Abstract
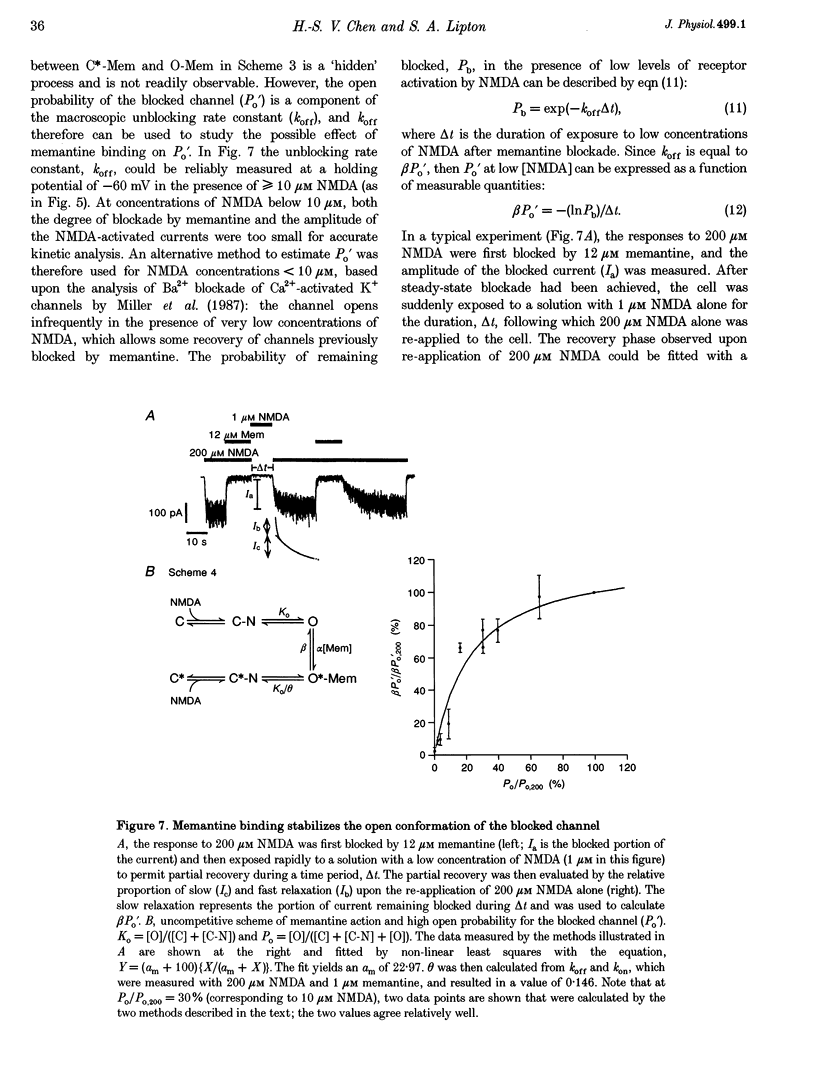
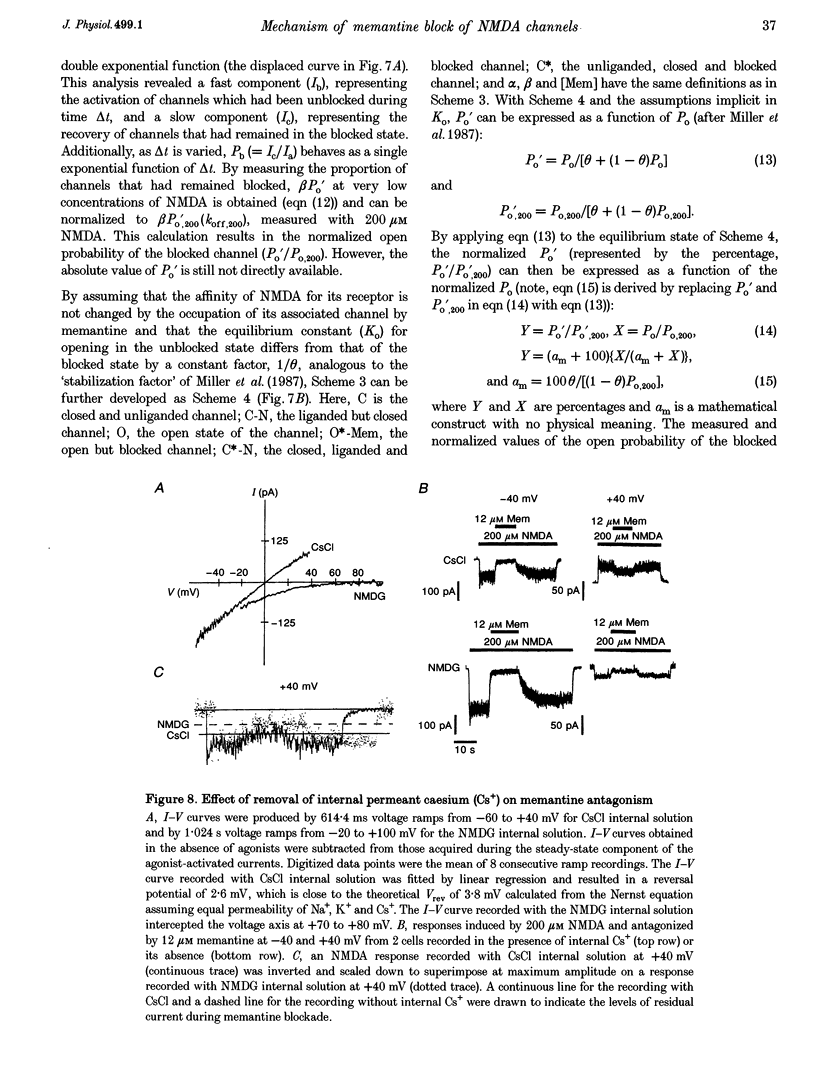
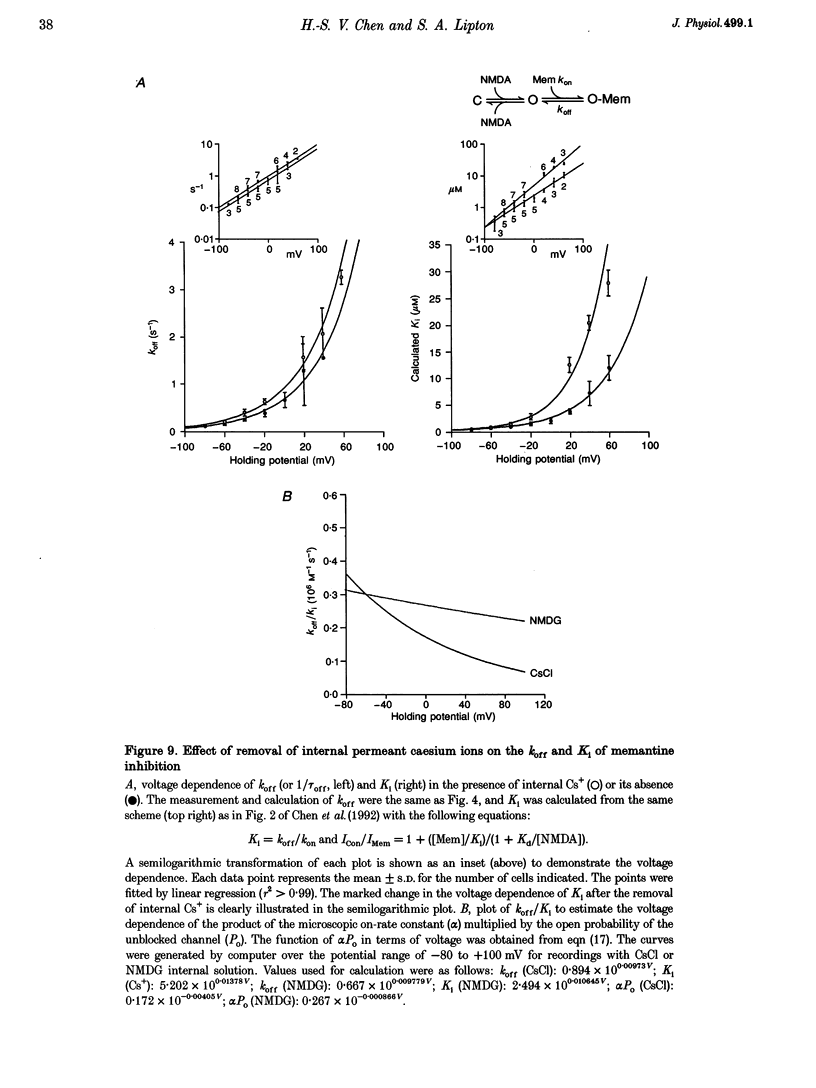
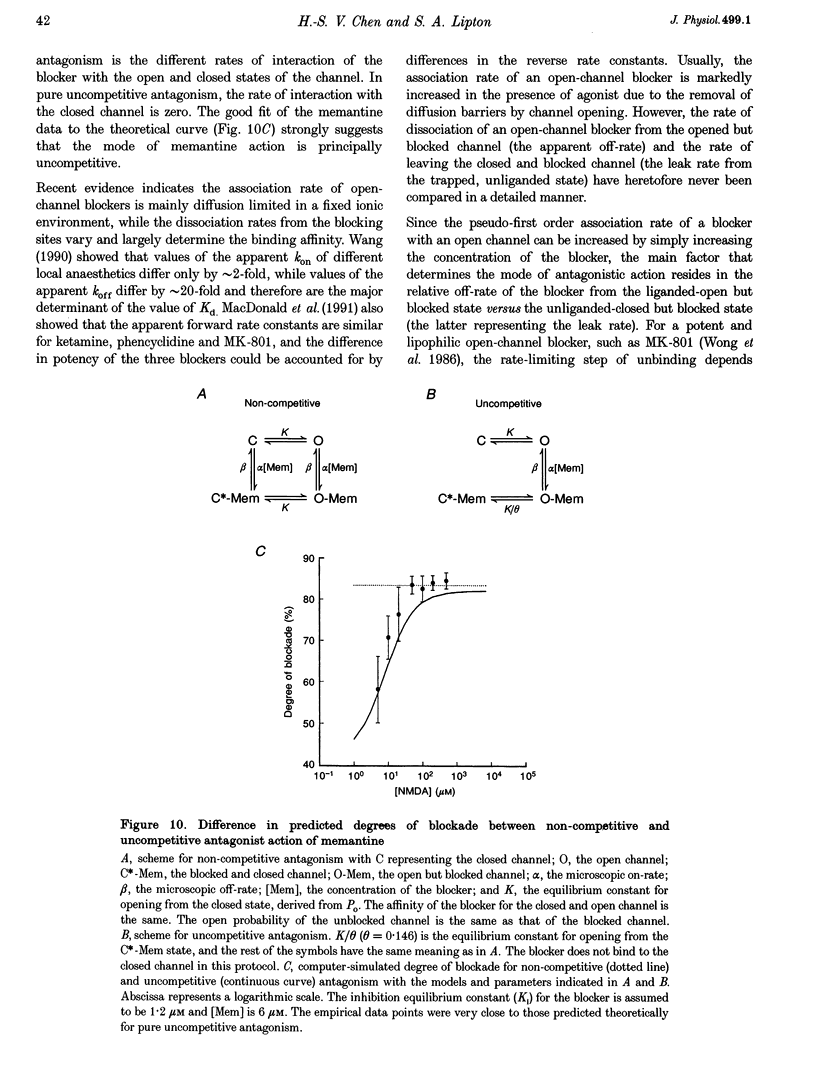
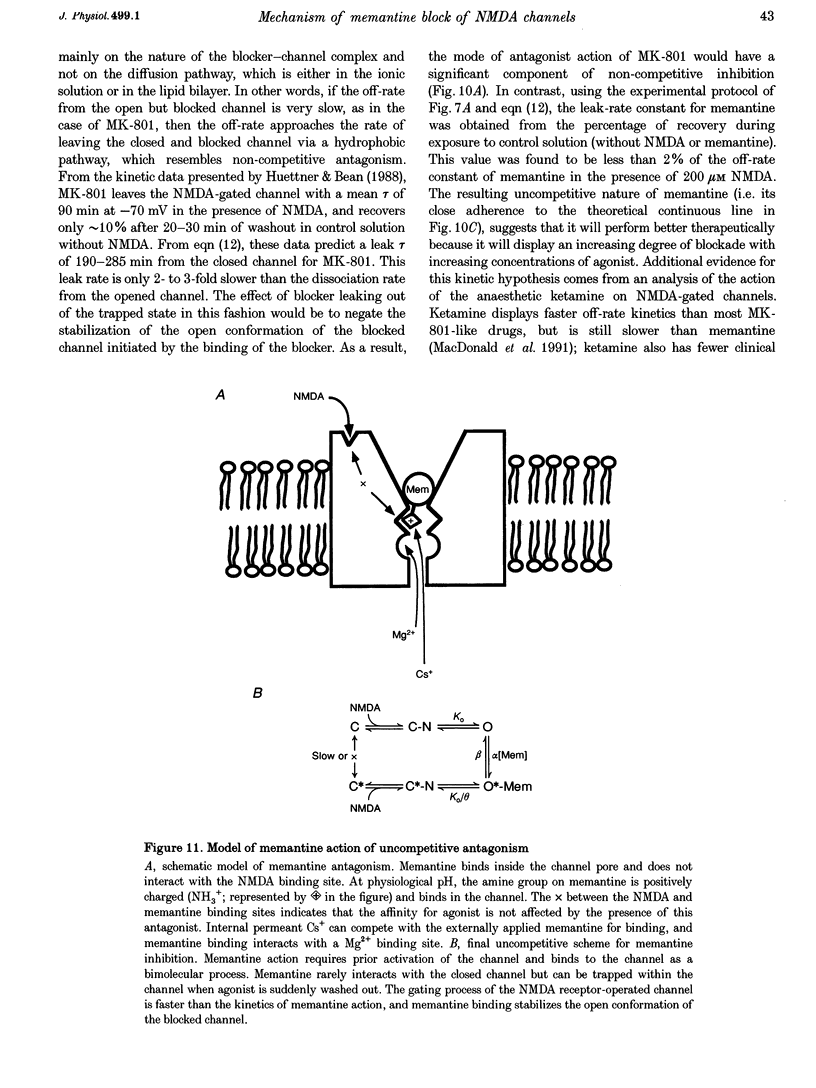
1. N-methyl-D-aspartic acid (NMDA)-activated currents were recorded from dissociated rat retinal ganglion cells using whole-cell recording. The NMDA open-channel blocking drug memantine was evaluated for non-competitive and/or uncompetitive components of antagonism. A rapid superfusion system was used to apply various drugs for kinetic analysis. 2. Dose-response data revealed that memantine blocked 200 microM NMDA-evoked responses with a 50% inhibition constant (IC50) of approximately 1 microM at -60 mV and an empirical Hill coefficient of approximately 1. The antagonism followed a bimolecular reaction process. This 1:1 stoichiometry is supported by the fact that the macroscopic blocking rate of memantine (kon) increased linearly with memantine concentration and the macroscopic unblocking rate (koff) was independent of it. The estimated pseudo-first order rate constant for macroscopic blockade was 4 x 10(5) M-1 S-1 and the rate constant for unblocking was 0.44 s-1. Both the blocking and unblocking actions of memantine were well fitted by a single exponential process. 3. The kon for 2 microM memantine decreased with decreasing concentrations of NMDA. By analysing kon behaviour, we estimate that memantine has minimal interaction with the closed-unliganded state of the channel. As channel open probability (Po) approached zero, a small residual action of memantine may be explained by the presence of endogenous glutamate and glycine. 4. Memantine could be trapped within the NMDA-gated channel if it was suddenly closed by fast washout of agonist. The measured gating process of channel activation and deactivation appeared at least 10-20-fold faster than the kinetics of memantine action. By combining the agonist and voltage dependence of antagonism, a trapping scheme was established for further kinetic analysis. 5. With low agonist concentrations, NMDA-gated channels recovered slowly from memantine blockade. By analysing the probability of a channel remaining blocked, we found that memantine binding appeared to stabilize the open conformation of the blocked channel and did not affect ligand affinity. Validity of the "trapping model' and stabilization of the open conformation were further suggested by agreement between the predicted dose-response curve for NMDA in the presence of 2 microM memantine and the empirically derived dose-response relationship. 6. Based on simple molecular schemes, the degree of blockade at various concentrations of agonist for "pure' non-competitive vs. uncompetitive inhibition was computer simulated. The measured degree of blockade by 6 microM memantine was close to ideal for pure uncompetitive antagonism. Taken together, we conclude that the predominant mechanism of open-channel blockade by memantine is uncompetitive. In general, the relative magnitude of the dissociation rate of an open-channel blocker from the open but blocked channel (the apparent off-rate) compared with the rate of leaving the closed and blocker-trapped state (the leak rate) will determine the contribution of uncompetitive vs. non-competitive actions, respectively. 7. Millimolar internal Cs+ competed with memantine for binding in the NMDA-gated channel, and reduced the association rate of memantine, but had no effect on the voltage dependence of the dissociation rate. After removal of Cs+, the calculated Ki for memantine remained voltage dependent. These observations would be difficult to reconcile with models in which memantine binds to a site outside the channel pore and instead strongly support the supposition that the blocking site for memantine is within the permeation pathway.
Full text
PDF



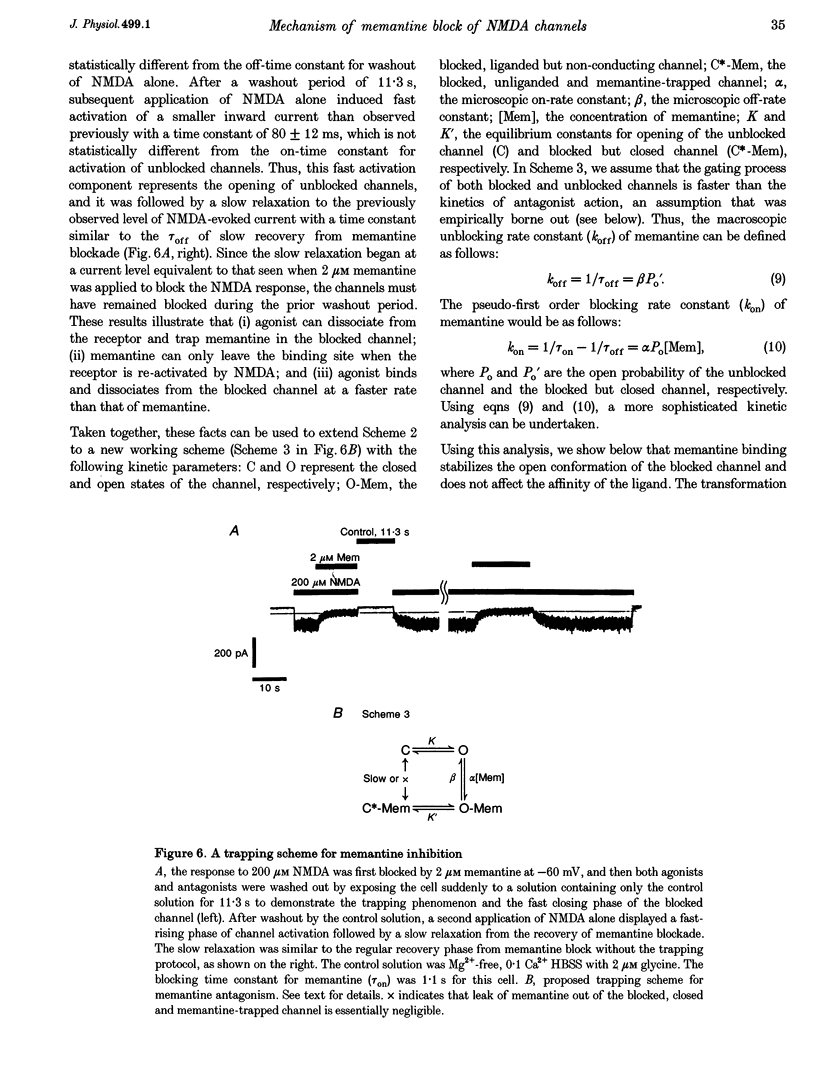






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizenman E., Frosch M. P., Lipton S. A. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol. 1988 Feb;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. S., MacKinnon R., Smith C., Miller C. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol. 1988 Mar;91(3):317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Memantine is a potent blocker of N-methyl-D-aspartate (NMDA) receptor channels. Eur J Pharmacol. 1989 Aug 3;166(3):591–592. doi: 10.1016/0014-2999(89)90385-3. [DOI] [PubMed] [Google Scholar]
- Chen H. S., Pellegrini J. W., Aggarwal S. K., Lei S. Z., Warach S., Jensen F. E., Lipton S. A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992 Nov;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Cull-Candy S. G., Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991 Jan;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A., Aizenman E., Lipton S. A. The interaction of agonists and noncompetitive antagonists at the excitatory amino acid receptors in rat retinal ganglion cells in vitro. J Neurosci. 1988 Aug;8(8):2895–2906. doi: 10.1523/JNEUROSCI.08-08-02895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin A., Lipton S. A. Calcium channels in solitary retinal ganglion cells from post-natal rat. J Physiol. 1989 Nov;418:379–396. doi: 10.1113/jphysiol.1989.sp017847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J., Bormann J., Retz W., Hübers M., Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur J Pharmacol. 1989 Aug 3;166(3):589–590. doi: 10.1016/0014-2999(89)90384-1. [DOI] [PubMed] [Google Scholar]
- Leifer D., Lipton S. A., Barnstable C. J., Masland R. H. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984 Apr 20;224(4646):303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Gendelman H. E. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995 Apr 6;332(14):934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lipton S. A. Prospects for clinically tolerated NMDA antagonists: open-channel blockers and alternative redox states of nitric oxide. Trends Neurosci. 1993 Dec;16(12):527–532. doi: 10.1016/0166-2236(93)90198-u. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Rosenberg P. A. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994 Mar 3;330(9):613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Tauck D. L. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987 Apr;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Bartlett M. C., Mody I., Pahapill P., Reynolds J. N., Salter M. W., Schneiderman J. H., Pennefather P. S. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991 Jan;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J Gen Physiol. 1988 Mar;91(3):335–349. doi: 10.1085/jgp.91.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M., Kumanishi T., Arakawa M., Sakimura K., Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S., Turski L., Schwarz M., Czuczwar S. J., Sontag K. H. Anticonvulsant action of 1,3-dimethyl-5-aminoadamantane. Pharmacological studies in rodents and baboon, Papio papio. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jan;332(1):93–97. doi: 10.1007/BF00633204. [DOI] [PubMed] [Google Scholar]
- Meldrum B., Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990 Sep;11(9):379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Miller C., Latorre R., Reisin I. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J Gen Physiol. 1987 Sep;90(3):427–449. doi: 10.1085/jgp.90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Trapping single ions inside single ion channels. Biophys J. 1987 Jul;52(1):123–126. doi: 10.1016/S0006-3495(87)83196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988 Nov;92(5):549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L. M., Wright J. M. Slow voltage-dependent changes in channel open-state probability underlie hysteresis of NMDA responses in Mg(2+)-free solutions. Neuron. 1992 Jan;8(1):181–187. doi: 10.1016/0896-6273(92)90119-x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993 Sep;14(9):325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Schneider E., Fischer P. A., Clemens R., Balzereit F., Fünfgeld E. W., Haase H. J. Wirkungen oraler Memantin-Gaben auf die Parkinson-Symptomatik. Ergebnisse einer placebo-kontrollierten Multicenter-Studie. Dtsch Med Wochenschr. 1984 Jun 22;109(25):987–990. doi: 10.1055/s-2008-1069311. [DOI] [PubMed] [Google Scholar]
- Schwab R. S., England A. C., Jr, Poskanzer D. C., Young R. R. Amantadine in the treatment of Parkinson's disease. JAMA. 1969 May 19;208(7):1168–1170. [PubMed] [Google Scholar]
- Sernagor E., Kuhn D., Vyklicky L., Jr, Mayer M. L. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989 Mar;2(3):1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Packer D. L., Grant A. O. Ligand binding to transiently accessible sites: mechanisms for varying apparent binding rates. J Theor Biol. 1987 Feb 7;124(3):335–341. doi: 10.1016/s0022-5193(87)80120-0. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Yeh J. Z., Tanguy J. A quantitative description of QX222 blockade of sodium channels in squid axons. Biophys J. 1986 Apr;49(4):913–920. doi: 10.1016/S0006-3495(86)83719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P., Béhé P., Schoepfer R., Colquhoun D. Single-channel conductances of NMDA receptors expressed from cloned cDNAs: comparison with native receptors. Proc Biol Sci. 1992 Dec 22;250(1329):271–277. doi: 10.1098/rspb.1992.0159. [DOI] [PubMed] [Google Scholar]
- Tauck D. L., Frosch M. P., Lipton S. A. Characterization of GABA- and glycine-induced currents of solitary rodent retinal ganglion cells in culture. Neuroscience. 1988 Oct;27(1):193–203. doi: 10.1016/0306-4522(88)90230-8. [DOI] [PubMed] [Google Scholar]
- Tominack R. L., Hayden F. G. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am. 1987 Jun;1(2):459–478. [PubMed] [Google Scholar]
- Vergara C., Latorre R. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers. Evidence for a Ca2+ and Ba2+ blockade. J Gen Physiol. 1983 Oct;82(4):543–568. doi: 10.1085/jgp.82.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignon J., Vincent J. P., Bidard J. N., Kamenka J. M., Geneste P., Monier S., Lazdunski M. Biochemical properties of the brain phencyclidine receptor. Eur J Pharmacol. 1982 Jul 30;81(4):531–542. doi: 10.1016/0014-2999(82)90342-9. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A., Priestley T., Knight A. R., Woodruff G. N., Iversen L. L. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. H., Kemp J. A. Sites for antagonism on the N-methyl-D-aspartate receptor channel complex. Annu Rev Pharmacol Toxicol. 1991;31:401–425. doi: 10.1146/annurev.pa.31.040191.002153. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. M., Kline P. A., Nowak L. M. Multiple effects of tetraethylammonium on N-methyl-D-aspartate receptor-channels in mouse brain neurons in cell culture. J Physiol. 1991 Aug;439:579–604. doi: 10.1113/jphysiol.1991.sp018683. [DOI] [PMC free article] [PubMed] [Google Scholar]


