Abstract
Gender influences the incidence and severity of some bacterial and viral infections and autoimmune diseases in animal models and humans. To determine a gender-based difference, comparisons were made between male and female mice inoculated with herpes simplex virus type 1 (HSV-1) by the corneal route. Mortality was higher in the male mice of the three strains tested: 129/Sv//Ev wild type, gamma interferon (IFN-γ) knockout (GKO), and IFN-γ receptor knockout (RGKO). Similarly, in vivo HSV-1 reactivation occurred more commonly in male mice, but the male-female difference in reactivation was restricted to the two knockout strains and was not seen in the 129/Sv//Ev control. Comparison among male mice of the three strains showed a higher mortality of the RGKO mice and a higher reactivation rate of the GKO and RGKO mice than of the 129/Sv//Ev males. In contrast, female RGKO and GKO mice did not differ from female 129/Sv//Ev controls in either mortality or reactivation. HSV-1 periocular and eyelid disease was also more severe in male and dihydrotestosterone (DHT)-treated female mice than in control female mice. These results show a consistent gender difference in HSV-1 infection, with a worse outcome in male mice. In addition, the results comparing GKO and RGKO mice to controls show differences only in male mice, suggesting that some effects of IFN-γ, a key immunoregulatory molecule, are gender specific.
It has long been appreciated that males and females differ in susceptibility to a variety of infections and diseases. Gender-determined differences in susceptibility to virus infections have been reported for encephalomyocarditis virus (8), vesicular stomatitis virus (3), and coxsackievirus B3 (CVB3) (20). Females mount more vigorous immune responses, especially humoral responses, and in general show higher resistance to bacterial and viral infections (1, 3, 22). Females are also disproportionately afflicted by autoimmune disease, such as lupus erythematosus, rheumatoid arthritis, myasthenia gravis, scleroderma, Sjögren's syndrome, multiple sclerosis, experimental allergic encephalomyelitis (EAE) (10, 28, 36), and herpes simplex virus (HSV). Despite early reports of differences in antibody titer (22) and severity of paralysis after HSV-1 inoculation of mice (18, 38), gender-based differences in HSV-1 infection or in HSV-1-associated diseases have not been well studied.
The mechanisms underlying gender- or, strictly, sex-based differences in susceptibility to infection and diseases remain ill defined (3, 35, 36); however, several recent studies suggest that differences in immune responses may involve gender. Gamma interferon (IFN-γ), an important immunoregulatory cytokine, plays a key role in the development of balanced Th1/Th2 responses that are essential for efficient control of infectious intracellular pathogens. Th1-type responses are generally regarded as more effective for controlling viral infections, but excessive responses can have serious immunopathological consequences (15, 16, 33). Indeed, IFN-γ can be either protective or deleterious during infection with HSV-1, depending on the target tissue (17). IFN-γ is associated with damaging corneal lesions in herpetic stromal keratitis but is required for control of cutaneous and reactivated HSV-1 infections. Although activity of the IFN-γ promoter is known to increase by treatment of lymphoid cells with estrogen (13), whether estrogen regulation occurs in vivo is unknown. To determine possible gender-specific effects of IFN-γ, we compared the outcomes of HSV-1 infection in male and female wild-type mice (129/Sv//Ev) and mutant mice lacking either the IFN-γ gene (IFN-γ−/−) (GKO) (11) or the IFN-γ receptor (IFN-γR) gene (IFN-γR−/−) (RGKO) (19).
Gender effects on HSV-1 acute infection.
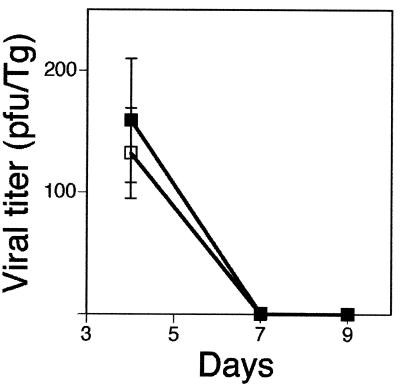
Male and female mutant GKO and RGKO mice and wild-type mice, all in the 129/Sv//Ev background (32), were used at 6 to 9 weeks of age for these studies. Because GKO mice were only available in the C57BL/6 background, we introduced the IFN-γ−/− null mutation into the 129/Sv//Ev background by using an ES cell clone (97E) heterozygous for the IFN-γ mutation as described previously (5). Male and female mice were housed in the same suite under specific-pathogen-free conditions. 129/Sv//Ev mice used as controls were obtained from a commercial supplier (Taconic, Inc., Germantown, N.Y.). To determine the effects of gender on mortality, male and female 129/Sv//Ev mice were inoculated with HSV-1 by corneal scarification as previously described (5), and mortality at the acute stage of infection (up to 2 weeks after inoculation) was recorded. As shown in Table 1, the rates of mortality in 129/Sv//Ev mice were 23% in males and 8% in females (P = 0.04). Since the IFN-γ promoter has been shown to be regulated by estrogen (13), it is conceivable that IFN-γ contributes to gender differences in infectious and inflammatory diseases. To determine if the HSV mortality difference noted in 129/Sv//Ev mice was similar in mice deficient in IFN-γ, we reviewed mortality records in over seven experiments with the null mutant RGKO and GKO mice (5; E. Cantin and J. Mann, Letter, J. Immunol. 162:6294–6295, 1999). As shown in Table 1, the mortality in female RGKO and GKO mice was 8% (the same mortality as in the female 129/Sv//Ev controls), whereas the mortality rates in both male null mutant mouse strains were significantly higher: 38% in RGKO mice (P = 0.0001) and 23% in GKO mice (P = 0.0002). These differences correspond to a 3.4- to 5.4-higher risk of death for males in both RGKO and GKO mutant and control mice. Viral titers determined in trigeminal ganglia and brain stem tissue homogenates at the peak of the acute infection on day 4 showed no significant male-female differences (not shown). A time course study monitoring HSV-1 titers in trigeminal ganglia of 129/Sv//Ev mice on days 4, 7, and 9 confirmed this result and further showed that the kinetics of clearance of HSV-1 from the ganglion was also not affected by gender (Fig. 1).
TABLE 1.
Effect of gender on HSV-induced mortality in mice
| Mouse strain | Total no. of mice | No. of deaths | Mortality
|
P valuec | |
|---|---|---|---|---|---|
| Rate (%) | Risk ratioa (95% CI)b | ||||
| Males | |||||
| 129/Sv/Ev | 150 | 35 | 23 | 3.41 (0.82–14.3) | 0.04 |
| GKO | 244 | 57 | 23 | 3.54 (1.62–7.75) | 0.0002 |
| RGKO | 380 | 144 | 38 | 5.43 (2.76–10.6) | 0.0001 |
| Females | |||||
| 129/Sv/Ev | 25 | 2 | 8 | NAd | |
| GKO | 93 | 7 | 8 | NA | |
| RGKO | 106 | 9 | 8 | NA | |
Risk in males versus that in females within each strain.
CI, confidence interval.
P value from univariate Cox regression analysis comparing male and female mice.
NA, not applicable.
FIG. 1.
Time course of HSV-1 replication in the trigeminal ganglion (Tg). Three mice per group were sacrificed at the indicated times, and HSV-1 titers were determined in ganglionic homogenates by plaque assay on CV-1 monolayers; titers are expressed as means ± standard errors.
The summary in Table 1 of mortality records from several experiments permitted comparisons among the three mouse strains that revealed interesting new findings. An analysis of only male mice showed a significantly higher mortality in RGKO mice than in GKO mice (P = 0.0001) or wild-type 129/Sv//Ev mice (P = 0.04), as previously reported (5). This result showing that the IFN-γR, more than IFN-γ itself, protects against mortality underpins speculation that in GKO mice binding of alternative ligand to the IFN-γR may mediate protection (5). Interestingly, the action of this putative alternative ligand does not appear to affect female mice, since the rates of mortality are the same in RGKO and GKO females. However, unlike GKO mice, male RGKO mice have a higher mortality than control male 129/Sv//Ev mice (38 versus 23%, P = 0.003). In contrast, lack of the IFN-γR in female mice did not affect mortality; there were no differences in mortality in female 129/Sv//Ev, GKO, or RGKO mice.
Gender effects on HSV-1 reactivation.
To determine if gender influences HSV-1 reactivation in vivo, the model of hyperthermic stress was used (30) with modification. Briefly, mice were subjected to three consecutive 10-min hyperthermia treatments (43°C) separated by 3-h intervals, a modification shown to enhance the efficiency of reactivation (R. L. Thompson, personal communication). The mice were thoroughly dried and kept warm under a heat lamp after each treatment to avoid hypothermia. After 24 h, the mice were euthanized, and then the trigeminal ganglia were removed, homogenized, and assayed for infectious HSV-1 (6). Spontaneous reactivation or viral persistence was assessed in latently infected mice not treated with hyperthermia by assaying ganglionic homogenates for infectious HSV. The reactivation data obtained in several experiments were pooled and analyzed statistically by using Pearson's chi-square test with Yates' continuity correction.
We showed previously that persistent ganglionic infection or spontaneous reactivation of HSV-1 did not occur in 129/Sv//Ev or GKO mice (4). For the present experiments, latently infected male and female GKO, RGKO, and 129/Sv//Ev control mice were subjected to hyperthermic stress at 30 to 60 days postinfection, and reactivated HSV-1 was detected by assay of infectious virus in ganglionic homogenates. As shown in Table 2, there was no difference in male and female129/Sv//Ev mice: reactivation frequencies were 11 and 12%, respectively. However, the observed frequency of reactivation in GKO mice was significantly greater in males: 51% compared to 12% for females (P = 0.0005). Similarly for RGKO mice, reactivation frequencies were higher in males than females (33 versus 21%), but the difference did not reach statistical significance (P = 0.17). As previously reported (4), reactivation frequency was higher in the GKO mice than in the 129/Sv//Ev controls. However, Table 2 shows that this difference was derived only from male mice; there was no statistically significant difference in reactivation in female RGKO or GKO mice compared to female 129/Sv//Ev mice (Table 2). This result, together with our mortality data (Table 1), suggests that, under certain circumstances, IFN-γ has a more important role in males than in females.
TABLE 2.
Differences in gender in induced in vivo reactivation of latent HSVa
| Mouse strain | No. of mice
|
Reactivation frequency (%) |
P value
|
||
|---|---|---|---|---|---|
| Total | Positive | Gender basedb | Strain basedcde | ||
| Males | |||||
| 129/Sv/Ev | 38 | 4 | 11 | >0.80 | |
| GKO | 37 | 19 | 51 | 0.0005 | 0.0003 |
| RGKO | 40 | 13 | 33 | 0.17 | 0.038 |
| Females | |||||
| 129/Sv/Ev | 26 | 3 | 12 | ||
| GKO | 42 | 5 | 12 | >0.46 | |
| RGKO | 29 | 6 | 21 | >0.46 | |
Cumulative results for seven experiments. Reactivation frequency was calculated from the ratio of positive to total mice.
Male versus female within each strain.
Male GKO versus male 129/Sv/Ev (P = 0.0003).
Male RGKO versus male 129/Sv/Ev (P = 0.038).
Female GKO and RGKO versus female 129/Sv/Ev (P > 0.46).
We suggested previously that rather than influencing the reactivation process itself (i.e., molecular latency), IFN-γ controlled reactivating virus, making it less likely that infectious HSV would be found in ganglionic homogenates or HSV antigens detected by immunohistochemistry (i.e., the IFN effect is on biological latency) (4). There are at least three possible explanations for the gender difference in reactivation shown in Table 2. First, the burden of HSV DNA at the latent stage, a suggested predictor of HSV reactivation (23, 25, 29), may be greater in male mice. Although we cannot exclude this possibility, it seems unlikely, since we found equivalent ganglionic HSV titers at the acute stage of infection in male and female mice (Fig. 1). Second, the effects of hyperthermic stress may be greater in males, so that reactivation from molecular latency occurs more frequently in males than in females. Failure to detect a gender difference in 129/Sv//Ev mice can explained by the dampening effect of IFN-γ maintaining biological latency in most of the 129/Sv//Ev males. However, in the absence of IFN-γ signaling, as in RGKO and GKO mice, the gender difference is readily apparent. The third possibility assumes that the levels of hyperthermia-induced reactivation from molecular latency are equivalent in male and female mice of all three strains, but female mice, with or without IFN-γ signaling, have a more efficient immunological repertoire to maintain biological latency. We have no data to differentiate between the second and third possibilities, but we favor immunological differences accounting for at least some of our results. Our observation of greater HSV-1 reactivation frequency in male mice (Table 2) is consistent with a report of a higher frequency of HSV-2 recurrence in men than in women (27). More detailed gender comparisons of HSV infection in humans would be of interest to detect differences similar to those we report here in mice. A recent report that a gD2 subunit vaccine was effective in preventing genital herpes disease in females, but not in males, is consistent with the notion that immune responses to HSV-1 are affected by gender (S. Spruance, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., addendum abstr. L6, p. 22, 2000).
Periocular eye disease is worse in testosterone-treated mice.
To determine a role for sex hormones in HSV-1 gender differences, we evaluated periocular skin and eye disease in male mice and female mice that received implants of either timed-release dihydrotestosterone (DHT) pellets or placebo pellets. In this trial, RGKO mice were used, since a consistent gender difference in mortality and reactivation had been demonstrated in RGKO mice (Tables 1 and 2), and use of IFN-γ null mutant mice eliminates any potential effect of estrogen on the IFN-γ promoter. We evaluated 56 mice for eye disease: 18 males, 20 DHT-treated females, and 18 placebo-treated females. As shown in Fig. 2, extensive periocular hair loss associated with skin breakdown and bleeding and lid edema producing eye closure were observed in male and DHT-treated female mice 2 weeks after HSV-1 inoculation, while the severity of these signs was noticeably reduced in the placebo-treated female mice. An observer skilled in assessing HSV eye disease, but unaware of the identity of the mouse groups, graded periocular hair loss and skin changes at 17 days after HSV-1 inoculation as follows: 0, no abnormality; 1, eyelid hair loss; 2, periocular hair loss extending <3 mm from the eyelid; 3, more extensive hair loss, but not involving the entire right side of the head; 4, hair loss on the entire right side of the head; and 5, skin breakdown with bleeding on the right side of the head. These clinical scores reflect the extent of HSV-1-induced lesions at the acute stage of infection. As shown in Fig. 3, there was considerable overlap in the eye disease scores in the three inoculation groups. However, none of the females had scores of 5, whereas three males and two DHT-treated females were scored at this highest level of disease severity.
FIG. 2.
HSV eyelid and periocular skin disease. A photograph of representative mice from each of the male, placebo-treated female, and DHT-treated female groups of mice shows severity of eyelid disease and periocular skin disease 2 weeks after corneal inoculation of HSV-1. Hair loss and skin lesions are prominent in the right eye of male and DHT-treated female mice, but not placebo-treated female mice (the affected area is indicated).
FIG. 3.
Clinical eye disease scores. Scores for periocular skin and eye disease in individual RGKO male, placebo-treated female, and DHT-treated female mice 2 weeks after HSV-1 inoculation of the right eye are presented. Horizontal bars indicate the mean scores.
To evaluate the hypothesis of more severe disease in male mice, the individual scores in the male group and the two groups of female mice were analyzed as ordered categories, by ordinal logistic regression to test for linear-by-linear association. The experimental groups were regarded as ordered categories with regard to testosterone levels, with DHT-treated females considered intermediate between males and placebo-treated females based on DHT treatment. Computing was done with SAS release 6.12 for Windows NT version 4. Where multiple versions of a test were computed (e.g., likelihood ratio and score tests), the more conservative result is given. The ordinal logistic regression of clinical scores revealed a positive association between skin disease severity and the ordering of the groups with regard to testosterone levels (P = 0.026), but corneal disease scores (not shown) were not significantly different. No departures from the logit-scale additivity assumed in the ordinal regression were detected (P > 0.6).
IFN-γ-dependent mechanisms have been reported to play a crucial role in the control of HSV periocular skin lesions (17, 31, 34). Consistent with these reports, we found more severe periocular skin and eyelid disease in male RGKO mice than in male 129/Sv//Ev mice. However, similar to our results on mortality (Table 1) and reactivation (Table 2), no clinical eye disease difference was noted in a comparison of female RGKO and female 129/Sv//Ev mice (not shown). These observations further underscore HSV gender differences and also show that some mechanism other than IFN-γ accounts for the milder eye disease in females.
Using a corneal disease rating scale without slit lamp biomicroscopy, we found more severe corneal disease in males, but unlike periocular skin disease, the gender difference for corneal disease did not reach significance. Published studies have shown that the development of corneal eye disease is strongly correlated with an inflammatory response characterized by CD4+ Th1 cytokine-producing T cells (14, 17, 26), whereas disease remission is accompanied by a shift to a Th2 profile (2, 9). To determine whether there are differences in specific cytokine production in our system, we have done quantitative reverse transcription-PCR assays of spleen cell cultures restimulated with HSV-1 antigen 72 h prior to the assay. Preliminary results with pooled RNA samples from mice in each group indicated that males and DHT-treated females had a Th1-like profile, producing IFN-γ and interleukin 12 (IL-12) mRNA transcripts. In contrast, placebo-treated females had a Th2-like profile, producing IL-4 and IL-10 and much lower levels of IFN-γ and IL-12 mRNA transcripts (not shown). Whether DHT reproducibly shifts cytokine profiles from Th2 to Th1 patterns in HSV-1-infected female mice awaits more definitive demonstration in individual as opposed to pooled mice.
The influence of sex hormones on the immune response has only recently been appreciated as a potentially important factor in the development of certain diseases (10, 37). In humans, myocarditis associated with coxsackievirus infection occurs predominantly in adolescent and adult males. Studies in the murine coxsackievirus model (CVB3) revealed that estrogen mediates resistance to CVB3-induced myocarditis, whereas elevated testosterone and progesterone levels in males and pregnant females confer susceptibility (24). Importantly, further studies revealed that testosterone treatment favored development of myocarditis by promoting induction of CD4+ Th1 cells secreting IFN-γ, whereas estrogen induced protective CD4+ Th2 cells secreting IL-4 (21). In another example of sex hormones influencing the immune response and disease outcome, testosterone treatment of female mice was shown to protect against the development of EAE through the induction of a biased Th2 response involving IL-10 production (10). The regulation of cytokine synthesis by steroid hormones in a variety of cell types suggests a possible role of sex hormones in regulating the balance between Th1- and Th2-type responses (7, 12). In principle, sex hormones could modulate the immune response and thereby affect the outcome of infection and disease. From limited studies, it is apparent that treatment with sex hormones in different disease or infection models can have vastly different outcomes, reflecting the complex interactions between sex hormones, the hypothalamic-pituitary-adrenal axis, and the immune system under the influence of the target tissues, the local physiological milieu, the genetic background, and the age of the animals (36).
In summary, we have shown that there are significant gender differences in mortality, virus reactivation, and clinical disease after HSV-1 inoculation of mice. There are probably multiple, yet to be described mechanisms for these differences. However, a novel finding of our study is that gender markedly affected the response of the GKO and RGKO mice to HSV-1 inoculation: in a comparison of null mutant to control mice, mortality and reactivation differences were seen only in males. This finding implies that some normal functions of IFN-γ are gender specific, in that IFN-γ plays a more important role in HSV-1 infection in male mice than in female mice. Although the IFN-γ promoter has been shown to be regulated by estrogen in vitro (13), to the best of our knowledge, this is the first report documenting in vivo gender-specific effects of IFN-γ.
Acknowledgments
We thank Michel Aguet (University of Zurich, Zurich, Switzerland) for the IFN-γR−/− mouse strain, Timothy Stewart (Genentech, Inc., San Francisco, Calif.) for the ES clone with a null mutation in the IFN-γ gene, and R. L. Thompson for communicating the modified hyperthermia procedure to us. We are grateful to Heather Adams (Animal Resources Center) for help with implantation of the DHT pellets in the mice.
This work was supported by Public Health Service grant MH55784 from the National Institute of Mental Health.
REFERENCES
- 1.Alexander J, Stimson W H. Sex hormones and the course of parasitic infection. Parasitol Today. 1988;4:189–193. [Google Scholar]
- 2.Babu J S, Kanangat S, Rouse B T. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J Immunol. 1995;154:4822–4829. [PubMed] [Google Scholar]
- 3.Barna M, Komatsu T, Bi Z, Reiss C S. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 4.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J Virol. 1999;73:5196–5200. doi: 10.1128/jvi.73.6.5196-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- 8.Curiel R E, Miller M H, Ishikawa R, Thomas D C, Bigley N J. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J Interferon Res. 1993;13:387–395. doi: 10.1089/jir.1993.13.387. [DOI] [PubMed] [Google Scholar]
- 9.Daheshia M, Kuklin N, Kanangat S, Manickan E, Rouse B T. Suppression of ongoing ocular inflammatory disease by topical administration of plasmid DNA encoding IL-10. J Immunol. 1997;159:1945–1952. [PubMed] [Google Scholar]
- 10.Dalal M, Kim S, Voskuhl R R. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 11.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 12.Daynes R A, Araneo B A, Hennebold J, Enioutina E, Mu H H. Steroids as regulators of the mammalian immune response. J Investig Dermatol. 1995;105:14S–19S. doi: 10.1111/1523-1747.ep12315187. [DOI] [PubMed] [Google Scholar]
- 13.Fox H S, Bond B L, Parslow T G. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- 14.Gangappa S, Manickan E, Rouse B T. Control of herpetic stromal keratitis using CTLA4Ig fusion protein. Clin Immunol Immunopathol. 1998;86:88–94. doi: 10.1006/clin.1997.4460. [DOI] [PubMed] [Google Scholar]
- 15.Gebhard J R, Perry C M, Harkins S, Lane T, Mena I, Asensio V C, Campbell I L, Whitton J L. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol. 1998;153:417–428. doi: 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 17.Hendricks R L, Tumpey T M, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 18.Hill T J, Yirrell D L, Blyth W A. Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J Gen Virol. 1986;67:309–320. doi: 10.1099/0022-1317-67-2-309. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 20.Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber S A, Kupperman J, Newell M K. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoblich A, Gortz J, Harle-Grupp V, Falke D. Kinetics and genetics of herpes simplex virus-induced antibody formation in mice. Infect Immun. 1983;39:15–23. doi: 10.1128/iai.39.1.15-23.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lekstrom-Himes J A, Pesnicak L, Straus S E. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J Virol. 1998;72:2760–2764. doi: 10.1128/jvi.72.4.2760-2764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leslie K, Blay R, Haisch C, Lodge A, Weller A, Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989;2:191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggioncalda J, Mehta A, Su Y H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 26.Niemialtowski G M, Rouse B T. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 27.Reeves W C, Corey L, Adams H G, Vontver L A, Holmes K K. Risk of recurrence after first episodes of genital herpes. Relation to HSV type and antibody response. N Engl J Med. 1981;305:315–319. doi: 10.1056/NEJM198108063050604. [DOI] [PubMed] [Google Scholar]
- 28.Sarvetnick N, Fox H S. Interferon-gamma and the sexual dimorphism of autoimmunity. Mol Biol Med. 1990;7:323–331. [PubMed] [Google Scholar]
- 29.Sawtell N M, Poon D K, Tansky C S, Thompson R L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciammas R, Kodukula P, Tang Q, Hendricks R L, Bluestone J A. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 33.Slifka K M, Whitton J L. Antigen-specific regulation of T cell-mediated cytokine production. Immunity. 2000;12:451–457. doi: 10.1016/s1074-7613(00)80197-1. [DOI] [PubMed] [Google Scholar]
- 34.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 35.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dis. 1991;13:1139–1150. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan D A. Sex hormones and Sjogren's syndrome. J Rheumatol. 1997;24(Suppl. 50):17–32. [PubMed] [Google Scholar]
- 37.Toda I, Wickham L A, Sullivan D A. Gender and androgen treatment influence the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary tissues of MRL/lpr mice. Clin Immunol Immunopathol. 1998;86:59–71. doi: 10.1006/clin.1997.4466. [DOI] [PubMed] [Google Scholar]
- 38.Yirrell D L, Blyth W A, Hill T J. The influence of androgens on paralysis in mice following intravenous inoculation of herpes simplex virus. J Gen Virol. 1987;68:2461–2464. doi: 10.1099/0022-1317-68-9-2461. [DOI] [PubMed] [Google Scholar]