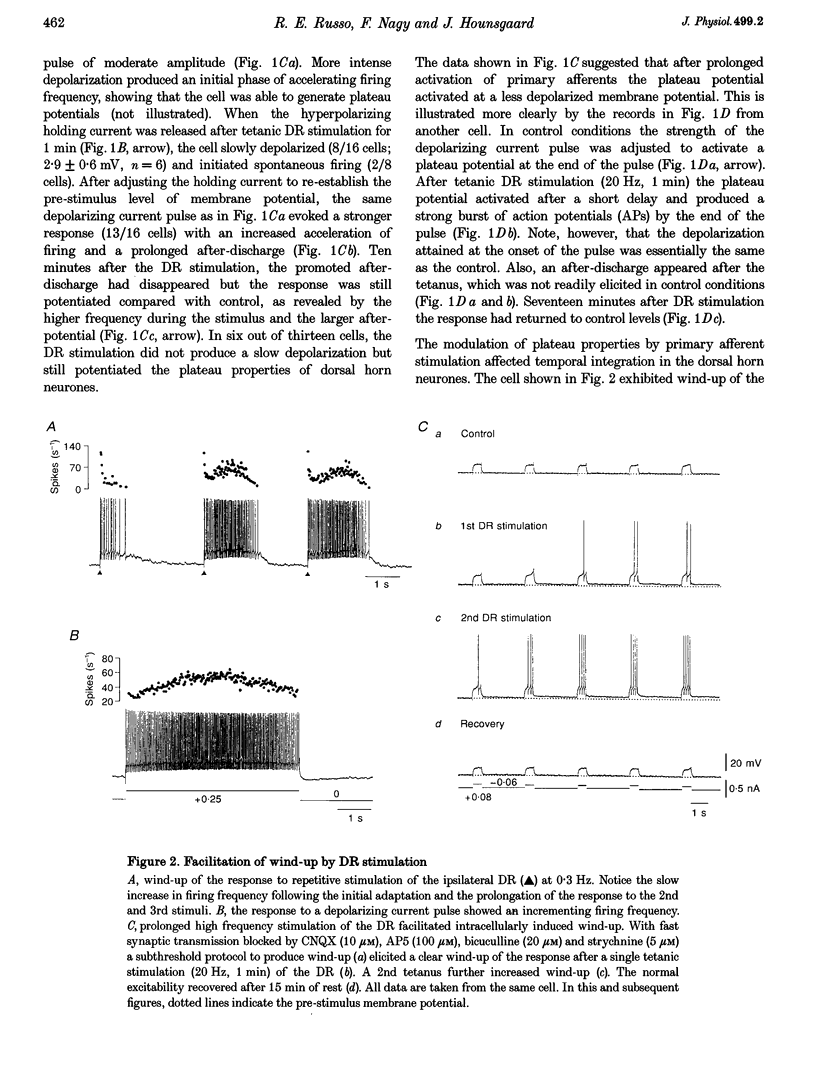
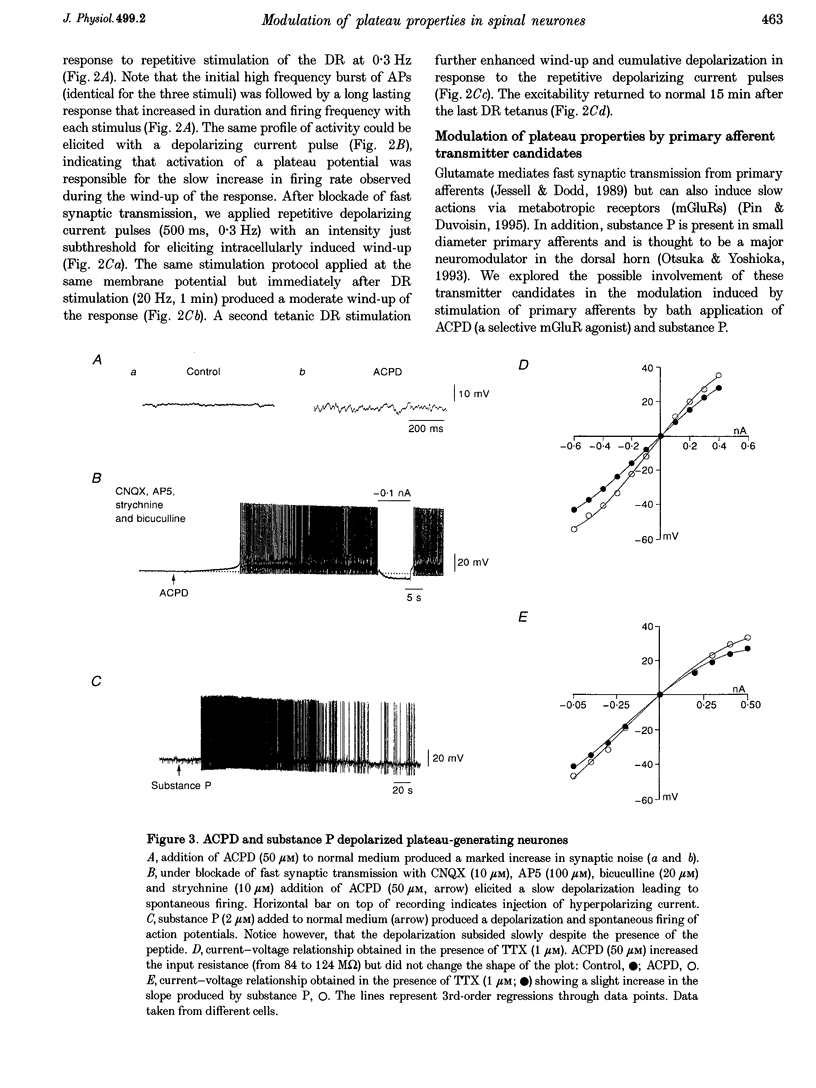
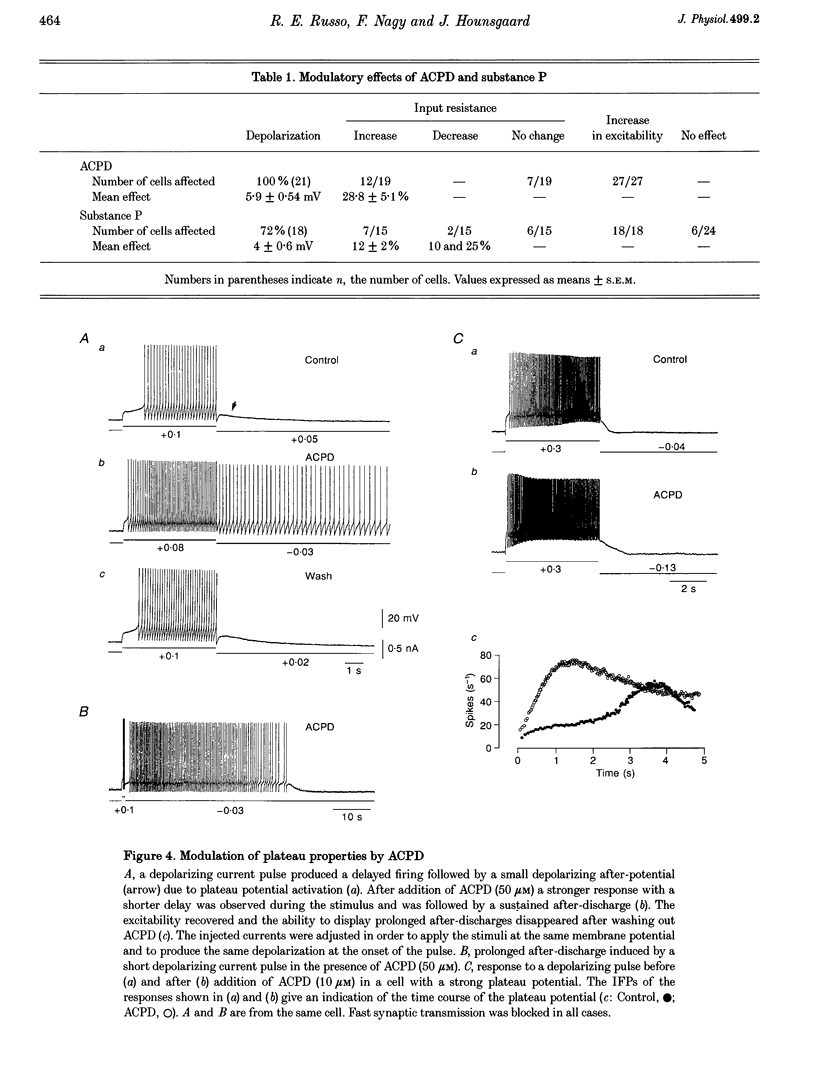
1. Modulation of plateau properties in dorsal horn neurones was studied in a transverse slice preparation of the spinal cord of the turtle. In plateau-generating neurones high frequency stimulation of the ipsilateral dorsal root (10-20 Hz, 0.5-2 min) produced a slow depolarization (2.9 +/- 0.6 mV, mean +/- S.E.M.; n = 6) and enhanced the properties mediated by dihydropyridine-sensitive Ca2+ channels. The tetanic stimulus facilitated wind-up and after-discharges even when fast synaptic transmission was blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10-20 microM), (+/-)-2-amino-5-phosphonopentanoic acid (AP5, 100 microM), bicuculline (10-20 microM) and strychnine (5-20 microM). 2. Application of cis-(+/-)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD, 10-50 microM) produced a slow depolarization (5.9 +/- 0.5 mV, n = 21) accompanied by an increase in input resistance (28.8 +/- 5.1%, n = 12). 3. ACPD increased the excitability by facilitating the plateau properties. In the presence of tetrodotoxin (TTX, 1 microM) a lower threshold and a slower decay of the plateau potential were observed. These effects resulted in facilitation of wind-up and prolonged after-discharges. 4. All ACPD-induced effects were blocked by alpha-methyl-4-carboxyphenylglycine (MCPG, 0.5-1 mM), a selective antagonist of metabotropic glutamate receptors. The selective agonist for the type I metabotropic glutamate receptor ((RS)-3,5-dihydrophenylglycine (DHPG, 50 microM)) reproduced all the effects of ACPD. 5. Application of a supposed neuromodulator, substance P (1-2 microM) produced a transient depolarization (4 +/- 0.6 mV) lasting 4-6 min during continued application of substance P. Variable effects on the input resistance were observed, a slight increase (12 +/- 2%) being the most frequent. In 61% of the cells, substance P induced a clear increase in excitability with no detectable change in input resistance or membrane potential. 6. The effects of substance P on plateau properties were indistinguishable from those produced by ACPD. Unlike the transient depolarization, the facilitation of the plateau properties persisted in the presence of the agonist. 7. The substance P-induced facilitation of the plateau potential was blocked by GR 82334 (5-10 microM), a selective NK-1 tachykinin-receptor antagonist, and was not affected by MEN 10376 (2 microM), a selective NK-2 antagonist. 8. The facilitation of plateau properties produced by dorsal root stimulation was also reduced by antagonists of metabotropic glutamate receptors and NK-1 tachykinin receptors. 9. We propose that modulation of postsynaptic plateau properties in dorsal horn neurones by activation of type I metabotropic glutamate receptors and NK-1 tachykinin receptors is involved in processing nociceptive information.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxall S. J., Thompson S. W., Dray A., Dickenson A. H., Urban L. Metabotropic glutamate receptor activation contributes to nociceptive reflex activity in the rat spinal cord in vitro. Neuroscience. 1996 Sep;74(1):13–20. doi: 10.1016/0306-4522(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Cerne R., Randic M. Modulation of AMPA and NMDA responses in rat spinal dorsal horn neurons by trans-1-aminocyclopentane-1,3-dicarboxylic acid. Neurosci Lett. 1992 Sep 14;144(1-2):180–184. doi: 10.1016/0304-3940(92)90745-s. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chavis P., Nooney J. M., Bockaert J., Fagni L., Feltz A., Bossu J. L. Facilitatory coupling between a glutamate metabotropic receptor and dihydropyridine-sensitive calcium channels in cultured cerebellar granule cells. J Neurosci. 1995 Jan;15(1 Pt 1):135–143. doi: 10.1523/JNEUROSCI.15-01-00135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P., Shinozaki H., Bockaert J., Fagni L. The metabotropic glutamate receptor types 2/3 inhibit L-type calcium channels via a pertussis toxin-sensitive G-protein in cultured cerebellar granule cells. J Neurosci. 1994 Nov;14(11 Pt 2):7067–7076. doi: 10.1523/JNEUROSCI.14-11-07067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. J., Woolf C. J., Wall P. D., McMahon S. B. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987 Jan 8;325(7000):151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty P. M., Palecek J., Palecková V., Willis W. D. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994 Oct;72(4):1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- Dubner R., Ruda M. A. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992 Mar;15(3):96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Hendry I. A., Morton C. R., Hutchison W. D., Zhao Z. Q. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988 Jun 7;451(1-2):261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- Egger M. D. Sensitization and habituation of dorsal horn cells in cats. J Physiol. 1978 Jun;279:153–166. doi: 10.1113/jphysiol.1978.sp012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérineau N. C., Gähwiler B. H., Gerber U. Reduction of resting K+ current by metabotropic glutamate and muscarinic receptors in rat CA3 cells: mediation by G-proteins. J Physiol. 1994 Jan 1;474(1):27–33. doi: 10.1113/jphysiol.1994.sp019999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Luthman J., Fernández A., Radmilovich M., Trujillo-Cenóz O. Immunohistochemical studies on the spinal dorsal horn of the turtle Chrysemys d'orbigny. Tissue Cell. 1991;23(4):515–523. doi: 10.1016/0040-8166(91)90009-i. [DOI] [PubMed] [Google Scholar]
- McMahon S. B., Lewin G. R., Wall P. D. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993 Aug;3(4):602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Meller S. T., Dykstra C. L., Gebhart G. F. Acute mechanical hyperalgesia is produced by coactivation of AMPA and metabotropic glutamate receptors. Neuroreport. 1993 Jul;4(7):879–882. doi: 10.1097/00001756-199307000-00010. [DOI] [PubMed] [Google Scholar]
- Morisset V., Nagy F. Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. J Neurophysiol. 1996 Oct;76(4):2794–2798. doi: 10.1152/jn.1996.76.4.2794. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Actions of substance P on rat spinal dorsal horn neurones. J Physiol. 1984 Jan;346:203–217. doi: 10.1113/jphysiol.1984.sp015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randić M. Substance P augments a persistent slow inward calcium-sensitive current in voltage-clamped spinal dorsal horn neurons of the rat. Brain Res. 1986 Feb 19;365(2):369–376. doi: 10.1016/0006-8993(86)91652-5. [DOI] [PubMed] [Google Scholar]
- Murase K., Ryu P. D., Randić M. Tachykinins modulate multiple ionic conductances in voltage-clamped rat spinal dorsal horn neurons. J Neurophysiol. 1989 Apr;61(4):854–865. doi: 10.1152/jn.1989.61.4.854. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Macdonald R. L. Substance P: ionic basis for depolarizing responses of mouse spinal cord neurons in cell culture. J Neurosci. 1982 Aug;2(8):1119–1128. doi: 10.1523/JNEUROSCI.02-08-01119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
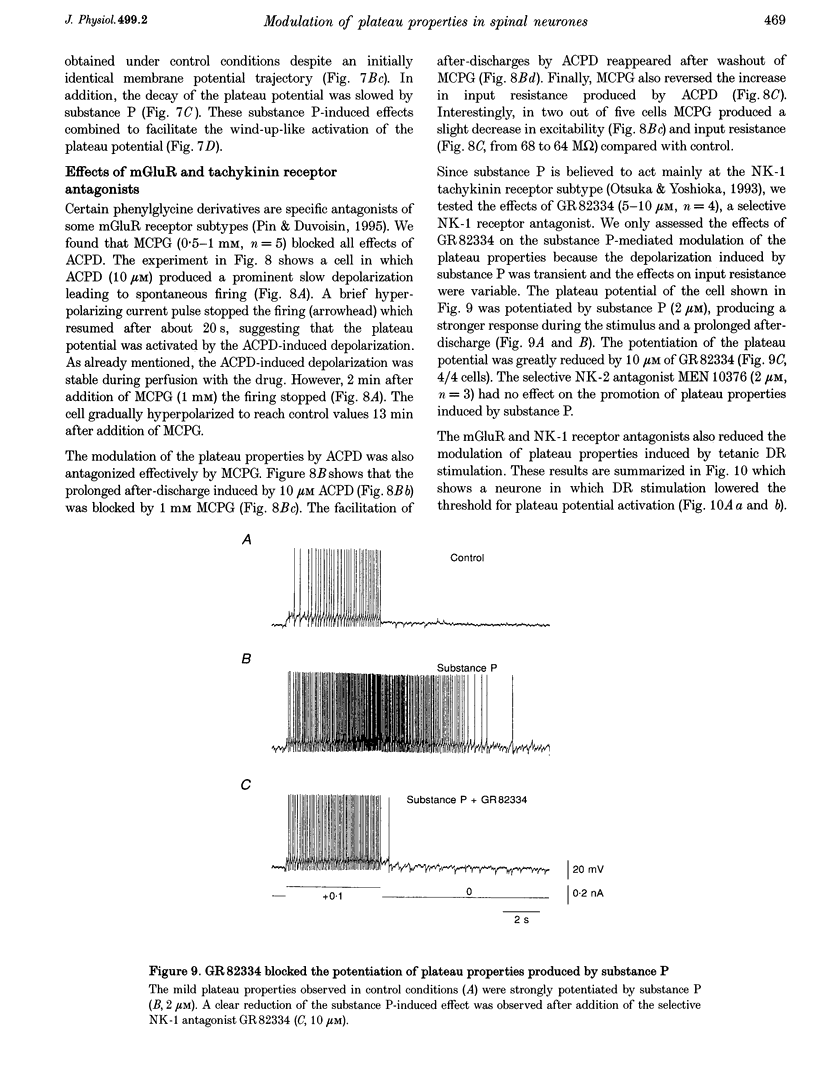
- Otsuka M., Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993 Apr;73(2):229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Pin J. P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995 Jan;34(1):1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Rusin K. I., Bleakman D., Chard P. S., Randic M., Miller R. J. Tachykinins potentiate N-methyl-D-aspartate responses in acutely isolated neurons from the dorsal horn. J Neurochem. 1993 Mar;60(3):952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
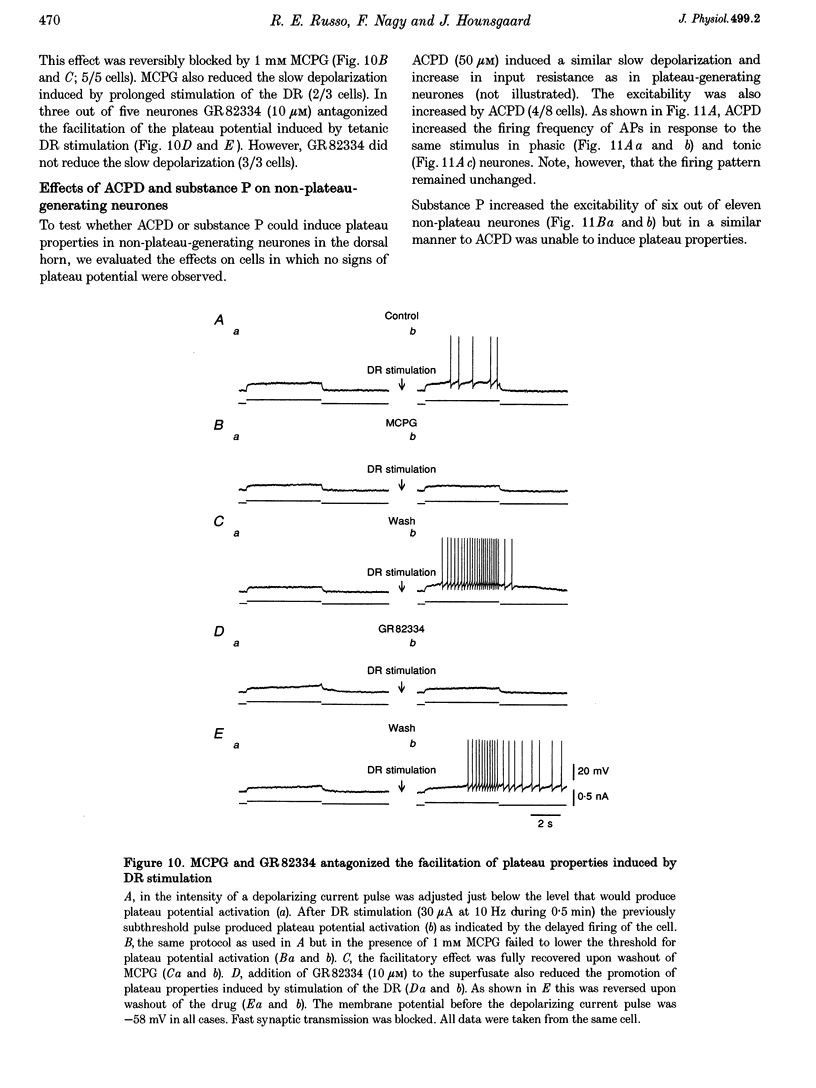
- Russo R. E., Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994 Jul;61(2):191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Katsumata K., Yogo H., Tan-No K., Sakurada S., Kisara K. Antinociception induced by CP 96,345, a non-peptide NK-1 receptor antagonist, in the mouse formalin and capsaicin tests. Neurosci Lett. 1993 Mar 19;151(2):142–145. doi: 10.1016/0304-3940(93)90006-7. [DOI] [PubMed] [Google Scholar]
- Sayer R. J., Schwindt P. C., Crill W. E. Metabotropic glutamate receptor-mediated suppression of L-type calcium current in acutely isolated neocortical neurons. J Neurophysiol. 1992 Sep;68(3):833–842. doi: 10.1152/jn.1992.68.3.833. [DOI] [PubMed] [Google Scholar]
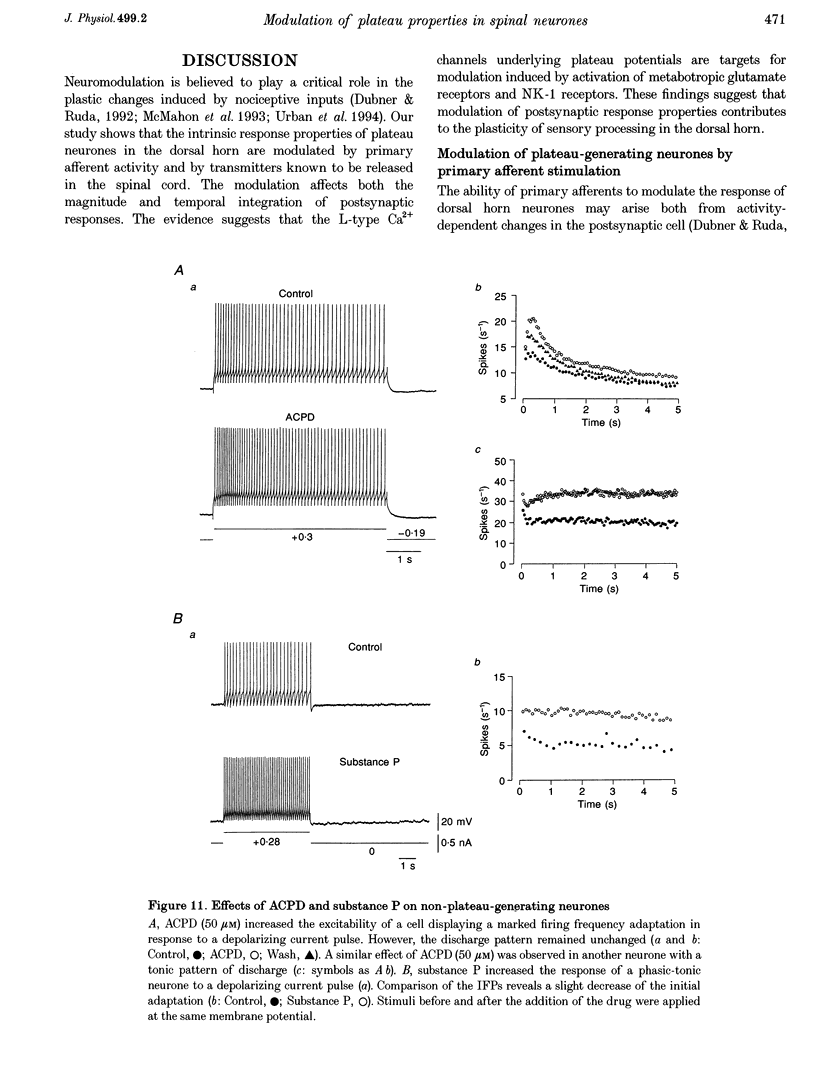
- Schmidt A. W., McLean S., Heym J. The substance P receptor antagonist CP-96,345 interacts with Ca2+ channels. Eur J Pharmacol. 1992 Sep 4;219(3):491–492. doi: 10.1016/0014-2999(92)90498-s. [DOI] [PubMed] [Google Scholar]
- Urban L., Thompson S. W., Dray A. Modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends Neurosci. 1994 Oct;17(10):432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Urbán L., Randić M. Slow excitatory transmission in rat dorsal horn: possible mediation by peptides. Brain Res. 1984 Jan 9;290(2):336–341. doi: 10.1016/0006-8993(84)90952-1. [DOI] [PubMed] [Google Scholar]
- Woolf C. J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 Dec 15;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Young M. R., Fleetwood-Walker S. M., Mitchell R., Munro F. E. Evidence for a role of metabotropic glutamate receptors in sustained nociceptive inputs to rat dorsal horn neurons. Neuropharmacology. 1994 Jan;33(1):141–144. doi: 10.1016/0028-3908(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Tulloch I. F. Effects of substance P on neurones in the dorsal horn of the spinal cord of the cat. Brain Res. 1979 Apr 27;166(2):273–282. doi: 10.1016/0006-8993(79)90213-0. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., Tölle T. R. The pharmacology of pain signalling. Curr Opin Neurobiol. 1993 Aug;3(4):611–618. doi: 10.1016/0959-4388(93)90063-5. [DOI] [PubMed] [Google Scholar]


