Abstract
Peptides derived from the heptad repeats of human immunodeficiency virus (HIV) gp41 envelope glycoprotein, such as T20, can efficiently inhibit HIV type 1 (HIV-1) entry. In this study, replication of HIV-1 was inhibited more than 100-fold in a T-helper cell line transduced with a retrovirus vector expressing membrane-anchored T20 on the cell surface. Inhibition was independent of coreceptor usage.
Despite aggressive combination therapy in human immunodeficiency virus (HIV) infection, latently infected cells persist and viral rebound is observed after prolonged treatment. Moreover, many patients are intolerant of the available drugs (3, 7, 17). Several gene therapeutic strategies have been proposed that could improve the therapy for HIV infection (25, 27). However, in most approaches, virus production from an integrated provirus is inhibited, whereas only a few antiviral gene products described so far, such as single-chain antibodies to HIV integrase, inhibit steps prior to retrovirus integration (18).
HIV replication is initiated by binding of the virus envelope glycoprotein gp120 to the CD4 receptor and a coreceptor on the target cell. The gp41 subunit of the HIV envelope glycoprotein then plays a key role in virus entry by mediating fusion of the viral lipid membrane and the plasma membrane of the target cell. In crystallographic studies, heptad repeat sequences were shown to form a coiled-coil structure during the conformational change of gp41 that is crucial for HIV membrane fusion (2, 28). Peptides derived from one of the heptad repeats most likely interact with this coiled coil, thereby locking gp41 in a fusion-incompetent conformation and inhibiting HIV type 1 (HIV-1) entry (30).
A potent peptide inhibitor is T20 (formerly DP178), which overlaps with the C-terminal heptad repeat and inhibits HIV infection at concentrations of less than 2 ng/ml (8). In a recent clinical study, short-term administration of T20 to HIV-infected patients was found to be safe and to potently suppress viremia (13). However, very large amounts of the peptide are required to achieve an antiviral effect. The peptides are not orally bioavailable and have an extremely short half-life, and large-scale production is expensive (5). The aim of the present study was to overcome these drawbacks by expressing the inhibitory peptide in the target cell of HIV-1 infection.
Design of retrovirus vectors.
Six retrovirus vectors designed to express secreted (M85 and M86) or membrane-bound (M87) T20 were constructed. The T20 sequences were cloned 5′ to an internal ribosome entry site (IRES)-neo cassette in retrovirus vector MPIN (Fig. 1). In M85, T20 was expressed as a fusion protein behind the signal peptide (SP) of the human low-affinity nerve growth factor receptor (LNGFR). In M86, the sequence coding for peptide RGD was introduced in frame between the SP and T20. The RGD motif binds integrins and thereby could increase local concentrations of T20 on the cell surface by trapping secreted peptides on the cell membrane. The LNGFR SP was amplified by PCR from the vector dLN, using the primers SPNOT+ (5′-gcggccgccatgggggcaggtgccaccggc-3′) and SPBgl− (5′-agatctggcacctccaagggacacccccag-3′) to introduce a NotI site at the 5′ end and a BglII site at the 3′ end (6). The sequence coding for T20 was amplified from NL4–3, using the primers T20Bgl+ (M85; 5′-agatcttacactagcttaatacactcctta-3′) or T20Bgl+RGD+ (M86; 5′-agatctagaggcgactacactagcttaatacactcctta-3′) and T20Hind− (5′-aagcttattaaaaccaattccaca aacttgccc-3′) to introduce a BglII site at the 5′ end and a HindIII site at the 3′ end. The fragments were ligated via the NotI and HindIII sites into pBluescript KS.
FIG. 1.
(A) Maps of retroviral vectors. The SP and the MSD are derived from the LNGFR. The hinge is derived from the IgG heavy chain. (B) Amino acid sequence of the membrane-anchored T20 expressed by M87. (C) Amino acid sequence of the SP and the T20 region in the mutated proteins expressed from M87m1 through M87m3.
In the construct M87, membrane-bound T20 was expressed as a fusion protein with the hinge region from the immunoglobulin G (IgG) heavy chain and the membrane-spanning domain (MSD) from the LNGFR. The MSD was amplified from dLN by using a 5′ primer that also contained the sequence coding for the hinge of the murine IgG heavy chain and a BglII site (hingeTMBgl+; 5′-agatctgttccaagagactgtggatgcaaaccctgtatatgtaccctcatccctgtctattgctccatcctggct gctg-3′) and a 3′ primer (U3−; 5′-cgcgcgaacagaagcgagaag-3′) (6). This PCR product was introduced together with the SP PCR product into pBluescript (NotI × BglII × HindIII), and the sequence for T20 was then introduced as a PCR product flanked by BglII sites (primers T20Bgl+ and T20Bgl−; 5′-agatctaaaccaattccacaaacttgccc-3′).
The genes coding for the different T20 fusion proteins were transferred as NotI-HindIII fragments from pBluescript together with the poliovirus IRES from SF1 MIN into MPIN (12).
In addition, mutations were introduced into M87 by site-directed mutagenesis (Fig. 1C). In mutant M87m1, the four C-terminal amino acids of the T20 peptide (WNWF) were mutated to ANAA. T20 peptides with these substitutions have been shown to be inactive (16). Two adjacent sequences in the coding region of M87 were amplified by PCR by using the following primers: PCR A, Af 5′-ttgtacaccctaagcctccgc-3′ and Ar 5′-cagatctagccgcattcgccaaacttgccc-3′; PCR B, Bf 5′-ttggcgaatgcggctagatctgttccaagagactgtggatgc-3′ and Br 5′-atggccgatcccatattggc-3′. The PCR products of PCR A and B were used as a template for PCR C, with the following primers: PCR C, Cf 5′-ttatccagccctcactccttc-3′ and Cr 5′-gagcggccgcaatccaattcgc-3′. The PCR C product was cloned into EcoRV of pBluescript and then introduced as a NotI fragment into M87 from which the original NotI fragment was removed. Three plasmid clones were sequenced. M87m1 had only the expected mutations. M87m2 had an additional G to A mutation in the fourth codon, resulting in a substitution of aspartic acid for glycine in the SP. M87m2 had an additional G to A mutation in the seventh codon, leading again to a substitution of aspartic acid for glycine. These additional mutations were most likely introduced by PCR.
Surface expression of membrane-anchored T20.
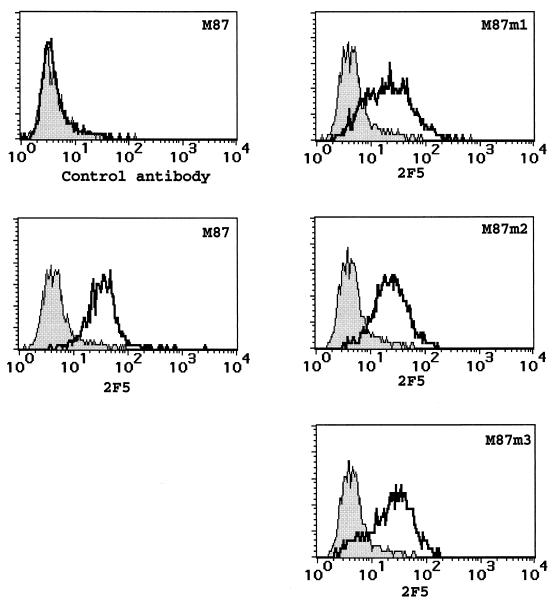
Retrovirus vectors were packaged by transfection of Phoenix packaging cells, and supernatants were used to transduce the T-helper cell line PM-1 (10, 19). As a control, the vector MPIN containing only neo was used. After G418 selection, PM-1/MPIN, PM-1/M87, and PM-1/M87m1–3 were stained with a human monoclonal antibody directed against a motif in gp41 (ELDKWA) that was present in T20 (2F5) and analyzed on a flow cytometer (FACScalibur; Becton Dickinson, Heidelberg, Germany). As a control, a human monoclonal antibody directed against HIV-1 gp120 was used (2G12). Both antibodies have been described in detail (1). The control antibody showed a similar level of background staining for PM-1/MPIN and PM-1/M87 (Fig. 2). In contrast, the anti-T20 antibody stained PM-1/M87 significantly. Interestingly, T20 could be detected on the cell surface of all M87 mutants, although in mutants M87m2 and M87m3 amino acid substitutions were present in the SP.
FIG. 2.
Expression of membrane-anchored T20. PM-1 cells were transduced with the retroviral vectors indicated and selected. The bulk cultures were stained either with a control human monoclonal antibody directed against gp120 (2G12) or with a human monoclonal antibody directed against a sequence within T20 (2F5) followed by a PE-conjugated anti-human IgG goat serum. Shaded curve, PM-1/MPIN; solid line, PM-1 transduced with the vector indicated.
Membrane-anchored T20 inhibits HIV-1 replication.
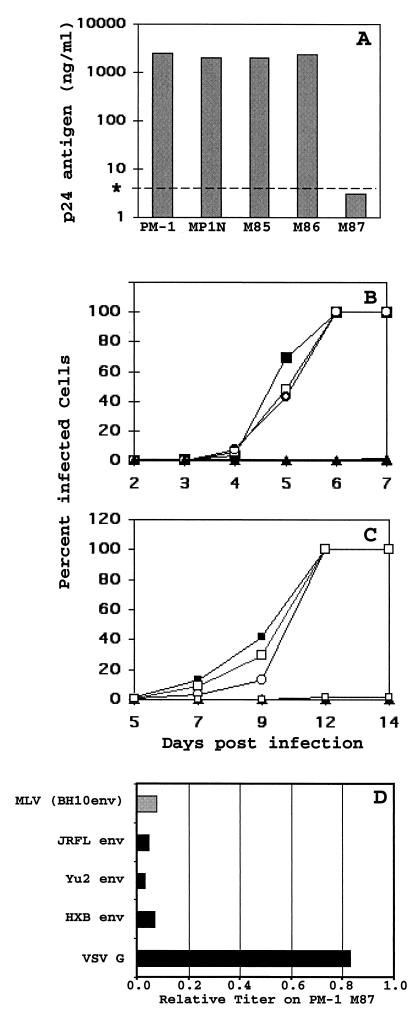
The different PM-1 cell lines were infected with HIV-1 produced from the proviral clone NL4–3 or NL4–3AGFP, at a multiplicity of infection of 0.1 50% tissue culture infective dose. In the latter virus, green fluorescent protein (GFP) was fused to the anchor domain of Nef, so that HIV-1 replication could be monitored by analysis of p24 antigen production as well as by flow cytometry (29). NL4–3 p24 antigen was measured in the culture supernatants as soon as a cytopathic effect became evident in the control cells (day 6 in the experiment shown in Fig. 3A) by enzyme-linked immunosorbent assay, as described previously (9, 15). Production of p24 was inhibited more than 500-fold in PM-1/M87. If the cultures were monitored further, NL4–3 finally broke through and a cytopathic effect became evident between days 14 to 20. Spread of NL4–3AGFP was monitored by flow cytometry (Fig. 3B). On day 6, when 100% of the control cells were GFP positive, less than 1% of the PM-1/M87 cells expressed GFP. Therefore, replication of NL4–3AGFP was reduced by more than 2 orders of magnitude by M87. The constructs designed to express secreted T20 (M85 and M86) had no antiviral activity (Fig. 3A and B). Most likely, the level of expression or secretion of the peptide was too low to achieve inhibitory concentrations. However, secreted peptide would be a highly attractive alternative to the membrane-anchored T20 for gene therapy applications, because of a possible bystander effect.
FIG. 3.
Inhibition of HIV-1 replication by membrane-anchored T20. (A) PM-1 cells were transduced with different retroviral vectors encoding T20 and then selected with G418. These bulk cultures were infected with NL4–3 at a multiplicity of infection of 0.1. On day 5, medium was replaced and the concentration of p24 antigen was determined in supernatants collected on day 6. ∗, detection limit. (B) In parallel, the selected PM-1 cultures were infected with NL4–3AGFP and the spread of virus was monitored by flow cytometry. × M85; □ M86; ▴ M87; ○ MPIN. (C) Selected cultures were infected with NL4–3AGFP, and the spread of virus was monitored by flow cytometry. ▴ M87; ○ MPIN; □ M87m1; ▪ M87m2; □ M87m3. (D) Inhibition of a single round of infection by membrane-anchored T20. PM-1 transduced with the control vector MPIN or the vector M87 expressing the membrane-anchored T20 was infected with fivefold dilutions of a replication-deficient virus NL4–3env−GFP pseudotyped either with VSV G protein or an HIV-1 envelope glycoprotein from one of the HIV strains depicted (solid bars). Cells were also transduced with an MLV vector with EGFP as a marker gene pseudotyed with the envelope glycoprotein of HIV-1 strain BH10 (shaded bar). Titers of different pseudotypes were determined on day 3 by flow cytometry for each of the PM-1 cultures. Titers on PM-1/M87 relative to the titer on PM-1/MPN are given.
We found that conditioned medium from cells expressing M87 did not inhibit HIV infection (data not shown). This indicates that the peptide inhibits infection while anchored to the target cell membrane and not after being shed into the supernatant. Interestingly, the peptide is anchored to the target membrane by a very short linker of only 13 amino acids. To interact with the membrane-anchored T20, the N-terminal coiled coil of gp41 must come very close to the target cell membrane, which would predict an elongated intermediate conformation of gp41.
Amino acid substitutions in the C-terminal domain of T20 inactivate the free peptide (16). Mutant M87m1, containing three amino acid substitutions in the C terminus of T20, also inhibited spread of NL4–3AGFP to the same degree as M87 (Fig. 3C). This region of T20 is not known to interact directly with the central gp41 coiled coil, and it is not clear why it is essential for efficient inhibition (2, 28). It is, therefore, not absolutely unexpected that when anchored to the cell membrane, the mutated T20 expressed by M87m1 is still active. On the other hand, an additional mutation in the N-terminal hydrophilic domain of the SP completely abolished the inhibitory activity of M87 (Fig. 3C, M87m2 and M87m3). Since the LNGFR SP does not have an inhibitory effect on HIV-1 replication itself (14), the effect of the SP mutations is most likely indirect. Charged amino acids in this domain are known to interfere with the translocation of proteins into the endoplasmic reticulum and favor type II orientation (20). A possible explanation for the lack of antiviral activity of M87m2 and M87m3 is that the N terminus of the proteins expressed is not translocated into the endoplasmic reticulum lumen completely.
Further, HIV-1 and HIV-2 isolates were tested on PM-1/MPIN and PM-1/M87 (World Health Organization primary isolates panel). The production of p24 in PM-1/M87 was inhibited by 95% for a subtype B HIV-1 isolate (BaL), 74% for a subtype D HIV-1 isolate (92UG35), 82% for a subtype E isolate (92TH22), 60% for a subtype O isolate (MVP2171), 87% for an HIV-2 isolate (prCBL23), and 64% for a different HIV-2 isolate (CBL23; tissue culture, laboratory adapted).
Cells were stained with anti-CD4, anti-CCR5, anti-CXCR4, and an isotype control antibody (Pharmingen, Hamburg, Germany). The level of expression for these surface proteins did not differ significantly between cells expressing membrane-anchored T20 (PM-1/M87) and the control containing neo vector only (PM-1/MPIN; data not shown). These results show that resistance of PM-1/M87 to HIV-1 infection is not simply explained by a lack of receptor or coreceptor.
To exclude overall toxicity of the membrane-anchored T20, growth curves were determined for PM-1/M87 and, as a control, PM-1/MPIN. The growth rates for these two cell lines did not differ significantly (data not shown).
M87 inhibits entry of HIV-1.
To determine the level at which HIV-1 replication was inhibited by M87, single-round infections were performed with the HIV clone NL4–3env−GFP. This HIV-1 clone is replication defective, due to a mutation in the env gene, and expresses GFP instead of nef. To produce infectious virus, NL4–3env−GFP was pseudotyped with Env proteins from three different HIV-1 clones (HXB, Yu2, and JRFL), as well as with the G protein of vesicular stomatitis virus (VSV-G), as described previously (11). HXB is classified as T-cell tropic with CXCR4 coreceptor usage, while Yu2 and JR-FL are macrophage tropic and use CCR5. The latter two Env proteins were cloned directly from primary HIV-1 isolates (11). In PM-1/M87 cells, infection via the three different HIV envelopes was inhibited more than 20-fold, while no significant inhibition was seen for infection with the VSV-G pseudotype (Fig. 3D). Similar to the results with free peptide, membrane-anchored T20 inhibited entry independently of coreceptor usage (8).
In addition, PM-1/M87 and PM-1/MPIN were transduced with a murine leukemia virus (MLV) vector pseudotyped with an HIV-1 envelope (24). This vector was produced with FLY cells (4) that express MLV gag and pol genes, the retrovirus vector pMX-EGFP (21), and the carboxyl-truncated version of HIV-1 BH10 Env. Transduction with this vector was also more than 20-fold less efficient for cells expressing membrane-anchored T20 than for control cells (Fig. 3D). These results clearly show that membrane-anchored T20 inhibits virus replication at the level of HIV Env-mediated entry, most likely membrane fusion, and that postentry events of HIV-1 replication were not affected.
A problem with all protein-based intracellular immunization strategies is possible immunogenicity. A cytotoxic T-lymphocyte (CTL) response to membrane-anchored T20 could be induced, although T20 does not overlap with dominant CTL epitopes in gp41 (26). In addition, antibodies may arise that neutralize the inhibitory effect of T20 or even lead to the elimination of genetically engineered cells by antibody-dependent cell lysis. However, such an immune response is not expected to have major adverse effects, as an anti-T20 immune response could help control HIV replication. In stem cell-based gene therapy, immunologic tolerance to the M87 gene product would be induced.
An advantage of entry inhibitors that target the heptad repeats of gp41 is the relatively low variability within these regions. In this study, HIV-1 strains were thus inhibited independently of coreceptor usage. Emergence of resistance is expected to be slow; however, HIV mutants resistant to T20 have been isolated in cell culture (23). In addition, across subtypes, the level of inhibition in this study did decrease with divergence in the amino acid sequence of the C-terminal heptad repeat. Inhibition was highest for subtype B HIV-1 NL4–3, from which M87 was derived, intermediate for the other subtype B isolates, and lowest for the non-B subtypes and for HIV-2. Within the 36 amino acids from the T20 region, JRFL, Yu2, and BaL (B subtypes) differ in only 3 amino acids from NL4–3, each. Subtype D differs in 5 amino acids, the subtype E isolate differs in 10 amino acids, the group O isolate differs in 16 amino acids, and the HIV-2 isolate (prCBL23) differs in 19 amino acids.
Entry inhibitors, such as membrane-anchored T20, have several advantages over gene products that suppress virus production from the integrated HIV provirus. Infected cells protected by the latter principle resemble latently infected cells and can escape immune surveillance. Moreover, many antiviral genes that inhibit HIV-1 gene expression still allow expression of early, potentially pathogenic viral genes such as tat. However, similar to drug therapy, a combination of antiviral genes is expected be most effective and, in addition, would prevent the emergence of resistant virus mutants.
Acknowledgments
We thank S. Roscher for excellent technical assistance.
M.H. is supported by a grant from the José Carreras Leukämie Stiftung. M.T.D. has a fellowship from the Bundesministerium für Bildung und Forschung. The Heinrich-Pette-Institut is supported by the Freie und Hansestadt Hamburg.
REFERENCES
- 1.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 2.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 3.Chun T-W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 4.Cosset F L, Takeuchi Y, Battini J L, Weiss R A, Collins M K. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert D M, Malashkevich V N, Hong L H, Carr P A, Kim P S. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell. 1999;99:103–115. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 6.Fehse B, Uhde A, Fehse N, Eckert H G, Clausen J, Ruger R, Koch S, Ostertag W, Zander A R, Stockschlader M. Selective immunoaffinity-based enrichment of CD34+ cells transduced with retroviral vectors containing an intracytoplasmatically truncated version of the human low-affinity nerve growth factor receptor (deltaLNGFR) gene. Hum Gene Ther. 1997;8:1815–1824. doi: 10.1089/hum.1997.8.15-1815. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Furuta R A, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 9.Grattinger M, Hohenberg H, Thomas D, Wilk T, Muller B, Krausslich H G. In vitro assembly properties of wild-type and cyclophilin-binding defective human immunodeficiency virus capsid proteins in the presence and absence of cyclophilin A. Virology. 1999;257:247–260. doi: 10.1006/viro.1999.9668. [DOI] [PubMed] [Google Scholar]
- 10.Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Lanfrancone L, Peschle C, Nolan G P, Pelicci P G. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- 11.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 12.Hildinger M, Fehse B, Hegewisch-Becker S, John J, Rafferty J, Ostertag W, Baum C. Dominant selection of human progenitor cells with retroviral mdr-1-coexpression vectors. Hum Gene Ther. 1998;9:33–42. doi: 10.1089/hum.1998.9.1-33. [DOI] [PubMed] [Google Scholar]
- 13.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 14.Klebba C, Ottmann O G, Scherr M, Pape M, Engels J W, Grez M, Hoelzer D, Klein S A. Retrovirally expressed anti-HIV ribozymes confer a selective survival advantage on CD4+ T cells in vitro. Gene Ther. 2000;7:408–416. doi: 10.1038/sj.gt.3301094. [DOI] [PubMed] [Google Scholar]
- 15.Konvalinka J, Litterst M A, Welker R, Kottler H, Rippmann F, Heuser A M, Krausslich H G. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. J Virol. 1995;69:7180–7186. doi: 10.1128/jvi.69.11.7180-7186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawless K M, Barney S, Guthrie K I, Bucy T B, Petteway S R, Jr, Merutka G. HIV-1 membrane fusion mechanism: structural studies of the interactions between biologically-active peptides from gp41. Biochemistry. 1996;35:13697–13708. doi: 10.1021/bi9606962. [DOI] [PubMed] [Google Scholar]
- 17.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R the Swiss HIV Cohort Study. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 18.Levy-Mintz P, Duan L, Zhang H, Hu B, Dornadula G, Zhu M, Kulkosky J, Bizub-Bender D, Skalka A M, Pomerantz R J. Intracellular expression of single-chain variable fragments to inhibit early stages of the viral life cycle by targeting human immunodeficiency virus type 1 integrase. J Virol. 1996;70:8821–8832. doi: 10.1128/jvi.70.12.8821-8832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 19.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM-1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martoglio B, Dobberstein B. Signal sequences: more than just greasy peptides. Trends Cell Biol. 1998;8:410–415. doi: 10.1016/s0962-8924(98)01360-9. [DOI] [PubMed] [Google Scholar]
- 21.Onishi M, Nosaka T, Misawa K, Mui A L, Gorman D, McMahon M, Miyajima A, Kitamura T. Identification and characterization of a constitutively active STAT5 mutant that promotes cell proliferation. Mol Cell Biol. 1998;18:3871–3879. doi: 10.1128/mcb.18.7.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimsky L T, Shugars D C, Matthews T J. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnierle B S, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstadter M, Kurth R, Groner B, Cichutek K. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sczakiel G, Pawlita M. Inhibition of human immunodeficiency type 1 replication in human cells stably expressing antisense RNA. J Virol. 1991;65:468–472. doi: 10.1128/jvi.65.1.468-472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar P, Fabry J A, Fong D M, Lieberman J. Three regions of HIV-1 gp160 contain clusters of immunodominant CTL epitopes. Immunol Lett. 1996;52:23–30. doi: 10.1016/0165-2478(96)02574-6. [DOI] [PubMed] [Google Scholar]
- 27.Sorg T, Methali M. Gene therapy for AIDS. Transfus Sci. 1997;18:277–289. doi: 10.1016/s0955-3886(97)00020-9. [DOI] [PubMed] [Google Scholar]
- 28.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 29.Welker R, Harris M, Cardel B, Krausslich H G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]