Abstract
Here we show that the potential to regulate NFAT is a conserved property of different Nef alleles and that Nef residues involved in membrane targeting and SH3 binding are critical for this function. Cotransfection of an activated protein kinase C-θ (PKC-θ) with Nef implicated PKC-θ as a possible physiological cofactor of Nef in promoting NFAT-dependent gene expression and T-cell activation.
Multiple cellular functions have been associated with Nef, such as enhancement of human immunodeficiency virus (HIV) replication and particle infectivity, down-regulation of cell surface expression of CD4 and major histocompatibility complex class I, and modulation of cellular signal transduction pathways (19).
We have recently shown that Nef can activate Ca2+/calcineurin-mediated signaling in T cells via a mechanism that is independent of the T-cell receptor (TCR) (15). Consequently, in Nef-expressing cells NFAT-dependent transcription directed by the ARRE2 element (antigen receptor response element of the interleukin-2 [IL-2] gene) can be abnormally activated by phorbol myristate acetate (PMA) ester treatment alone. Stimulation of the mitogen-activated protein kinase (MAPK) cascade by PMA activates transcription factors, most notably activator protein 1 (AP-1), whose cooperation is necessary for NFAT to activate its target genes, such as the IL-2 gene (17).
Sustained elevation of free Ca2+ caused by calcium influx is required to maintain NFAT in the nucleus, whereas transient Ca2+ pulses generated solely by calcium release from intracellular stores fail to accomplish this (5, 24). Provided that activation of the MAPK pathway is ensured (e.g., by PMA stimulation), ARRE2-dependent transcription serves as a good indicator for any effects that can cause such sustained elevation of intracellular calcium characteristic of T-cell activation.
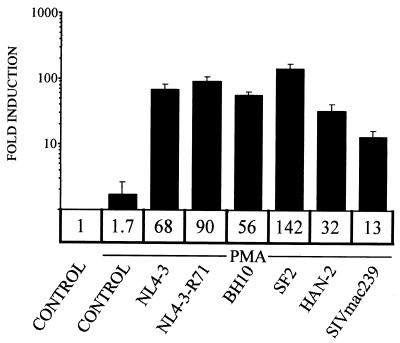
To confirm that the potential to regulate NFAT is a conserved property of divergent Nef proteins, we compared a panel of different HIV type 1 (HIV-1) Nef alleles, including NL4-3-R71 (20), NL4-3-T71 (1), BH10 (6), SF2 (11), and HAN-2 (21) alleles, as well as the simian immunodeficiency virus (SIV) mac239 allele (9) expressed from an EF-1α promoter-driven vector (16), for their capacities to activate ARRE2-dependent luciferase expression in transiently transfected Jurkat T cells. Transfections and luciferase assays were done as described previously (15). As shown in Fig. 1, all tested HIV-1 Nef alleles potently cooperated with the MAPK cascade in NFAT activation. PMA treatment in cells expressing SIVmac239 Nef consistently led to a smaller but yet marked (13-fold) induction of NFAT. Thus, the ability to contribute to the activation of the Ca2+/calcineurin signaling pathway is a well-conserved property of Nef, and thereby potentially important for its pathogenic effect in AIDS.
FIG. 1.
Synergistic activation of NFAT is a conserved function of Nef. Jurkat T cells were transfected with an NFAT-dependent ARRE2 luciferase reporter plasmid together with an empty control vector (control) or with vectors for different HIV-1 or SIV Nef proteins as indicated. Twenty hours after transfection one of the control vector-transfected cultures was left untreated while the other, together with the rest of the cultures, was treated with 100 ng of PMA/ml for 5 h. The mean induction (fold increase) in relative luciferase activity in each case is shown and represents data from four or more independent transfection experiments. Variation between different experiments is indicated by standard error bars.
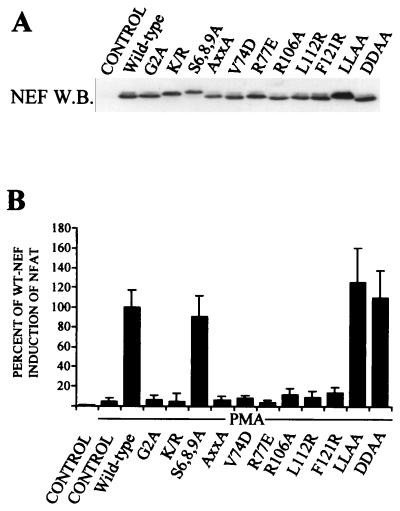
To address the structural and mechanistic bases of this effect, we tested a panel of Nef mutants (Fig. 2B). Immunoblotting analysis of the steady-state expression levels of these Nef mutants was done as described previously (14) and is shown in Fig. 2A. One group of mutations (Nef-G2A and Nef-K/R) affected membrane localization of Nef. Nef-K/R carried changes in the basic residues, i.e., K4V, K5A, R17A, R19A, R21A, and R22A, which together with the myristoylated Gly2 form the bipartite membrane targeting signal of Nef (26). Although defective in membrane association for different reasons, both Nef-G2A and Nef-K/R proteins were equally unable to contribute to NFAT activation.
FIG. 2.
Mutagenesis analysis of Nef residues involved in NFAT activation. (A) Steady-state expression levels of different Nef proteins examined by Western blotting (W.B.). Results are representative of three independent transfection experiments. (B) Jurkat T cells were transfected with an NFAT (ARRE2)-luciferase reporter together with an empty control vector (control), a wild-type (WT) allele, or different mutant HIV-1 Nef alleles as indicated. Twenty hours later one of the control vector-transfected cultures was left untreated while the other, together with the rest of the cultures, was treated with 100 ng of PMA/ml for 5 h. Luciferase activity of the PMA-treated, wild-type Nef-transfected cells was set to 100%, and the activities of other samples are shown relative to this. The luciferase data represent mean values from at least four independent transfection experiments. Variation between these experiments is indicated by standard error bars.
Because a cluster of serines in the amino terminus of Nef has recently been implicated as a target for PKC (Harris et al., unpublished data), we tested the potential of a Nef variant carrying the amino acid changes S6A, S8A, S9A and (Nef-S6, 8, 9A) to regulate NFAT but found this mutant to be fully functional. Nef variants carrying amino acid changes L164A and L165A (Nef-LLAA) and D174A and D175A (Nef-DDAA), which have been reported to block Nef-induced CD4 down-regulation by interfering with interactions connecting Nef to the endocytic machinery (4, 13), were also found to have an undiminished capacity to activate NFAT.
Another group of mutations disrupted the PxxP motif (Nef-AxxA) or other critical determinants of the SH3 domain binding surface of Nef (Nef-V74D and Nef-R77E). With the exception of CD4 down-regulation, SH3 binding has been found to be necessary for virtually all of the cellular functions of HIV-1 Nef and for association with many cellular signaling proteins (19). All three SH3 binding-deficient mutants were found to be completely defective in inducing NFAT (Fig. 2B). The identity of the relevant SH3-containing partner of Nef raises an interesting question. Our previous data do not support a role for the Src family of tyrosine kinases or other SH3-containing TCR-proximal signaling molecules, such as Vav (15). Thus, the collection of SH3-containing cellular partners of Nef might include a yet-unidentified protein involved in cellular calcium metabolism.
Although SH3 binding is also required for Nef to associate with NAK/PAK2 (14, 18), mutations affecting a number of other Nef residues, such as Arg106, originally noted by Sawai et al. (22), as well as Leul 12 and Phe121 can abrogate PAK2 association without affecting SH3 binding by Nef (14). We were therefore interested to test the capacity of Nef-R106A, Nef-L112R, and Nef-F121R to activate NFAT and found all of these three mutants to be inactive in this function. Although this result could indicate a role for the PAK2 interaction in regulation of NFAT by Nef, this correlation could also be due to other reasons. For example, recent studies have suggested that Arg106 as well as a hydrophobic patch on the surface of Nef, which comprises both Leul12 and Phe121 (10, 14), are involved in the oligomerization of Nef (3, 12), which might be independently important for both PAK2 association and NFAT activation.
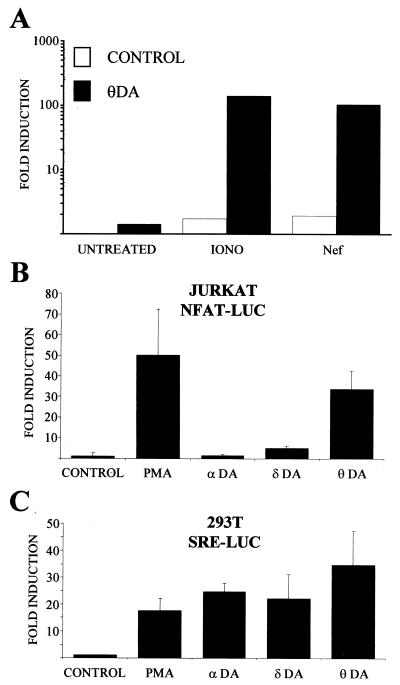
Phorbol ester treatment activates several cellular signaling pathways, in particular those controlled by members of the protein kinase C (PKC) family (8). To study if the observed synergistic effect of PMA on ARRE2-dependent transcription activated by Nef was indeed due to PKC-mediated induction of the MAPK cascade, we examined if it would be possible to replace PMA stimulation by cotransfection of an activated PKC-θ mutant (PKC-θ-A148E). As shown in Fig. 3A, dominant-active PKC-θ induced a robust activation of NFAT-dependent transcription in Nef-expressing as well as in cells treated with the calcium ionophore ionomycin.
FIG. 3.
Overexpression of a constitutively active form of PKC-θ-A148E efficiently synergizes with Nef to activate NFAT-dependent gene expression. (A) Jurkat T cells were transfected with an NFAT (ARRE2)-luciferase reporter with (solid bars) or without (open bars) a PKC-θ-A148E (θDA) expression plasmid. When indicated, the cells were also cotransfected with Nef. An empty control plasmid was used to normalize the total amount of DNA used per transfection. Twenty hours later some of the cultures that were not transfected with Nef were treated with 1 μg of ionomycin (iono)/ml for 5 h while the others (untreated) as well as the Nef-transfected cultures were left untreated. These data are plotted as fold induction relative to luciferase activity measured from untreated cells transfected with the control plasmid. This experiment was repeated four times with similar results. (B) Jurkat T cells were transfected with an NFAT (ARRE2)-luciferase reporter and Nef together with constitutively active PKC isoforms PKC-α-A25E (αDA), PKC-δ-A147E (δDA), and PKC-θ-A148E (θDA) or an empty vector (control). Twenty hours later one of the control transfected samples was treated with 100 ng of PMA/ml for 5 h (PMA). These data are plotted as in panel A and are from four independent transfection experiments. (C) 293T fibroblast cells were transfected with a serum response element (SRE)-luciferase reporter construct together with constitutively active PKC isoforms and treated as explained above. These data represent results from two independent transfection experiments.
To examine if other PKC isoforms would also show this effect, we engineered the corresponding activating mutations into PKC-α and PKC-δ and coexpressed these with Nef using the same EF-1α promoter-driven vector (16) used for PKC-θ. As shown in Fig. 3B, PKC-δ-A147E was considerably less potent than PKC-θ-A148E in cooperating with Nef in NFAT induction and PKC-α-A25E was virtually unable to do so. By contrast, all these three activated PKC isoforms were able to activate serum response element-driven transcription when expressed in 293T, HeLa, or HepG2 fibroblast cell lines (Fig. 3C and data not shown).
Considering this preferential ability of PKC-θ to cooperate with Nef, it is interesting to note that Smith et al. have reported that PKC-θ, but not other PKC isoforms, can interact with Nef in Jurkat cells (23). However, we observed a similar functional hierarchy among these PKC isoforms (θ > δ > α) when their cooperation with ionomycin in NFAT activation was examined (data not shown). Therefore, and in the light of previous work on PKC-θ (25, 27), the general compatibility of PKC-θ to cooperate with Ca2+/calcineurin signaling in T cells, rather than a specific capacity for functional interplay with Nef, is more likely to explain the observed preference for PKC-θ as a T-cell-activating partner of Nef. Moreover, consistent with a central role of PKC-θ in T-cell activation, expression of a dominant-negative PKC-θ mutant (PKC-θ-K409R) potently inhibited αCD3-triggered activation of NFAT (data not shown).
Nonetheless, these data indicate that PKC-θ can efficiently substitute for PMA in cooperating with Nef in NFAT activation. Therefore, in Nef-expressing T cells any stimuli that would activate PKC-θ or another appropriate component in the PKC/Ras/MAPK cascade might lead to abnormal triggering of the T-cell activation program independently of antigenic stimulation via TCR. Interestingly, the strong replicative advantage of Nef+ versus Nef− HIV and SIV strains in herpesvirus saimiri-immortalized IL-2-dependent T lymphocytes, which can be explained by Nef-induced IL-2 expression (2), might represent an in vitro model for the above scenario. Immortalization by this herpesvirus depends on its STP-C protein, which can bind and activate cellular Ras (7) and which, in Nef-expressing cells, could thus provide the MAPK-inducing stimulus required for activation of NFAT target genes, such as the IL-2 gene. In addition to the IL-2 gene, NFAT is known to regulate a number of genes important for T-cell function, such as IL-4, tumor necrosis factor alpha, and FasL genes (17), all of which might also be involved in mediating the pathogenic effects of Nef in HIV infection.
Acknowledgments
We are grateful to Amnon Altman for PKC-θ cDNA, Mark Harris for sheep α-Nef serum, Nef-S6,8,9A, and sharing unpublished information, Hans-Georg Kräusslich for Nef-K/R, Sabine Lang for SIVmac239 Nef, Vladimir Ovod for α-Nef monoclonal antibodies, and Olli Silvennoinen for PKC-α and PKC-δ cDNAs. We thank Kristina Lehtinen for expert technical assistance.
This study was supported by grants to K.S. from the Academy of Finland (project 44499) and the Medical Research Fund of Tampere University Hospital (project 9A061). G.H.R. was an EU Biomed Marie Curie program Fellow and is currently supported by a postdoctoral fellowship from the Academy of Finland.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arold S, Hoh F, Domergue S, Birck C, Delsuc M A, Jullien M, Dumas C. Characterization and molecular basis of the oligomeric structure of HIV-1 nef protein. Protein Sci. 2000;9:1137–1148. doi: 10.1110/ps.9.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 5.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 6.Harada S, Kobayashi N, Koyanagi Y, Yamamoto N. Clonal selection of human immunodeficiency virus (HIV): serological differences in the envelope antigens of the cloned viruses and HIV prototypes (HTLV-III B, LAV, and ARV) Virology. 1987;158:447–451. doi: 10.1016/0042-6822(87)90219-4. [DOI] [PubMed] [Google Scholar]
- 7.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazanietz M G. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 10.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 11.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 12.Liu L X, Heveker N, Fackler O T, Arold S, Le Gall S, Janvier K, Peterlin B M, Dumas C, Schwartz O, Benichou S, Benarous R. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J Virol. 2000;74:5310–5319. doi: 10.1128/jvi.74.11.5310-5319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, Yu H, Liu S H, Brodsky F M, Peterlin B M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 14.Manninen A, Hiipakka M, Vihinen M, Lu W, Mayer B J, Saksela K. SH3-domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology. 1998;250:273–282. doi: 10.1006/viro.1998.9381. [DOI] [PubMed] [Google Scholar]
- 15.Manninen A, Renkema G H, Saksela K. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol Chem. 2000;275:16513–16517. doi: 10.1074/jbc.M910032199. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 18.Renkema G H, Manninen A, Mann D A, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr Biol. 1999;9:1407–1410. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 19.Renkema G H, Saksela K. Interactions of HIV-1 NEF with cellular signal transducing proteins. Front Biosci. 2000;5:D268–D283. doi: 10.2741/renkema. [DOI] [PubMed] [Google Scholar]
- 20.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauermann U, Schneider J, Mous J, Brunckhorst U, Schedel I, Jentsch K D, Hunsmann G. Molecular cloning and characterization of a German HIV-1 isolate. AIDS Res Hum Retroviruses. 1990;6:813–823. doi: 10.1089/aid.1990.6.813. [DOI] [PubMed] [Google Scholar]
- 22.Sawai E T, Baur A S, Peterlin B M, Levy J A, Cheng-Mayer C. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J Biol Chem. 1995;270:15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- 23.Smith B L, Krushelnycky B W, Mochly-Rosen D, Berg P. The HIV nef protein associates with protein kinase C theta. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman L A, Clipstone N A, Ho S N, Northrop J P, Crabtree G R. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- 25.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 26.Welker R, Harris M, Cardel B, Krausslich H G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]