Abstract
The neurotropic JHM strain of mouse hepatitis virus (MHV) causes acute encephalitis and chronic demyelinating encephalomyelitis in rodents. Previous results indicated that CD8 T cells infiltrating the central nervous system (CNS) were largely antigen specific in both diseases. Herein we show that by 7 days postinoculation, nearly 30% of the CD4 T cells in the acutely infected CNS were MHV specific by using intracellular gamma interferon (IFN-γ) staining assays. In mice with chronic demyelination, 10 to 15% of the CD4 T cells secreted IFN-γ in response to MHV-specific peptides. Thus, these results show that infection of the CNS is characterized by a large influx of CD4 T cells specific for MHV and that these cells remain functional, as measured by cytokine secretion, in mice with chronic demyelination.
Recent studies have shown that the CD8 T-cell response is largely directed at the inciting agent in many human and experimental infections (1, 3, 7, 14, 17). However, much less is known about the specificity of CD4 T cells in these infections. CD4 T cells have a crucial role in the pathological process in experimental models of demyelination and in the human disease multiple sclerosis (MS). Mouse hepatitis virus (MHV) strain JHM (MHV-JHM) causes acute and chronic neurological diseases, including demyelinating encephalomyelitis, in susceptible strains of rodents (9, 19). Although demyelination in this model system was initially believed to result from direct viral lysis of oligodendrocytes, demyelination was demonstrated to be largely immune mediated in more recent reports (8, 10, 24, 26, 29). Both the acute encephalitis and chronic demyelinating diseases caused by MHV-JHM are characterized by extensive infiltration of the central nervous system (CNS) by mononuclear cells.
As part of the process of assigning a specific role to CD4 T cells in the induction of either acute or chronic CNS disease, it is critical to determine the number, kinetics of appearance, and specificity of CD4 T cells in the CNS. Previously, CD4 T cells recognizing MHV-specific epitopes were identified by using bulk populations of CNS-derived lymphocytes in gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays (30), but conditions were not optimized for quantification in these experiments. At least three CD4 T-cell epitopes are recognized in the CNS of mice with MHV-induced neurological disease. These encompass residues 134 to 147 of the transmembrane (M) protein [epitope M(134–147)] and residues 333 to 347 and 358 to 372 of the surface (S) glycoprotein [epitope S(333–347) and epitope S(358–372)]. CD4 T cells responding to epitope M(134–147) are most abundant in the CNS of mice with either acute encephalitis or chronic demyelination (30). In order to obtain more precise quantification, intracellular IFN-γ staining was used to analyze functionally activated, MHV-specific CD4 T cells in the CNS of infected mice.
A large fraction of the CD4 T cells infiltrating the CNS is MHV specific.
Lymphocytes were harvested from the CNS of mice with acute encephalitis as previously described (4). Typically 0.8 × 106 to 1.0 × 106 lymphocytes were harvested from each brain and spinal cord by day 7 after intranasal inoculation with MHV. Lymphocytes were stained for intracellular IFN-γ production as previously described (28). In order to quantify accurately the frequency of virus-specific CD4 T cells in the CNS, we optimized the intracellular IFN-γ staining assay. CHB3 cells (I-Ab), derived from a B-cell tumor (2), were used to present MHV-derived peptides, because we detected suboptimal stimulation after in vitro culture with naïve splenocytes. We determined that a ratio of 5 CHB3 cells to 1 lymphocyte resulted in optimal stimulation of CD4 T cells in this short-term in vitro culture.
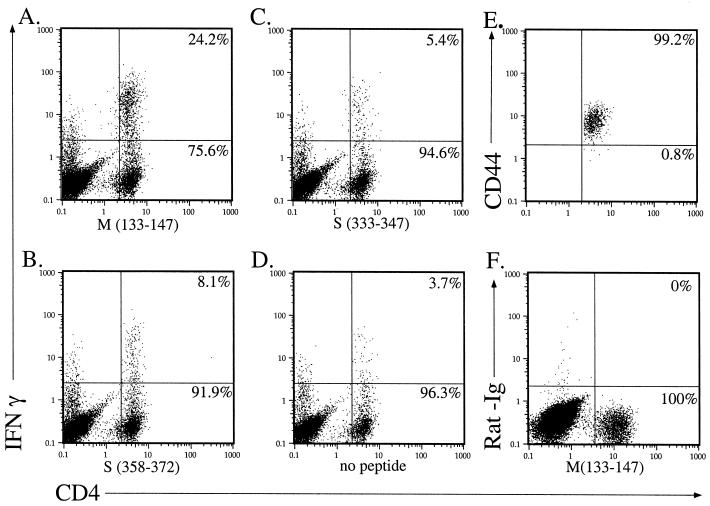
A small proportion of CD4 T cells expressed IFN-γ in the absence of added peptide (Fig. 1D). Although the basis of this expression is not known, it may result from stimulation by infected microglia or macrophages present in the CNS cell preparation (31). To calculate the percentage of MHV-specific CD4 T cells, the frequency of cells that spontaneously expressed IFN-γ was subtracted from the frequency observed after peptide stimulation. This may underestimate the number of MHV-specific cells, since it is likely that at least some of the cells that spontaneously expressed IFN-γ were MHV specific. By days 6 to 7 postinfection (p.i.), a large infiltrate of CD4 T cells was detectable (Fig. 1). Approximately 15 to 20% of the CD4 T-cell population was specific for epitope M(133–147) (Fig. 1A). As expected, these epitope M(133–147)-specific cells were CD44hi, indicative of current or past activation (Fig. 1E). An additional 6% of the CNS-derived CD4 T cells were specific for epitopes S(333–347) and S(358–372) (Fig. 1B and C). These results showed that approximately 25 to 30% of the CD4 T cells in the acutely infected CNS were MHV specific. The actual percentage of MHV-specific CD4 T cells may be even higher, since we identified cells recognizing only three MHV-specific CD4 T-cell epitopes, and it is likely that additional stimulatory epitopes are present.
FIG. 1.
Substantial numbers of CD4 T cells specific for MHV-specific epitopes were detected in the acutely infected CNS. Six-week-old C57BL/6 mice (National Cancer Institute, Bethesda, Md.) were inoculated intranasally with MHV-JHM (4 × 104 PFU). Lymphocytes were harvested from the CNS of moribund mice (6 to 7 days p.i.). Cells from four mice with acute encephalitis were pooled and analyzed for IFN-γ expression after stimulation with peptide M(133–147) (A), peptide S(358–372) (B), peptide S(333–347) (C), or no peptide (D). All of the epitope M(133–147)-specific CD4 T cells in the acutely infected CNS were CD44hi (E), consistent with an activated phenotype. No staining was detected if an isotype-matched phycoerythrin-conjugated control antibody (PharMingen, San Diego, Calif.) was used in lieu of anti-IFN-γ antibody (F). Numbers indicate the percentage of CD4 T cells in each quadrant. This experiment is representative of 10 individual experiments.
TCR Vβ usage by M(133–147)-specific CD4 T cells is extremely diverse
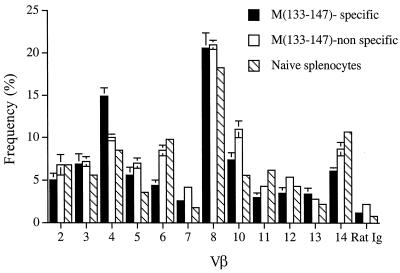
Studies of MHV-JHM-infected mice as well as those using other infectious models have shown that the CD8 T-cell response to dominant epitopes is generally polyclonotypic (6, 12, 17), but little is known about the diversity of epitope-specific CD4 T-cell responses in viral infections. To determine the diversity of T-cell receptor (TCR) expression within the epitope M(133–147)-specific CD4 T-cell population, surface staining for individual TCR Vβ regions was used in conjunction with intracellular staining for IFN-γ followed by fluorescence-activated cell sorter (FACS) analysis. M(133–147)-specific CD4 T cells expressed a diverse group of β chains with only a small amount of skewing relative to Vβ usage by CD4 T cells harvested from the naïve spleen (Fig. 2). Previous results showed that the MHV-specific CD8 T-cell response was polyclonotypic, although it was characterized by preferential usage of a few Vβ segments (17). The data in Fig. 2 indicate that Vβ expression by MHV-specific CD4 T cells was even more diverse than that by virus-specific CD8 T cells.
FIG. 2.
Comparison of the Vβ phenotypes of epitope M(133–147)-specific and nonspecific CD4 T cells isolated from the infected CNS and splenocytes harvested from naïve mice. Lymphocytes were harvested from the CNS of four to six infected mice 7 days p.i. and pooled. Vβ surface staining, using a previously described panel of biotinylated monoclonal antimouse Vβ antibodies (PharMingen) (17), and intracellular staining for IFN-γ were done after stimulation with peptide M(133–147). The bars represent the percentage of CNS-derived peptide M(133–147)-specific, nonspecific, and splenic CD4 T cells that were also positive for the indicated Vβ element. Rat Ig is a biotinylated control antibody (Caltag, Burlingame, Calif.). Values shown for CNS-derived CD4 T cells are the average of six experiments. Error bars show the standard error for each value. Values shown for naïve splenic CD4 T cells are the average of two independent experiments.
Both MHV-specific CD4 and CD8 T cells infiltrated the CNS at early times p.i.
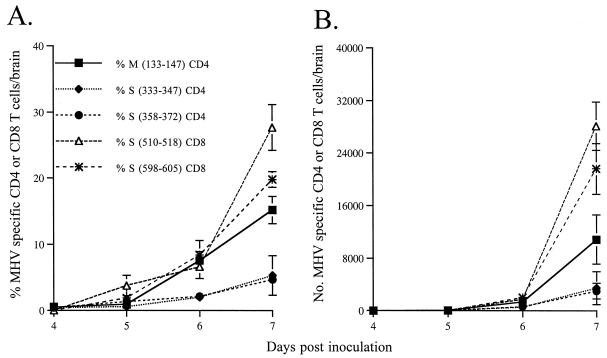
Previous results suggest that CD4 T cells are necessary for the survival of CD8 T cells in the infected CNS (18). To determine if MHV-specific CD4 T cells were detected prior to CD8 T cells, the appearance of both types of T cells in the CNS, draining cervical lymph nodes (CLN), and spleen was monitored. Lymphocytes were purified from mice at 3 to 7 days p.i. MHV-specific CD8 T cells were detected with intracellular IFN-γ assays as described previously (28), by using EL-4 cells (H-2Db, Kb) to present antigen (ratio of 1 EL-4 cell to 50 lymphocytes). By day 6 p.i., approximately 8% of the total CD4 T cells in the CNS were M(133–147) specific (1,300 to 1,400 cells). The number of virus-specific CD4 T cells continued to increase rapidly until mice were moribund (Fig. 3). By 7 days p.i., approximately 15% of the CD4 T cells in the CNS were M(133–147) specific, equivalent to an absolute number of 11,000 cells per infected brain. Cells responding to the subdominant CD4 T-cell epitopes S(333–347) and S(358–372) accumulated with nearly the same kinetics shown for epitope M(133–147), but exhibited proportionately lower frequencies and absolute cell numbers (Fig. 3).
FIG. 3.
Kinetics of appearance of MHV-specific CD4 and CD8 T cells in the CNS. Intracellular staining for IFN-γ was done on lymphocytes isolated from the brains of mice on days 3 to 7 p.i. The percentage of MHV-specific T cells was calculated by subtracting the percentage of lymphocytes that express IFN-γ spontaneously (CD4 T cells) or in response to irrelevant peptide (CD8 T cells) from the percentage of lymphocytes that expressed IFN-γ when stimulated with an MHV-specific peptide. The absolute number of MHV-specific T cells per mouse was calculated as follows: total number of CNS-derived lymphocytes × the percentage of CD4 or CD8 T cells × (the percentage of MHV-specific T cells − the percentage of cells that express IFN-γ spontaneously or in response to irrelevant peptide). The percentage (A) and absolute number (B) of MHV-specific T cells are shown. Values shown are averages of three to five experiments. Error bars show the standard error for each value.
The appearance of CD8 T cells in the CNS paralleled that of CD4 T cells. By day 6 p.i., approximately 15% of the CD8 T cells (3,000 to 4,000 cells) were specific for epitopes S(510–518) and S(598–605). A vast expansion of antigen-specific CD8 T cells occurred between 6 and 7 days p.i., similar to the expansion observed in the CD4 T-cell population (Fig. 3). By day 7 p.i., 50% of the total CD8 T cells were S(510–518) or S(598–605) specific. The expansion of CD8 T cells from day 6 to day 7 p.i. was greater than that of CD4 T cells, but the resulting number of epitope S(510–518)-specific CD8 T cells was only two- to threefold greater than the number of M(133–147)-specific CD4 T cells at day 7 p.i. These results indicated that the MHV-specific CD4 and CD8 T-cell responses were both robust in the infected CNS.
In contrast, MHV-specific CD4 T cells were detected in the CLN only at low frequency. Initially, we could not detect any MHV-specific CD4 T cells in the CLN at any time p.i. However, by first selecting CD4 T cells with an activated phenotype (CD44hi or CD62Llo), low numbers of MHV-specific CD4 T cells were detectable in the CLN. They were detected simultaneously with, or just prior to, their appearance in the CNS, and the number continued to increase over the course of the infection in parallel with the rapid expansion observed in the CNS. MHV-specific CD4 T cells were present in the spleen only at very low frequencies (<1.0%), even after selection for activated cells (data not shown).
A reduced fraction of CD4 T cells in the chronically infected CNS was MHV specific.
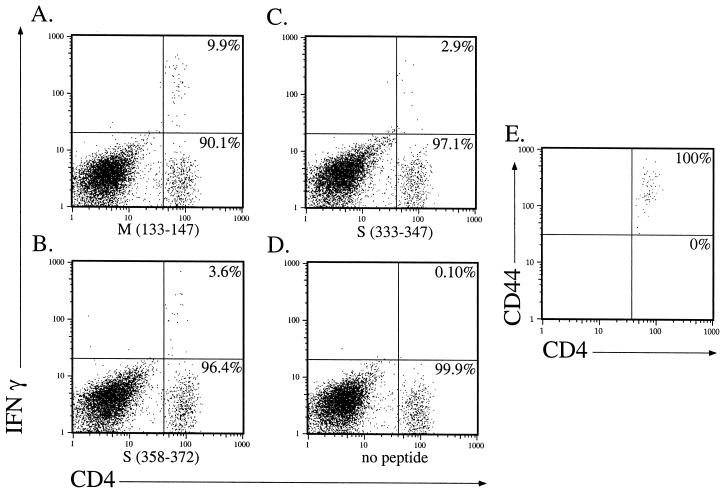
Suckling mice inoculated intranasally with MHV develop a fatal encephalitis unless they are nursed by dams previously immunized with live MHV. Under these conditions, 40 to 90% of mice develop clinical signs of hindlimb paralysis and histological evidence of demyelination at 3 to 8 weeks p.i. with high titers of infectious virus present in the CNS of these animals (16). CD4 T cells have an important role in the development of MHV-induced demyelination (11, 28), but in other virus infections, CD4 T cells become unresponsive during the course of persistence (15). Next, we determined the state of responsiveness of MHV-specific CD4 T cells in the chronically infected CNS as measured by IFN-γ expression (Fig. 4). Data from analyses of five individual mice with chronic demyelination (harvested 24 to 36 days p.i.) showed that 7.8% (± 1.5%), 1.5% (± 0.5%), and 2.1% (± 0.8%) responded to peptides M(133–147), S(333–347), and S(358–372), respectively. These percentages were lower than what was detected in mice with acute encephalitis (Fig. 1). Whether this represents an actual decrease in number of cells responding to the three MHV-specific epitopes or a decreased ability to produce IFN-γ remains to be determined. The decrease in number of CD4 T cells making IFN-γ is not due to a cytokine profile switch to a Th2 phenotype, since no M(133–147)-specific CD4 T cells stained positive for IL-4 after peptide stimulation (data not shown). All of the epitope M(133–147)-specific CD4 T cells in the chronically infected CNS are CD44hi (Fig. 4E).
FIG. 4.
MHV-specific CD4 T cells were detected in the CNS of mice with chronic demyelination. Lymphocytes were harvested from the brain and spinal cord of a mouse with hindlimb paralysis at day 36 p.i. Intracellular staining for IFN-γ was done after stimulation with peptide M(133–147) (A), peptide S(358–372) (B), peptide S(333–347) (C), or no peptide (D). Numbers indicate the percentage of CD4 T cells in each quadrant. All of the epitope M(133–147)-specific CD4 T cells in the chronically infected CNS were CD44hi (E), consistent with an activated phenotype. These data are representative of five experiments performed with lymphocytes harvested from the CNS of five individual mice with chronic demyelination at days 24 to 36 p.i.
The frequency of CD4 T cells responding to a single epitope [M(133–147)] is substantially higher than values obtained from prior studies. Many of these studies used limiting dilution assays to identify antigen-specific cells, and, as is the case for CD8 T cells, use of this method substantially underestimates this number (5, 20, 21, 23). The recent development of more sensitive methods to measure antigen-specific T cells has led to the reevaluation of antigen specificity of CD4 T cells in other viral infections. Approximately 3 to 10% of CD4 T cells in the spleen and lymph nodes of mice infected with lymphocytic choriomeningitis virus (LCMV) are virus specific, as measured by intracellular staining for IFN-γ (21, 22, 27). This is in marked contrast to results obtained with limiting dilution assays, in which <1% of CD4 T cells were determined to be LCMV specific (23). As shown above, the antigen-specific CD4 T-cell response was even higher in the CNS of mice infected with MHV-JHM, reflecting the localized nature of the infection caused by this virus. Few MHV-specific CD4 or CD8 T cells were detected in the CLN or spleen, probably as a consequence of this localization.
Although a substantial portion of the CD4 T-cell population in the infected CNS was MHV specific, this did not account for 100% of the lymphocytes at this site, particularly in mice chronically infected with the virus (Fig. 4). Nothing is known about the specificity of the CD4 T cells that do not recognize the three known MHV CD4 T epitopes. CD4 T cells responsive to myelin-specific epitopes have been identified in mice with demyelination induced by Theiler's murine encephalomyelitis virus (13). Although autoreactive CD4 T cells have been identified only infrequently in MHV-infected rodents (25), such cells might be present in this putative pool of non-MHV-specific CD4 T cells and might contribute to the chronic demyelination observed in persistently infected mice.
The kinetics of appearance of CD4 and CD8 T cells responding to each MHV-specific epitope within the CNS were very similar, but not identical. This was particularly evident at days 6 and 7 p.i., when the expansion of CD8 T cells responding to epitopes S(510–518) and S(598–605) was greater than that of M(133–147)-specific CD4 T cells. These differences may reflect the critical role of CD8 T cells as direct antiviral effector cells. Alternatively, they may represent differences in trafficking or proliferative capabilities of the two subsets of cells or quantitative differences in antigen presentation. Another possibility is that the number of precursor CD4 T cells able to recognize epitope M(133–147) is significantly greater in the naïve T-cell population than the number responding to either CD8 T-cell epitope. As a consequence, the response to epitope M(133–147) would initially be more rapid, although eventually the MHV-specific CD8 T-cell response would eclipse it. The data presented in Fig. 2, showing little skewing of Vβ usage in the epitope M(133–147)-specific CD4 T-cell population compared to that of naïve splenocytes, are consistent with this possibility.
In conclusion, these results show that MHV-specific CD4 T cells comprise a large fraction of the CD4 T-cell population in the infected CNS and remain functional in persistently infected animals. Further investigations will be directed at determining the precise role of these MHV-specific, as well as any MHV-nonspecific, CD4 T cells in the process of chronic demyelination.
Acknowledgments
We thank Charles Lutz and Morris Dailey for critically reviewing the manuscript and Justin Fishbaugh for help with FACS analysis.
This research was supported in part by grants from the National Institutes of Health (NS36592 and AI43497) and the National Multiple Sclerosis Society (RG2864-A-2). J.S.H. was supported by an NIH institutional training grant (P32 AI 07533).
REFERENCES
- 1.Bergmann C C, Altman J D, Hinton D, Stohlman S A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 2.Bishop G A, Ramirez L M, Koretzsky G A. Growth inhibition by a B cell clone mediated by ligation of IL-4 receptors or membrane IgM. J Immunol. 1993;150:2565–2574. [PubMed] [Google Scholar]
- 3.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro R F, Evans G D, Jaszewski A, Perlman S. Coronavirus-induced demyelination occurs in the presence of virus-specific cytotoxic T cells. Virology. 1994;200:733–743. doi: 10.1006/viro.1994.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen J P, Doherty P C. Quantitative analysis of the acute and long-term CD4+ T-cell response to a persistent gammaherpesvirus. J Virol. 1999;73:4279–4283. doi: 10.1128/jvi.73.5.4279-4283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole G, Hogg T, Woodland D. T cell recognition of the immunodominant Sendai virus NP324–332/Kb epitope is focused on the center of the peptide. J Immunol. 1995;155:2841–2848. [PubMed] [Google Scholar]
- 7.Flynn K J, Belz G, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 8.Houtman J J, Fleming J O. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 9.Houtman J J, Fleming J O. Pathogenesis of mouse hepatitis virus-induced demyelination. J Neurovirol. 1996;2:361–376. doi: 10.3109/13550289609146902. [DOI] [PubMed] [Google Scholar]
- 10.Lampert P W, Sims J K, Kniazeff A J. Mechanism of demyelination in JHM virus encephalomyelitis. Acta Neuropathol. 1973;24:76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane T E, Liu M T, Chen B P, Asensio V C, Samawi R M, Paoletti A D, Campbell I L, Kunkel S L, Fox H S, Buchmeier M J. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y M, Welsh R M. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller S D, Vanderlugt C, Begolka W, Pao W, Yauch R, Neville K, Katz-Levy Y, Carrizosa A, Kim B. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Altman J D, Suresh M, Sourdive D, Zajac A, Miller J, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 15.Oxenius A, Zinkernagel R M, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 1998;9:449–457. doi: 10.1016/s1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 16.Perlman S, Schelper R, Bolger E, Ries D. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb Pathog. 1987;2:185–194. doi: 10.1016/0882-4010(87)90020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pewe L, Heard S B, Bergmann C C, Dailey M O, Perlman S. Selection of CTL escape mutants in mice infected with a neurotropic coronavirus: quantitative estimate of TCR diversity in the infected CNS. J Immunol. 1999;163:6106–6113. [PubMed] [Google Scholar]
- 18.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 19.Stohlman S A, Bergmann C C, Perlman S. Mouse hepatitis virus. In: Ahmed R, Chen I, editors. Persistent viral infections. New York, N.Y: John Wiley & Sons, Ltd.; 1998. pp. 537–557. [Google Scholar]
- 20.Topham D J, Doherty P C. Longitudinal analysis of the acute Sendai virus-specific CD4+ T cell response and memory. J Immunol. 1998;161:4530–4535. [PubMed] [Google Scholar]
- 21.Varga S, Welsh R. Detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J Immunol. 1998;161:3215–3218. [PubMed] [Google Scholar]
- 22.Varga S M, Welsh R M. High frequency of virus-specific interleukin-2-producing CD4+ T cells and Th1 predominance during lymphocytic choriomeningitis virus infection. J Virol. 2000;74:4429–4432. doi: 10.1128/jvi.74.9.4429-4432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga S M, Welsh R M. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J Immunol. 1998;161:367–374. [PubMed] [Google Scholar]
- 24.Wang F, Stohlman S A, Fleming J O. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol. 1990;30:31–41. doi: 10.1016/0165-5728(90)90050-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe R, Wege H, ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983;305:150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner L P. Pathogenesis of demyelination induced by a mouse hepatitis virus (JHM virus) Arch Neurol. 1973;28:298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- 27.Whitmire J K, Asano M S, Murali-Krishna K, Suresh M, Ahmed R. Long-term CD4 Th1 and Th2 memory following acute lymphocytic choriomeningitis virus infection. J Virol. 1998;72:8281–8288. doi: 10.1128/jvi.72.10.8281-8288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Dandekar A, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- 29.Wu G F, Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue S, Perlman S. Antigen specificity of CD4 T cell response in the central nervous system of mice infected with mouse hepatitis virus. Virology. 1997;238:68–78. doi: 10.1006/viro.1997.8819. [DOI] [PubMed] [Google Scholar]
- 31.Xue S, Sun N, van Rooijen N, Perlman S. Depletion of blood-borne macrophages does not reduce demyelination in mice infected with a neurotropic coronavirus. J Virol. 1999;73:6327–6334. doi: 10.1128/jvi.73.8.6327-6334.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]