Abstract
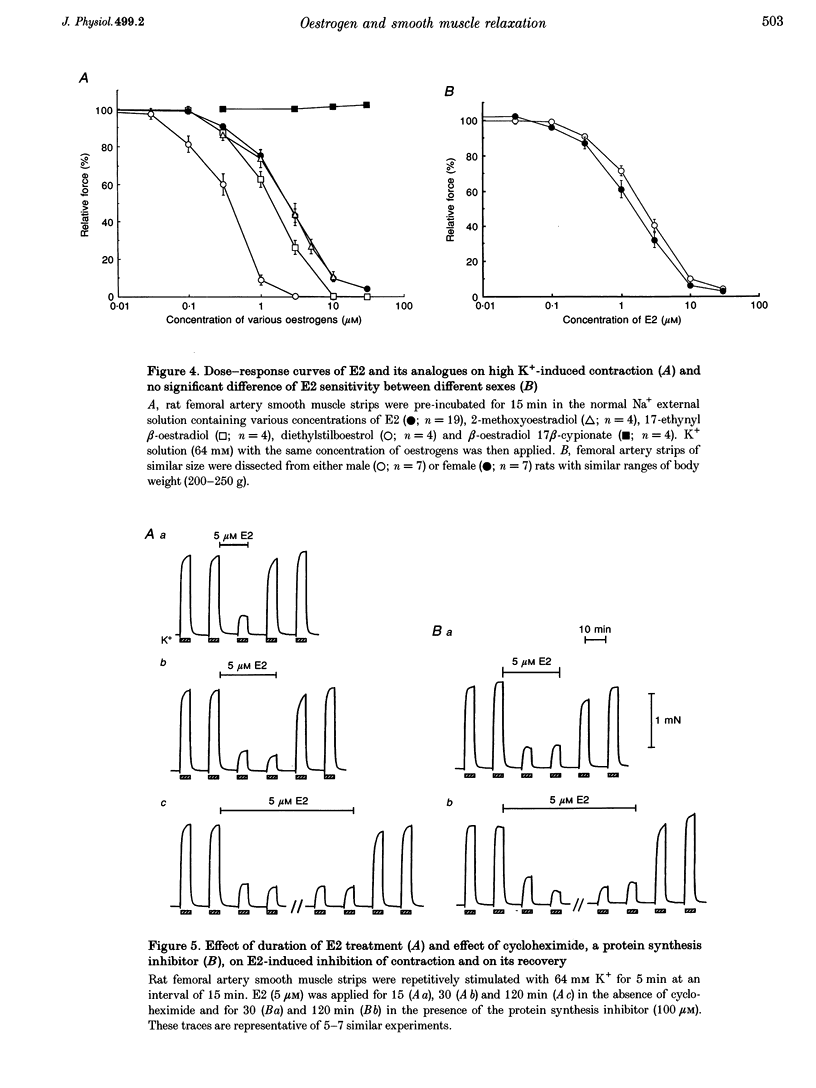
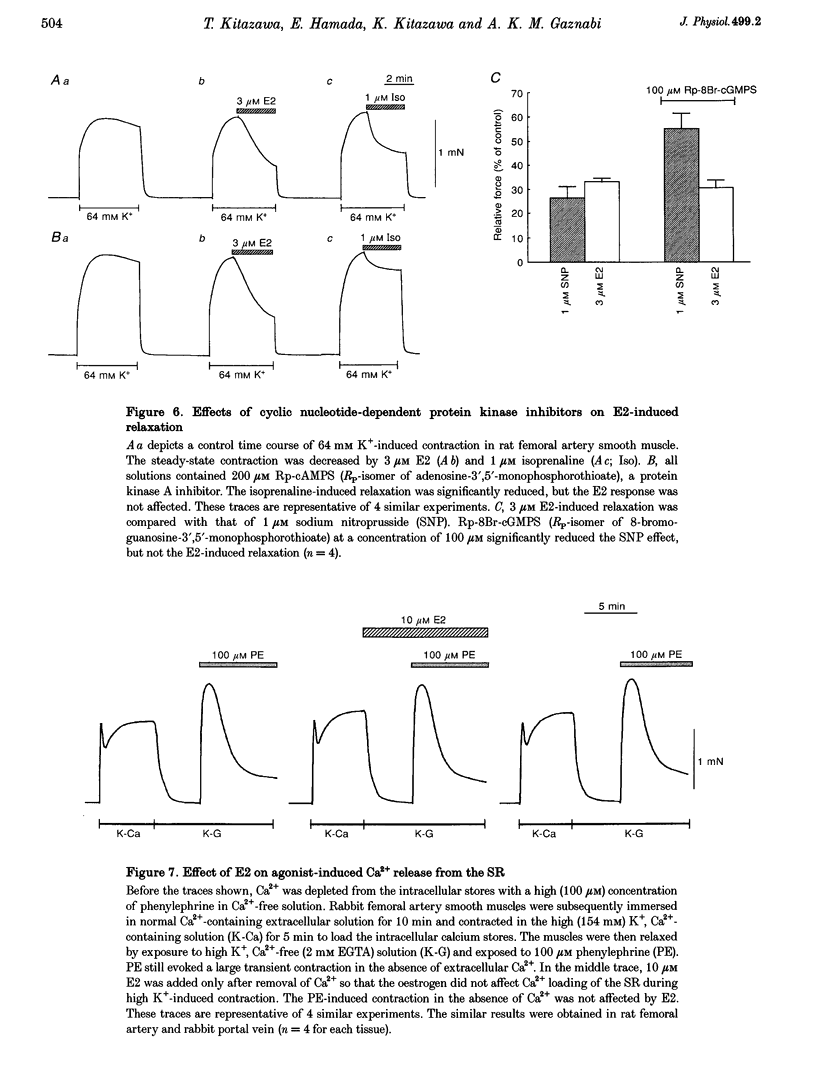
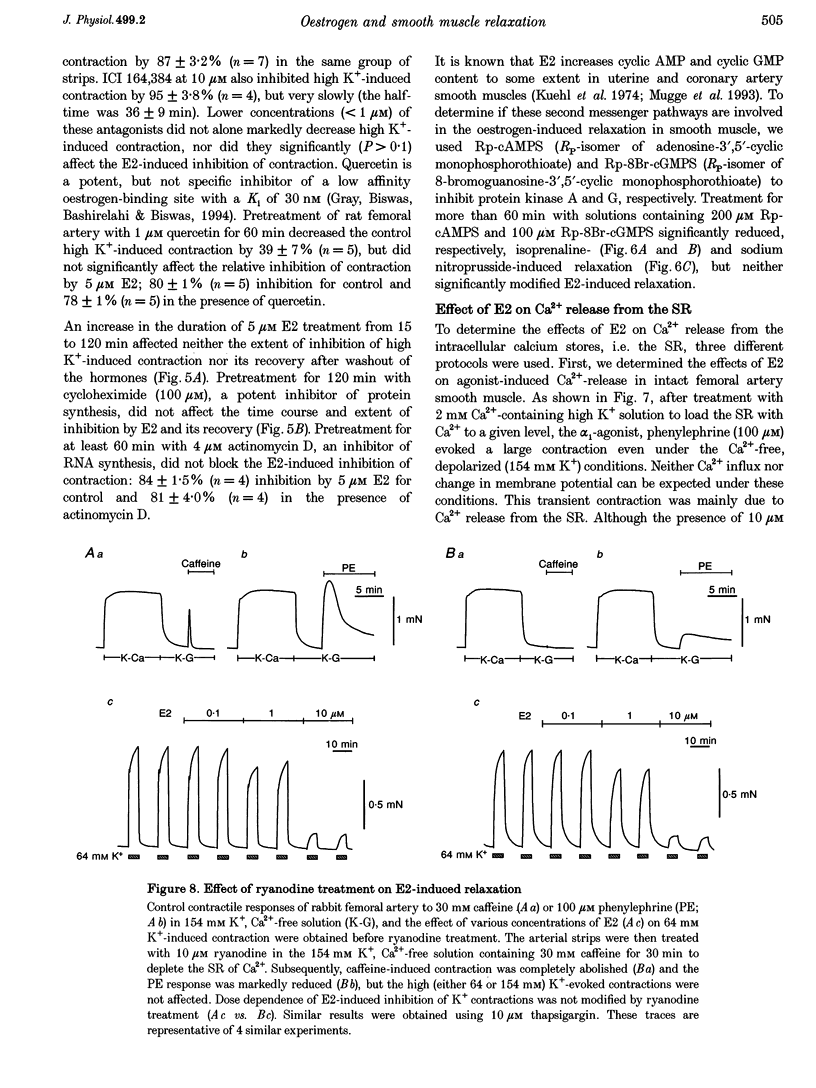
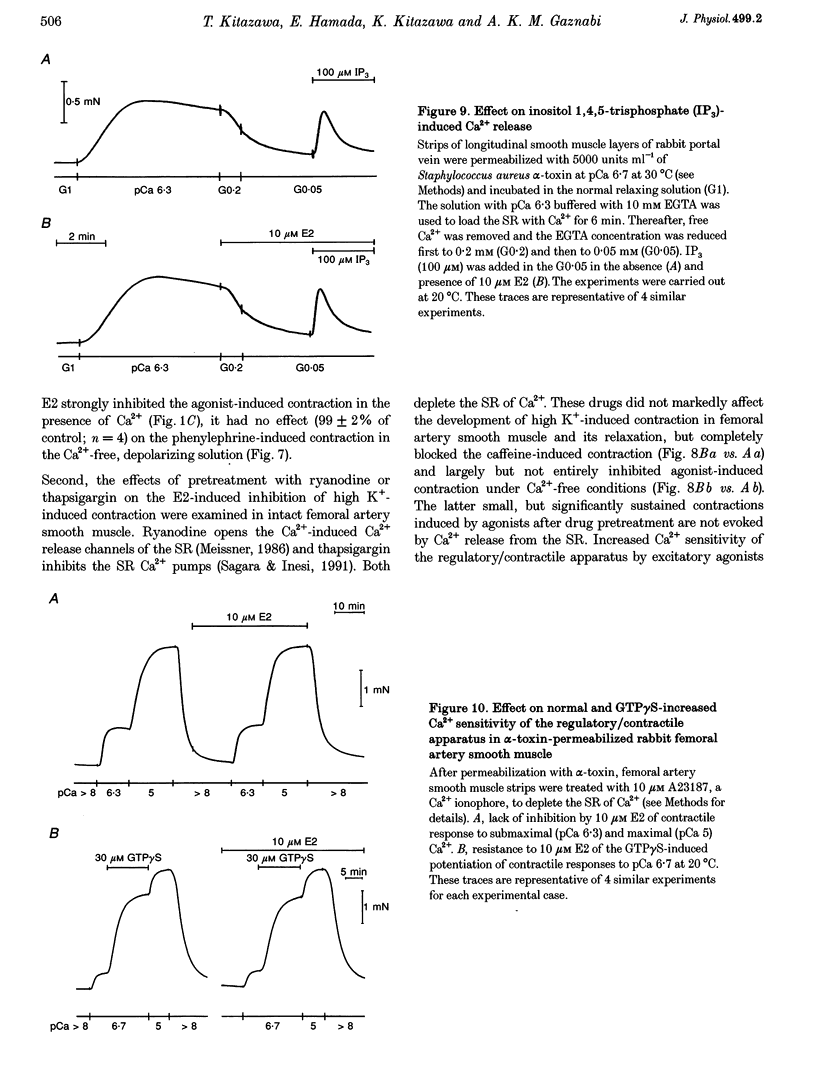
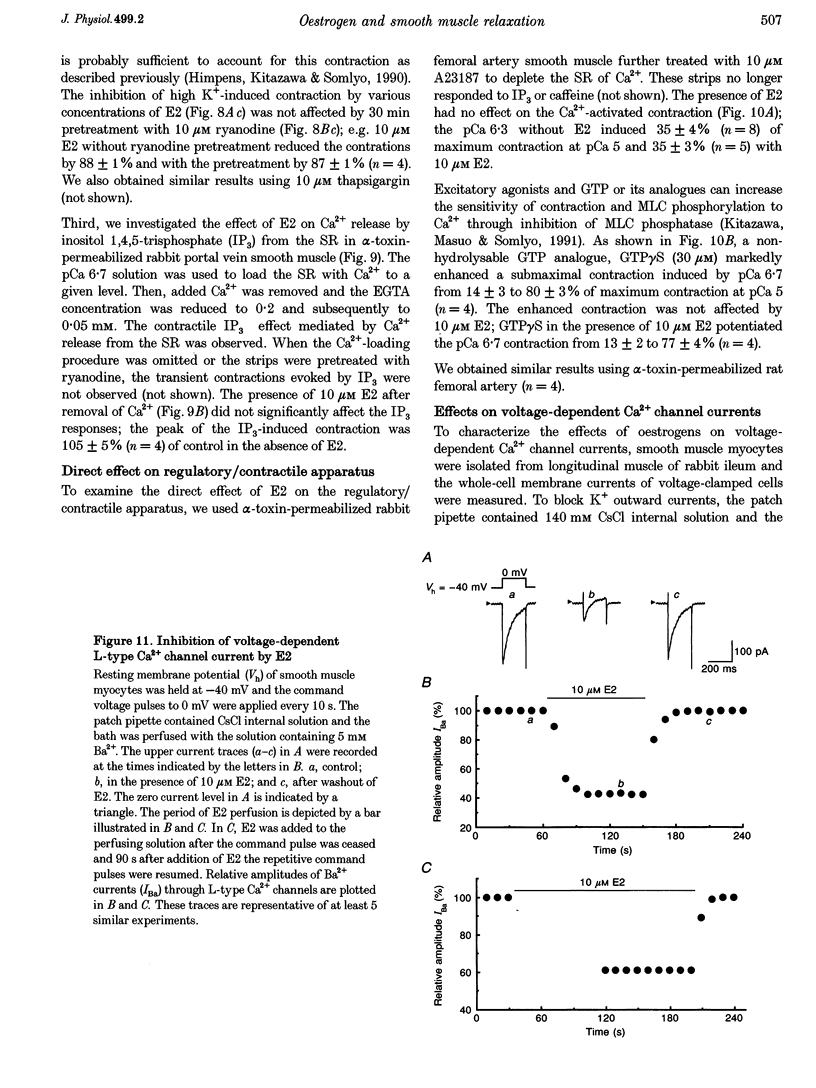
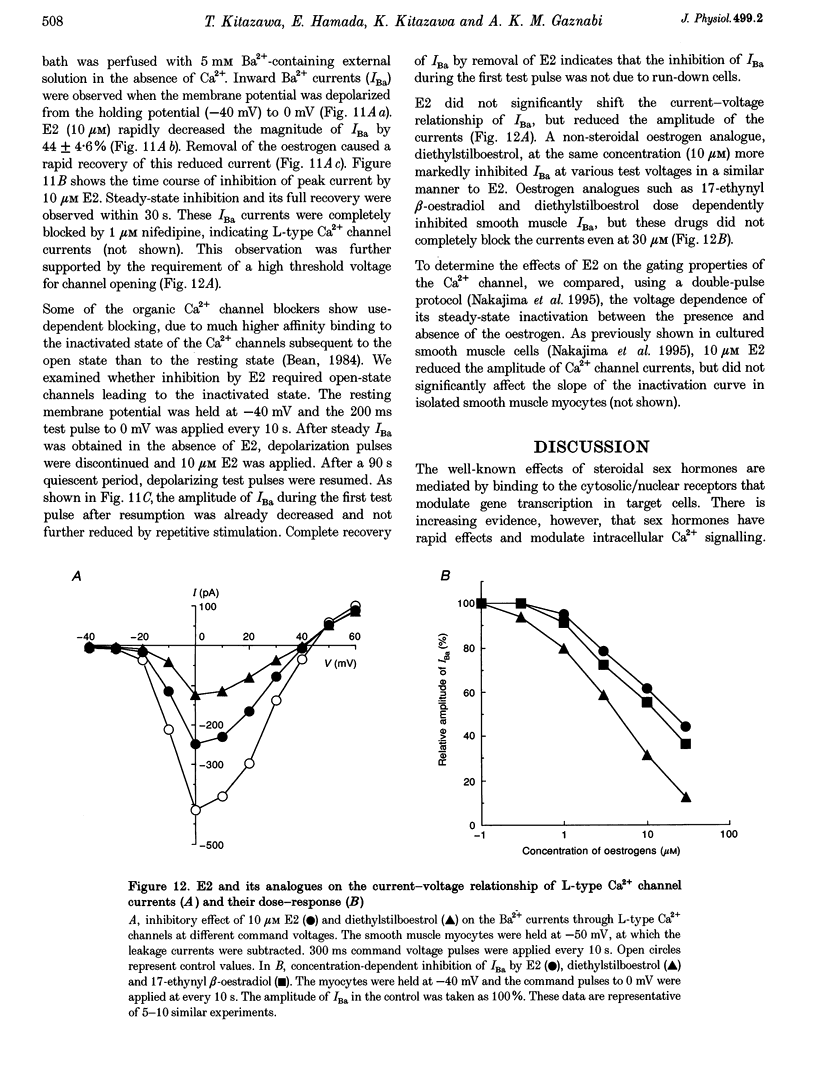
17 beta-Oestradiol (E2) at 0.1-10 microM directly inhibited various tonic and phasic smooth muscle contractions. The mechanism(s) of oestrogen-induced inhibition of contraction was studied using intact and permeabilized strips and isolated single cells of smooth muscle. 2. In endothelium-denuded vascular smooth muscle, E2 attenuated high K(+)-induced force development and myosin light chain phosphorylation, and produced rapid and reversible relaxation. There were no significant differences in these inhibitory effects between tissue types (femoral artery vs. portal vein), species (rat vs. rabbit) or sexes. 3. The inhibitory potencies of several steroidal and non-steroidal oestrogen analogues were examined and their effects were for the most part stereo-specific. However, two steroids with negligible affinities for the nuclear oestrogen receptor also strongly inhibited high K(+)-induced contraction. 4. Genomic modulators including a protein synthesis inhibitor, an RNA synthesis inhibitor, and oestrogen receptor antagonists did not affect the inhibitory actions of E2. Inhibitors of cyclic nucleotide-dependent protein kinases did not reduce the E2 effect. 5. Ca2+ release from intracellular stores by agonists and by inositol 1,4,5-trisphosphate (IP3) does not appear to be modulated by E2. Neither pretreatment with ryanodine nor with thapsigargin affected the E2-induced inhibition of high K(+)-induced contraction. 6. E2 had no effect on either normal or GTP gamma S-increased Ca2+ sensitivity of the regulatory and contractile apparatus. 7. E2 and its analogues rapidly inhibited voltage-dependent L-type Ca2+ channel currents in isolated smooth muscle cells. Repetitive stimulation was not required for E2-induced inhibition of the currents. 8. This study strongly suggests that at pharmacological concentrations oestrogen primarily reduces Ca2+ influx through inhibition of L-type Ca2+ channels in a non-genomic manner and decreases myosin light chain phosphorylation and contraction of smooth muscle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett-Connor E., Bush T. L. Estrogen and coronary heart disease in women. JAMA. 1991 Apr 10;265(14):1861–1867. [PubMed] [Google Scholar]
- Batra S., Bengtsson B. Effects of diethylstilboestrol and ovarian steroids on the contractile responses and calcium movements in rat uterine smooth muscle. J Physiol. 1978 Mar;276:329–342. doi: 10.1113/jphysiol.1978.sp012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P. F., Neulen J., Lattanzio F., Beebe S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991 Oct 5;266(28):18655–18659. [PubMed] [Google Scholar]
- Collins P., Rosano G. M., Jiang C., Lindsay D., Sarrel P. M., Poole-Wilson P. A. Cardiovascular protection by oestrogen--a calcium antagonist effect? Lancet. 1993 May 15;341(8855):1264–1265. doi: 10.1016/0140-6736(93)91158-i. [DOI] [PubMed] [Google Scholar]
- Dawson R. F., Robson J. M. The pharmacological actions of diethyl-stilboestrol and other oestrogenic and non-oestrogenic substances. J Physiol. 1939 Apr 14;95(3):420–430. doi: 10.1113/jphysiol.1939.sp003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat M. Y., Lavigne M. C., Ramwell P. W. The vascular protective effects of estrogen. FASEB J. 1996 Apr;10(5):615–624. [PubMed] [Google Scholar]
- Gray W. G., Biswas E. E., Bashirelahi N., Biswas S. B. A low-affinity estrogen-binding site in pregnant rat uteri: analysis and partial purification. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11502–11506. doi: 10.1073/pnas.91.24.11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Himpens B., Kitazawa T., Somlyo A. P. Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Arch. 1990 Sep;417(1):21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- Horiuti K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. J Physiol. 1988 Apr;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz A. R., Liu S. T. Determination of aqueous solubility and pKa values of estrogens. J Pharm Sci. 1977 May;66(5):624–627. doi: 10.1002/jps.2600660504. [DOI] [PubMed] [Google Scholar]
- Jiang C. W., Sarrel P. M., Lindsay D. C., Poole-Wilson P. A., Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991 Dec;104(4):1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Sarrel P. M., Poole-Wilson P. A., Collins P. Acute effect of 17 beta-estradiol on rabbit coronary artery contractile responses to endothelin-1. Am J Physiol. 1992 Jul;263(1 Pt 2):H271–H275. doi: 10.1152/ajpheart.1992.263.1.H271. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A. Comparative binding affinities of estrogen derivatives. Cancer Treat Rep. 1978 Aug;62(8):1243–1249. [PubMed] [Google Scholar]
- Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991 Jan 25;266(3):1708–1715. [PubMed] [Google Scholar]
- Kitazawa T., Masuo M., Somlyo A. P. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Ham E. A., Zanetti M. E., Sanford C. H., Nicol S. E., Goldberg N. D. Estrogen-related increases in uterine guanosine 3':5'-cyclic momophosphate levels. Proc Natl Acad Sci U S A. 1974 May;71(5):1866–1870. doi: 10.1073/pnas.71.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberherr M., Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994 Mar 11;269(10):7217–7223. [PubMed] [Google Scholar]
- Magness R. R., Parker C. R., Jr, Rosenfeld C. R. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993 Nov;265(5 Pt 1):E690–E698. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- Martucci C., Fishman J. Uterine estrogen receptor binding of catecholestrogens and of estetrol (1,3,5(10)-estratriene-3,15alpha,16alpha,17beta-tetrol). Steroids. 1976 Mar;27(3):325–333. doi: 10.1016/0039-128x(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Morley P., Whitfield J. F., Vanderhyden B. C., Tsang B. K., Schwartz J. L. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992 Sep;131(3):1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- Mügge A., Riedel M., Barton M., Kuhn M., Lichtlen P. R. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993 Nov;27(11):1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Kitazawa T., Hamada E., Hazama H., Omata M., Kurachi Y. 17beta-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 1995 Dec 29;294(2-3):625–635. doi: 10.1016/0014-2999(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Notides A. C. The binding affinity and specificity of the estrogen receptor of the rat uterus and anterior pituitary. Endocrinology. 1970 Nov;87(5):987–992. doi: 10.1210/endo-87-5-987. [DOI] [PubMed] [Google Scholar]
- Orimo A., Inoue S., Ikegami A., Hosoi T., Akishita M., Ouchi Y., Muramatsu M., Orimo H. Vascular smooth muscle cells as target for estrogen. Biochem Biophys Res Commun. 1993 Sep 15;195(2):730–736. doi: 10.1006/bbrc.1993.2106. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Estrogen receptors in uterine plasma membrane. J Steroid Biochem. 1979 Oct;11(4):1471–1483. doi: 10.1016/0022-4731(79)90124-9. [DOI] [PubMed] [Google Scholar]
- Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr Rev. 1990 Feb;11(1):80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991 Jul 25;266(21):13503–13506. [PubMed] [Google Scholar]
- Salas E., López M. G., Villarroya M., Sánchez-García P., De Pascual R., Dixon W. R., García A. G. Endothelium-independent relaxation by 17-alpha-estradiol of pig coronary arteries. Eur J Pharmacol. 1994 Jun 2;258(1-2):47–55. doi: 10.1016/0014-2999(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Shan J., Resnick L. M., Liu Q. Y., Wu X. C., Barbagallo M., Pang P. K. Vascular effects of 17 beta-estradiol in male Sprague-Dawley rats. Am J Physiol. 1994 Mar;266(3 Pt 2):H967–H973. doi: 10.1152/ajpheart.1994.266.3.H967. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Tesarik J., Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995 Apr;80(4):1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- Weatherill P. J., Wilson A. P., Nicholson R. I., Davies P., Wakeling A. E. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Steroid Biochem. 1988;30(1-6):263–266. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Zhang F., Ram J. L., Standley P. R., Sowers J. R. 17 beta-Estradiol attenuates voltage-dependent Ca2+ currents in A7r5 vascular smooth muscle cell line. Am J Physiol. 1994 Apr;266(4 Pt 1):C975–C980. doi: 10.1152/ajpcell.1994.266.4.C975. [DOI] [PubMed] [Google Scholar]