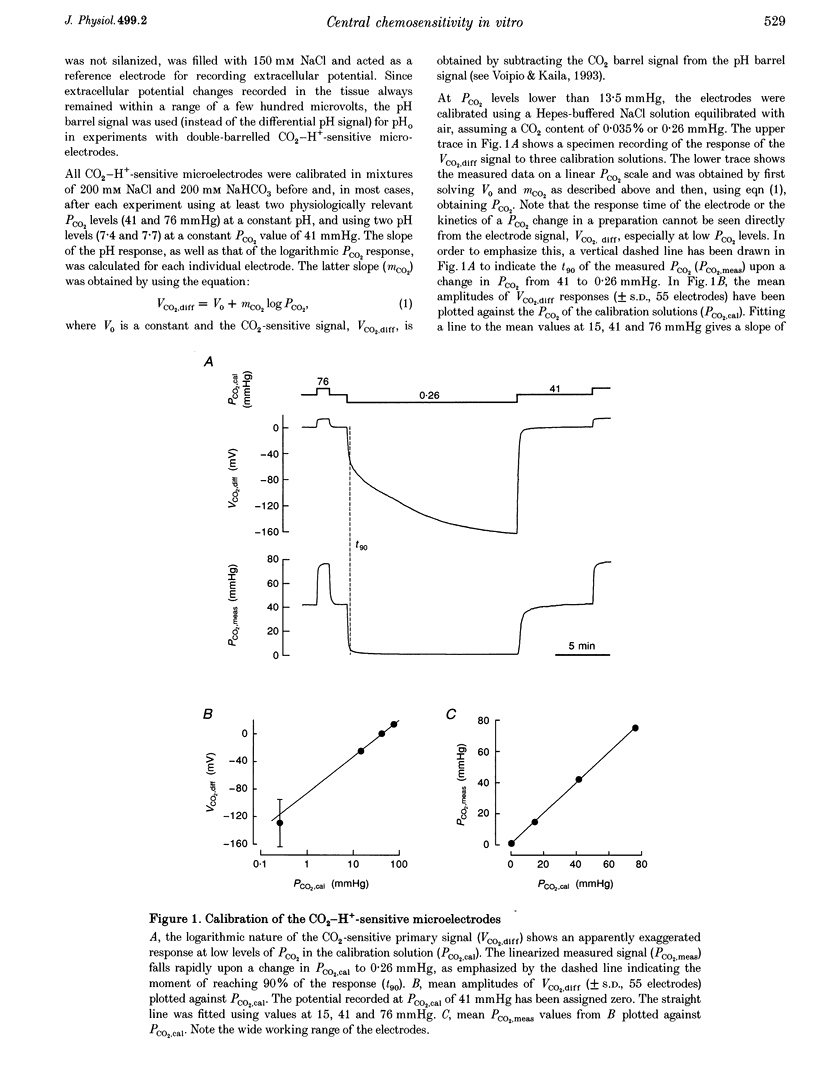
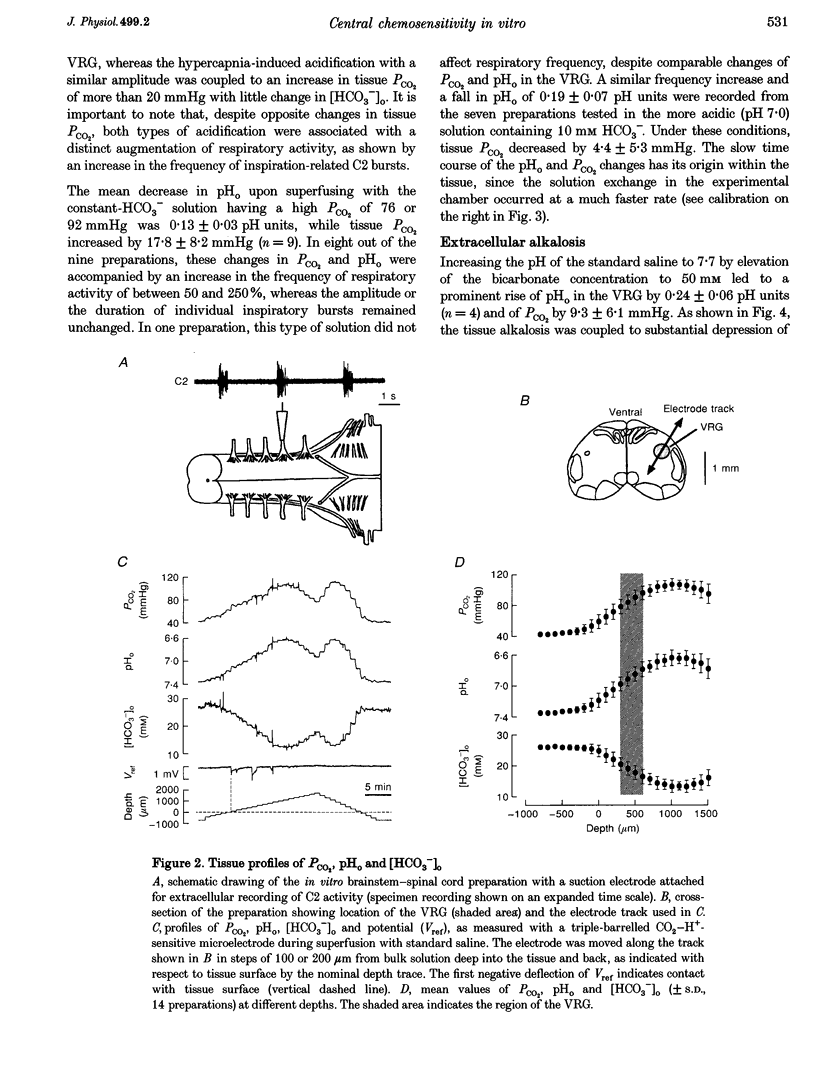
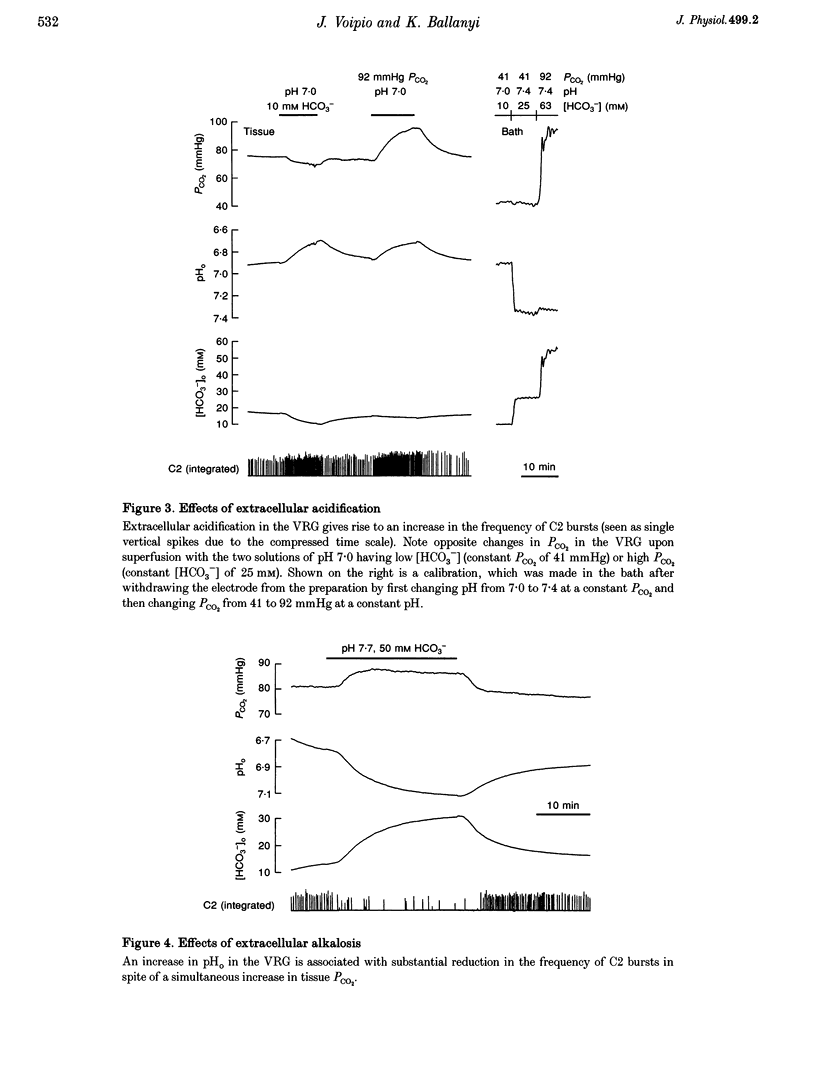
Abstract
1. CO2-H(+)-sensitive microelectrodes were used for simultaneous measurements of the partial pressure of CO2 (PCO2) and extracellular pH (pHo) in the ventral respiratory group (VRG) of the isolated brainstem-spinal cord of neonatal rats. Some of the data were analysed using diffusion equations. 2. With increasing recording depth within the boundaries of the VRG (300-600 microns below the tissue surface), PCO2 increased from 77 to 95 mmHg and pHo fell from 7.0 to 6.8 at steady state in standard saline equilibrated with 5% CO2 and 95% O2. 3. Elevating bath CO2 from 5 to 10-12.5% produced a mean increase in PCO2 of 18 mmHg, a fall in pHo of 0.13 pH units, and a 50-250% increase in the frequency of respiration-related spinal (C2) nerve bursts. Similar effects on C2 activity and pHo were observed upon lowering bath [HCO3-] from 25 to 10 mM, leading to a mean decrease in PCO2 of 4.4 mmHg in the VRG. 4. Raising bath [HCO3-] to 50 mM produced a substantial frequency decrease, a rise in pHo of 0.24 pH units and an elevation in PCO2 of 9.3 mmHg. C2 activity was not profoundly affected upon doubling the CO2-HCO3- content, leading to a mean increase in pHo of 0.13 pH units and elevation of PCO2 by 30 mmHg. 5. In a CO2-HCO3(-)-free, Hepes-buffered solution, PCO2 decreased to 18 mmHg in the VRG and pHo fell by 0.15 pH units with no major effect on rhythmic activity. Subsequent anoxic exposure for more than 15 min produced a further fall in PCO2 to below 1 mmHg, a decrease in pHo of 0.55 pH units, and blockade of respiration-related activity. In three out of the six preparations tested, C2 activity could be restored by reapplication of CO2-HCO3- in the absence of O2. 6. C2 activity persisted at a reduced frequency, even up to 30 min, during anoxia in the CO2-HCO(-)-buffered saline,leading to an elevation in PCO2 of 15 mmHg and a fall in pHo of 0.18 pH units. 7. The diffusion coefficient of CO2 in the tissue was found to be equal to that in saline. Two mean estimates for anoxic tissue of the function lambda 2/ alpha of tortuosity (lambda) and extracellular volume fraction (alpha), affecting extracellular diffusion of bicarbonate, were 4.7 and 4.1. The mean rate of acid production by anoxic tissue was 1.1 mequiv 1-1 min-1. 8. The results suggest that extracellular H+ is the primary stimulating factor in central chemosensitivity, which may often mask the less evident effects of CO2. A model of diffusion of acid equivalents in brain tissue is proposed.
Full text
PDF


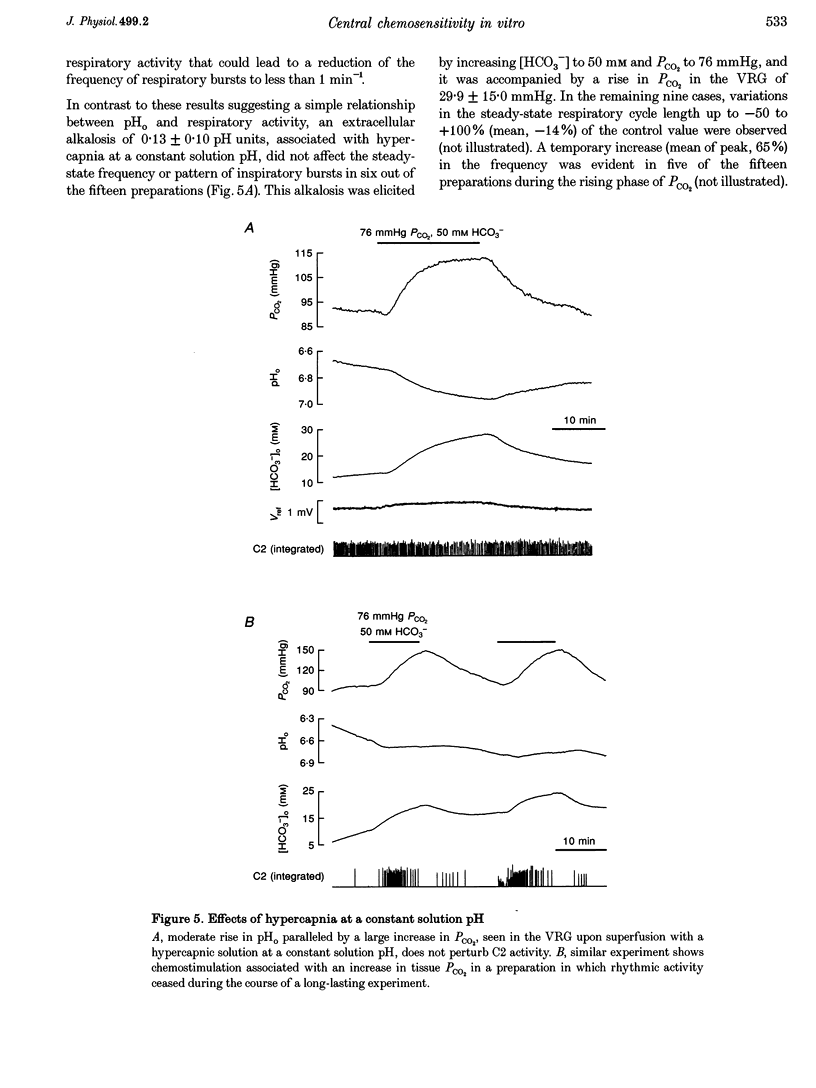






Images in this article
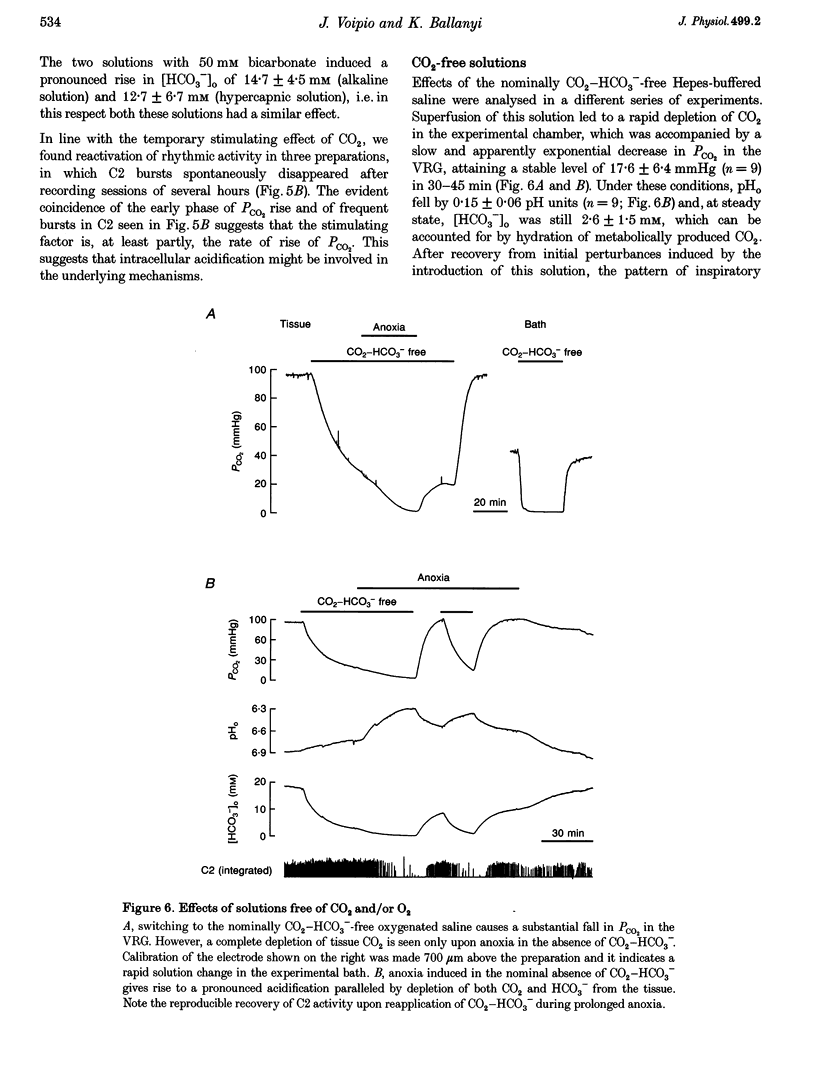
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arita H., Ichikawa K., Kuwana S., Kogo N. Possible locations of pH-dependent central chemoreceptors: intramedullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res. 1989 Apr 24;485(2):285–293. doi: 10.1016/0006-8993(89)90572-6. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Völker A., Richter D. W. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. Neuroreport. 1994 Dec 30;6(1):165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Völker A., Richter D. W. Functional relevance of anaerobic metabolism in the isolated respiratory network of newborn rats. Pflugers Arch. 1996 Aug;432(4):741–748. doi: 10.1007/s004240050193. [DOI] [PubMed] [Google Scholar]
- Brockhaus J., Ballanyi K., Smith J. C., Richter D. W. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J Physiol. 1993 Mar;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bômont L., Bilger A., Boyet S., Vert P., Nehlig A. Acute hypoxia induces specific changes in local cerebral glucose utilization at different postnatal ages in the rat. Brain Res Dev Brain Res. 1992 Mar 20;66(1):33–45. doi: 10.1016/0165-3806(92)90137-l. [DOI] [PubMed] [Google Scholar]
- Chesler M., Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992 Oct;15(10):396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
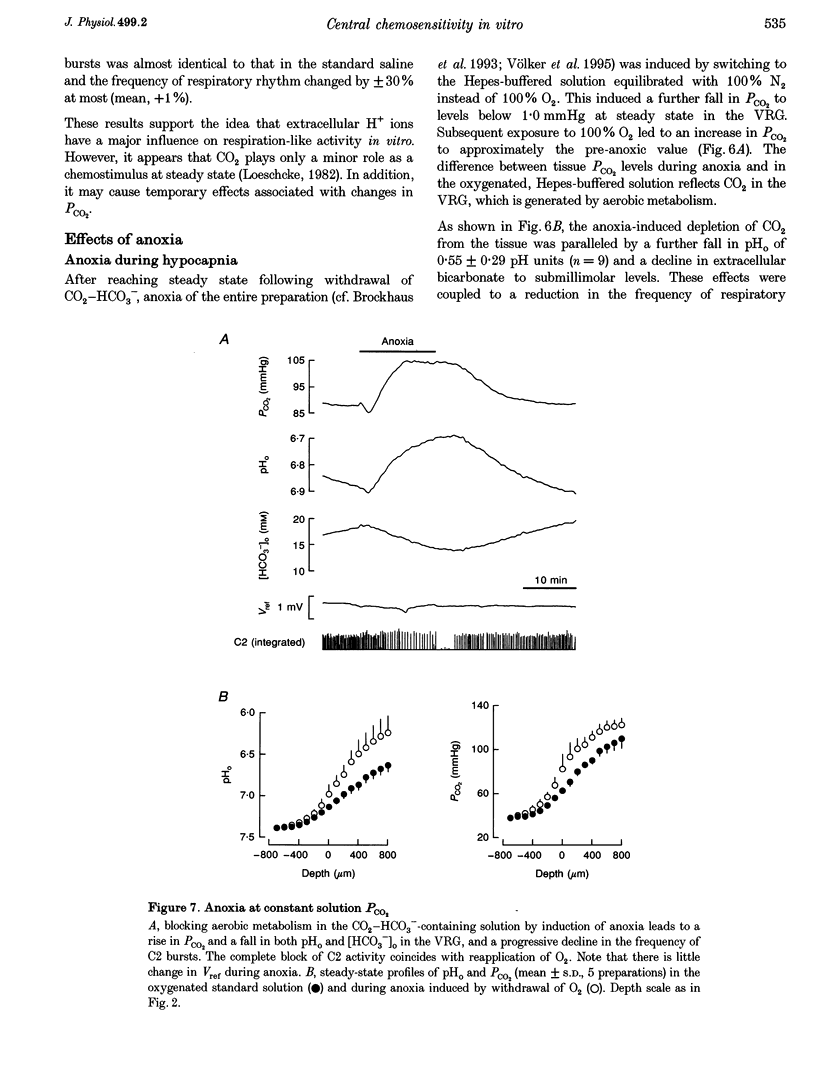
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- Coates E. L., Li A., Nattie E. E. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol (1985) 1993 Jul;75(1):5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Dean J. B., Lawing W. L., Millhorn D. E. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76(3):656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Duffy T. E., Kohle S. J., Vannucci R. C. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1975 Feb;24(2):271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Gros G., Moll W. Facilitated diffusion of CO2 across albumin solutions. J Gen Physiol. 1974 Sep;64(3):356–371. doi: 10.1085/jgp.64.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
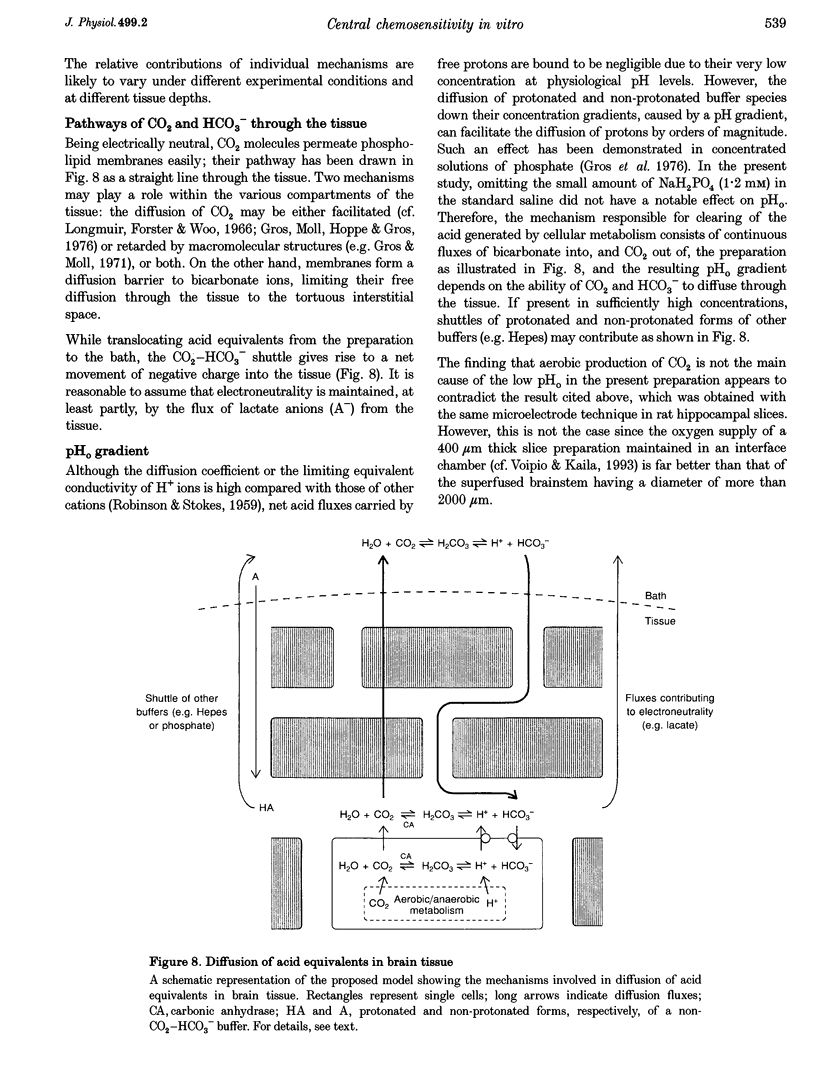
- Gros G., Moll W., Hoppe H., Gros H. Proton transport by phosphate diffusion--a mechanism of facilitated CO2 transfer. J Gen Physiol. 1976 Jun;67(6):773–790. doi: 10.1085/jgp.67.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros G., Moll W. The diffusion of carbon dioxide in erythrocytes and hemoglobin solutions. Pflugers Arch. 1971;324(3):249–266. doi: 10.1007/BF00586422. [DOI] [PubMed] [Google Scholar]
- Grote J., Zimmer K., Schubert R. Effects of severe arterial hypocapnia on regional blood flow regulation, tissue PO2 and metabolism in the brain cortex of cats. Pflugers Arch. 1981 Sep;391(3):195–199. doi: 10.1007/BF00596170. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Hansen A. J. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985 Jan;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kuno M., Wang Y. Z. Differential effects of carbon dioxide and pH on central chemoreceptors in the rat in vitro. J Physiol. 1985 Nov;368:679–693. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa F. G., Remmers J. E. Identification of a subsurface area in the ventral medulla sensitive to local changes in PCO2. J Appl Physiol (1985) 1992 Feb;72(2):439–446. doi: 10.1152/jappl.1992.72.2.439. [DOI] [PubMed] [Google Scholar]
- John W. M., Wang S. C. Response of medullary respiratory neurons to hypercapnia and isocapnic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):812–821. doi: 10.1152/jappl.1977.43.5.812. [DOI] [PubMed] [Google Scholar]
- Kaila K., Paalasmaa P., Taira T., Voipio J. pH transients due to monosynaptic activation of GABAA receptors in rat hippocampal slices. Neuroreport. 1992 Jan;3(1):105–108. doi: 10.1097/00001756-199201000-00028. [DOI] [PubMed] [Google Scholar]
- Kawai A., Ballantyne D., Mückenhoff K., Scheid P. Chemosensitive medullary neurones in the brainstem--spinal cord preparation of the neonatal rat. J Physiol. 1996 Apr 1;492(Pt 1):277–292. doi: 10.1113/jphysiol.1996.sp021308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmenkühler A., Syková E., Svoboda J., Zilles K., Nicholson C. Extracellular space parameters in the rat neocortex and subcortical white matter during postnatal development determined by diffusion analysis. Neuroscience. 1993 Jul;55(2):339–351. doi: 10.1016/0306-4522(93)90503-8. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. The general physiology of reactions catalyzed by carbonic anhydrase and their inhibition by sulfonamides. Ann N Y Acad Sci. 1984;429:568–579. doi: 10.1111/j.1749-6632.1984.tb12389.x. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol (1985) 1986 Oct;61(4):1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Phillips J. M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol. 1981 Dec;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C., Rice M. E. The migration of substances in the neuronal microenvironment. Ann N Y Acad Sci. 1986;481:55–71. doi: 10.1111/j.1749-6632.1986.tb27139.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Mückenhoff K., Holtermann G., Acker H., Scheid P. Depth profiles of pH and PO2 in the isolated brain stem-spinal cord of the neonatal rat. Respir Physiol. 1993 Sep;93(3):315–326. doi: 10.1016/0034-5687(93)90077-n. [DOI] [PubMed] [Google Scholar]
- Okada Y., Mückenhoff K., Scheid P. Hypercapnia and medullary neurons in the isolated brain stem-spinal cord of the rat. Respir Physiol. 1993 Sep;93(3):327–336. doi: 10.1016/0034-5687(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syková E., Svoboda J., Polák J., Chvátal A. Extracellular volume fraction and diffusion characteristics during progressive ischemia and terminal anoxia in the spinal cord of the rat. J Cereb Blood Flow Metab. 1994 Mar;14(2):301–311. doi: 10.1038/jcbfm.1994.37. [DOI] [PubMed] [Google Scholar]
- Trapp S., Lückermann M., Brooks P. A., Ballanyi K. Acidosis of rat dorsal vagal neurons in situ during spontaneous and evoked activity. J Physiol. 1996 Nov 1;496(Pt 3):695–710. doi: 10.1113/jphysiol.1996.sp021720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voipio J., Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2/H(+)-sensitive microelectrode based on a PVC-gelled membrane. Pflugers Arch. 1993 May;423(3-4):193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]
- Voipio J., Paalasmaa P., Taira T., Kaila K. Pharmacological characterization of extracellular pH transients evoked by selective synaptic and exogenous activation of AMPA, NMDA, and GABAA receptors in the rat hippocampal slice. J Neurophysiol. 1995 Aug;74(2):633–642. doi: 10.1152/jn.1995.74.2.633. [DOI] [PubMed] [Google Scholar]
- Völker A., Ballanyi K., Richter D. W. Anoxic disturbance of the isolated respiratory network of neonatal rats. Exp Brain Res. 1995;103(1):9–19. doi: 10.1007/BF00241960. [DOI] [PubMed] [Google Scholar]