Abstract
During the recent COVID-19 pandemic, wastewater-based epidemiological (WBE) surveillance played a crucial role in evaluating infection rates, analyzing variants, and identifying hot spots in a community. This expanded the possibilities for using wastewater to monitor the prevalence of infectious diseases. The full potential of WBE remains hindered by several factors, such as a lack of information on the survival of pathogens in sewage, heterogenicity of wastewater matrices, inconsistent sampling practices, lack of standard test methods, and variable sensitivity of analytical techniques. In this study, we review the aforementioned challenges, cost implications, process automation, and prospects of WBE for full-fledged wastewater-based community health screening. A comprehensive literature survey was conducted using relevant keywords, and peer reviewed articles pertinent to our research focus were selected for this review with the aim of serving as a reference for research related to wastewater monitoring for early epidemic detection.
Keywords: wastewater-based epidemiology (WBE), operational challenges, disease surveillance, pandemic preparedness, automation, artificial intelligence
1. Introduction
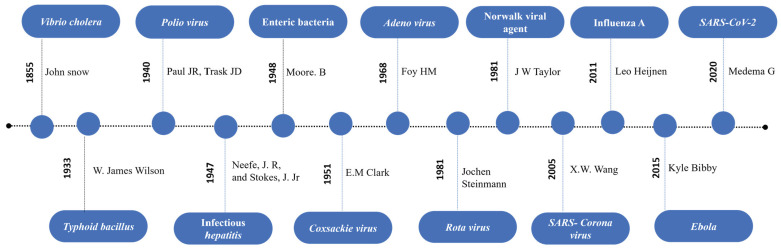
Wastewater is mostly considered a source of environmental pollution, and most established tests are performed to determine the pollutant load and to assess the treatment efficacy. However, it also provides useful information including biological traces, lifestyle drugs [1,2], and a variety of disease markers that threaten our well-being [3]. In 1854, John Snow first documented the waterborne transmission of Cholera and was able to identify the exact source [4]. Since then, epidemiologists studied water to understand how diseases spread and how they can be tracked to contain disease outbreaks, such as polio [5] and hepatitis A virus (HAV) [6]. A study of influenza in wastewater was performed during the H1N1 (swine) influenza virus outbreak. While influenza A viruses were found in sewage, the H1N1 pandemic virus was not detected in wastewater. This study confirmed the ability of quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) testing to detect influenza viruses in sewage samples [7]. Similarly, Aeromonas hydrophilia, E. coli, [8] Enterococcus spp., Staphylococcus spp., and coliform bacteria [9] were successfully detected from wastewater using a PCR-based method. In the following years, Enteric viruses such as noroviruses (NoVs), sapovirus (SV), hepatitis A and E viruses, adenoviruses (AdVs), rotaviruses (RVs), astroviruses (AstVs) [10,11,12], and respiratory viruses [13] were detected from wastewater samples. Recent reports of Zika and Ebola virus in wastewater suggest that these viruses could also be potential targets for continuous wastewater surveillance [14,15,16]. The milestones and pathogens reported from wastewater are summarized in Figure 1 below.
Figure 1.
Chronological representation of major milestones of wastewater-based epidemiology [17,18,19,20,21,22,23,24,25,26,27].
Recently, WBE has been widely used to monitor severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections [27,28]. Several countries deployed wastewater-based surveillance for SARS-CoV-2, as this method overcomes limitations of clinical surveillance, helps screening both symptomatic and asymptomatic individuals, and is more economical. Table 1 provides a detailed list of countries conducting wastewater surveillance for pathogens, along with links to their respective data dashboards. WBE has the potential to complement clinical infectious disease surveillance and can act as an early warning system for disease outbreaks [29]. This narrative review discusses the challenges faced in wastewater surveillance for infectious disease and recent advancements and explores prospects of full-fledged wastewater-based communal screening.
Table 1.
List of available wastewater surveillance databases.
| S. No | Country | Testing Agency | Target Tested | Data/Dashboard Link |
|---|---|---|---|---|
| 1 | United State of America | CDC’s National Wastewater Surveillance System (NWSS) | SARS-CoV-2, Respiratory syncytial virus (RSV), Influenza A, Mpox | NWSS Wastewater Monitoring in the U.S. (https://www.cdc.gov/nwss/index.html, accessed on 14 October 2024) |
| 2 | Republic of Korea | Department of Analysis of High-Risk Pathogens | SARS-CoV-2 | Korea Disease Control and Prevention Agency (https://kdca.go.kr/, accessed on 14 October 2024) |
| 3 | Japan | National Institute of Infectious Diseases | SARS-CoV-2 | NIJIs—Novel Coronavirus Survey Project in Sewage (https://nijis.jp/#links, accessed on 14 October 2024) |
| 4 | New Zealand | ESR (Environmental Science and Research) | SARS-CoV-2 | ESR Wastewater Surveillanc (https://esr-cri.shinyapps.io/wastewater/#region=Wellington&log_or_linear=linear&period=twelveMonthsButton, accessed on 14 October 2024) |
| 5 | Australia | Government of Western Australia, | SARS-CoV-2 and its variants | COVID-19 wastewater surveillance (https://www.health.wa.gov.au/articles/a_e/coronavirus/covid19-wastewater-surveillance accessed on 14 October 2024) |
| New South Wales (NSW), health | Influenza and SARS-CoV-2 | NSW respiratory surveillance—COVID-19 and influenza (https://www.health.nsw.gov.au/Infectious/covid-19/Pages/reports.aspx accessed on 14 October 2024) | ||
| 6 | India | CSIR-National Chemical Laboratory (NCL), Symbiosis School of Biological Sciences (SSBS), and the Indian Institute Science Education and Research (IISER) Pune | H1N1, H3N2, Influenza A, and SARS-CoV-2 | Wastewater Surveillance Dashboard For Infectious Diseases (COVID-19, H1N1, H3N2, Influenza-A) (https://www.pkc.org.in/pkc-focus-area/health/waste-water-surveillance/wws-covid-dashboard-pune/ accessed on 14 October 2024) |
| 8 | Turkey | Turkish Water Institute | SARS-CoV-2 | Türkiye Genelinde COVID-19 Yayılımının Atık Sularda SARS-CoV-2 Analizleri ile Takibi (https://covid19.tarimorman.gov.tr/Home/Index accessed on 14 October 2024) |
| 9 | Switzerland | ETH, Zurich | SARS-CoV-2, Influenza A, B and RSV | Wastewater Surveillance (https://wise.ethz.ch/ accessed on 14 October 2024) |
| 10 | European Union | Digital European Exchange Platform (DEEP)/European Commission’s Health Emergency Preparedness and Response Authority (HERA) | SARS-CoV-2 and Variants of concern | Official SARS-CoV-2 sewage surveillance in the EU (https://wastewater-observatory.jrc.ec.europa.eu/#/dashboards/1/47 accessed on 14 October 2024) |
| 11 | Canada | Provincial, territorial, and academic partners across Canada | SARS-CoV-2, Influenza, RSV | Respiratory viruses: Wastewater monitoring dashboard: Respiratory virus activity (https://health-infobase.canada.ca/wastewater/ accessed on 14 October 2024) |
| 12 | South Africa | National Institute for communicable Diseases | SARS-CoV-2 and Variants of concern | Wastewater-Based Epidemiology For SARS-CoV-2 In South Africa—NICD (https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/weekly-reports/wastewater-based-epidemiology-for-sars-cov-2-in-south-africa/ accessed on 14 October 2024) |
| 13 | Global Database-Wastewater SPHERE (SARS Public Health Environmental Response) and COVID poops 19 | Data contributed by multiple organizations | SARS-CoV-2 | About: Wastewater SPHERE (https://sphere.waterpathogens.org/about accessed on 14 October 2024) |
A comprehensive literature review was conducted to gather relevant data for this study. Key search terms included “wastewater surveillance pathogen”, “public health”, “sampling for wastewater monitoring”, and “automation in wastewater surveillance” and were used across databases including PubMed, Pro Scopus, ScienceDirect, and JSTOR. Articles were selected by two independent reviewers (WE and BKS) based on their relevance and connection to the study’s focus. Only peer reviewed and published articles were considered in this review.
1.1. Advances in Understanding the Key Factors for Wastewater-Based Epidemiology
1.1.1. Determining the Scale of Sampling
Traditionally, sampling sites and scales are chosen based on high-risk or vulnerable locations identified by epidemiologists. Choosing a suitable sampling technique and scale for wastewater analysis is crucial for obtaining a true representation [33]. Each sampling scale or site has its own merits and disadvantages. Screening at an inlet of a centralized wastewater treatment facility helps understand the disease dynamics of the catchment area. However, it fails to accurately specify the hotspot of infection (outbreak region), and a detailed map and a well-established sewer network is needed to facilitate narrowing down the areas of interest and enable a more accurate interpretation [34]. Alternatively, samples collected from sewer line manholes can provide geographically more precise data [35]. The tagging of manholes with geographical information (GIS) and access to population data can improve the correlation of results. This approach requires multiple sampling points to cover a region, and accessing a manhole located in a high-traffic location requires special permission from authorities. Other sampling sites, such as those from closed communities, are more precise, with most studies and simulations performed in such closed setups aiming toward a better understanding and correlation with clinical testing [36,37,38,39]. Overall, sampling at a centralized wastewater treatment facility is more practical and economical for routine analysis; upon identification of a biomarker or agent of interest, the source can be further investigated upstream and can be located via more intense sampling at the level of neighborhood sewer manholes, blocks, and buildings, followed by clinical surveillance for the implementation of mitigation efforts.
Wastewater samples from cruise ships and long-haul passenger aircrafts have also been studied in recent times [40,41]. As air travel is one of the prime means of infectious disease spreading worldwide, the probability of detecting pathogens from aircraft samples relies on lavatory usage. According to survey results conducted by Davey. L Jones et al. [42], approximately 13% of passengers defecate during short-haul flights, and it rises to 36% for long-haul flights. Wastewater collected from the aircraft lavatories provided an earlier detection when compared to respiratory swab testing on 30% of travelers for SARS-CoV-2 screening [43].
1.1.2. Sampling Techniques Adopted for Wastewater-Based Surveillance
WBE necessitates standardization in several aspects, including the selection of appropriate sampling techniques. Several attempts have been made to develop a simple, yet efficient sampling method to capture the entire microbiome of wastewater [44,45]. The three widely studied sampling methods are grab sampling (sample collected at a preset timing) from the source, composite sampling using an autosampler (a composite sample is made up of aliquots that are collected over a period, often 24 h), and passive sampling using a membrane attached to a suitable support (deployment of a device with a special membrane that interacts/attracts the biomarker present in the wastewater). In most cases, a grab sampling approach is used to cover larger populations (~100,000 to 300,000), while a composite sampling approach is employed for smaller catchment populations.
1.1.3. Comparison of Different Sampling Methods—Passive Sampling
Passive sampling is achieved by exposing a collecting medium to wastewater, thereby enabling the exchange of analytes between the medium and the sample. Rafiee et al. (2021) [46] compared different sampling methods and suggested that the passive sampling by Moore [21,47] is equally efficient as composite sampling for SARS-CoV-2 virus detection and variant profiling in sewage [48,49]. Similarly, Schang et al. (2021) [50] reported that passive and composite sample methods are more effective at providing meaningful results for catchment areas [51]. Recently, different passive sampling devices have been designed and tested for wastewater. Melissa et al. (2022) [52], used a torpedo-style 3D-printed passive sampler device containing cotton swabs and an electronegative filter membrane and reported greater sensitivity than grab and composite (autosampler) sampling [53]. Acer et al. (2022) [54] attempted a passive sampling approach using tampons made from rayon with a polyester string and showed a positive correlation between wastewater viral load and positive caseload in the sampling region [55]. In a similar study, Michael et al. (2024) [56] attempted passive sampling using cost-effective sorption materials, such as cheesecloths, gauze swabs, electronegative filters, glass wool, and tampons housed in a torpedo-style setup, compared with standard composite sampling. Their research aimed to detect SARS-CoV-2, influenza A and B viruses, crAssphage, and human AdV. The authors concluded that the selected passive sampling method/material was more effective for detecting other viruses studied than for SARS-CoV-2. Bivin et al. (2022) [44] undertook a comprehensive review of passive sampling and demonstrated a heterogeneous correlation with concentrations from paired composite samples of individual studies. Further studies have recommended that the usage of electronegative membranes over nitrocellulose serves effectively for monitoring SARS-CoV-2 viruses. Although passive samples are comparable to other sampling methods, the main drawbacks of passive sampling are that the medium tends to saturate with time and the specificity of material used toward the pathogen of interest, and the results acquired are mostly used for qualitative or semi-quantitative analysis.
1.1.4. Composite Sampling and Grab Sampling
A composite sample is a single sample volume made up of aliquots, collected over a predetermined amount of time. The method is further divided into time-dependent and flow-dependent applications, where the aliquot volume is altered in a way that is proportional to the wastewater flow, or the sample time is altered based on the application. Ahmed et al. (2021) [57] demonstrated in their study that 24 h composite samples offered increased sensitivity and decreased variability compared to 1 h composite samples when monitoring wastewater for pathogenic viruses with low infection rates within a community. In contrast, a study conducted by Augusto et al. (2022) [58] on SARS-CoV-2 detection showed that grab samples collected during peak flow matches the mean value of the 24 h composite samples. The release of biomarkers in wastewater changes throughout the day due to diurnal activity. Therefore, obtaining a grab sample at a predetermined time is likely to capture most of the predominant pathogens from wastewater. However, this approach might fail to detect pathogens of low concentration. Although the grab sample method is easy to operate but prone to variability in results, the composite sample method provides stable and accurate data. However, its prohibitive cost and operational difficulties make it unsuitable for small-scale studies [59]. Furthermore, for WBE applications, the usage of 24 h composite samples and passive sampling techniques with electronegative mesh are recommended, as a more precise method to provide accurate results. Both sampling methods are reliable, whereas passive sampling is more practical, efficient, and cost-effective. Table 2 provides a summary of various sampling methods used for wastewater surveillance.
Table 2.
Summary of different sampling methods for detection of pathogen from wastewater.
| Sampling | Volume/Duration of Exposure | Pathogen | Detected Concentration/Positive Ratio | Ref. | Remarks |
|---|---|---|---|---|---|
| Grab | - | SARS-CoV-2 | N1 gene-5.5 log 10 copies/L N2 gene-6.4 log 10 copies/L |
[58] | The grab samples, collected between 8 a.m. and 10 a.m., showed less variability than composite sample. |
| Composite | Varied based on the wastewater flow rate. | SARS-CoV-2 | N1 gene-5.3 log 10 copies/L N2 gene-6.3 log 10 copies/L |
||
| Passive sample using cotton buds, electronegative membranes, and medical gauze Composite sampling |
24 h 24 h |
SARS-CoV-2 | 25% 41% 31% 50% |
[50] | Passive samples made of affordable materials can be used as an economic alternative to expensive auto samplers. The author further used 3D-printed housing units to maintain the mass transfer efficiency. |
| Composite sampling | 1 h |
|
log 10 Gene copies/mL
|
[57] | The author advocates that 24 h composite samples are likely to be superior to 1 h composite samples. |
| Composite sampling | 24 h |
|
log 10 Gene copies/mL
|
||
Passive sampling using
|
One week | SARS-CoV-2 | Mean gene copies/L
|
[49] | The study depicts a positive correlation with composite samples, tested municipal WWTP, and passive samples collected at a city scale. |
| Passive sampling using tampons | 24 h | SARS-CoV-2 | Median gene copies/day N2 = 1.29 × 109 N1 = 1.04 × 109 |
[54] | The author quantified viral RNA by two methods, N1 and N2, and recommends passive sampling approach due to its ease of operation. |
Passive sampling using
|
24 h | SARS-CoV-2 | E gene copies/24 h
|
[56] | The passive sample approach and the medium used was more suitable for other pathogen studies in the work (AdV and Influenza virus). |
| Composite sample: 24 h composite sample |
24 h | E gene copies/24 h 5.4 × 107 |
1.1.5. Shelf Life and the Importance of Turnaround Time
The persistence of bacteria in wastewater is affected by several factors such as temperature, dissolved oxygen, pH, nutrient availability, and salinity [60]. Bacteria tends to survive longer in wastewater than in surface water under similar storage conditions [61]. Shuxin Zhang et al. (2023) [62] investigated the decay of two gastrointestinal pathogens, Campylobacter jejuni and Campylobacter coli, in wastewater. The decay under different sewer conditions was investigated in a reactor, and the result signifies that at a higher spike concentration (10−6/mL), about 9.76% recovery was achieved after 36 h. Considerable variations in the recovery were also observed within the same genus. Unlike bacteria, viruses need a host cell to multiply, and therefore the viral concentration present in wastewater would be proportional to the concentrations excreted by the corresponding population.
Diversity in the wastewater matrix, dilution effects, and the presence of reactive chemicals may affect the viral particle concentration and integrity [63]. Thus, the interpretation of results requires an understanding of the stability of the virus in wastewater. The effect of temperature on SARS-CoV-2 RNA detection in wastewater surveillance has not been considered in most reported studies [64]. Non-enveloped viruses have been reported to be more stable at varying temperatures than enveloped viruses [65,66]. In winter months, the viruses are more stable in wastewater and can be detected for up to 100 h, whereas in summer months, it is reduced to 20 h. These temperature-related variations were comparatively lower in locations with lower water temperatures, as the viral RNA was preserved for a longer duration [67]. Lisa et al. (2009) [68] evaluated the survival of two surrogates of coronaviruses, transmissible gastroenteritis (TGEV), and mouse hepatitis (MHV). At 4 °C, there was a <1 log10 decrease in infectivity for both viruses after four weeks. In pasteurized sewage, a 99% reduction was reported in 9 days for TGEV and 7 days for MHV. In a study conducted by Markt et al. in 2021 [69], the author compared the impact of storing 24 h composite wastewater samples at temperatures of −20 °C and ≤4 °C and observed that the storage of wastewater for up to 9 days at 4 °C did not have a significant impact on the number of detectable SARS-CoV-2 fragments. Similarly, Ahmed et al. (2020) [70] compared different storage conditions and different matrix effects and reported a decay rate of approximately 8% per day at 4 °C. The impact of storing SARS-CoV-2 in wastewater sludge at varying storage temperatures (4 °C, −2 °C, and −80 °C) was studied by Simpson et al. (2021) [71]. The author observed no change in concentration by 7 to 8 days when stored at 4 °C, and by 35 to 122 days, about a 60% reduction was observed. The author further reports that SARS-CoV 2 RNA in sludge is more stable in a freeze–thaw cycle than in a liquid effluent. Bonnie et al. (2022) [72] reported a seven-fold decrease in viral load when stored at−80 °C for 92 days compared to without freezing, and the author further observed that storage in a glycine-releasing buffer resulted in four-fold inhibitions. Attempts were made to filter the sample and store the filter paper containing virus particles; at −80 °C, this procedure had no significant loss in viral concentration for up to a month [73]. Several studies have used population biomarkers in wastewater to normalize the pathogen load [74,75]. Normalizing SARS-CoV-2 RNA concentrations by concentrations of PMMoV RNA, an endogenous wastewater virus, can correct for changes during storage, as the effect of storage parameters on PMMoV RNA is like that on SARS-CoV-2 RNA [76]. Finding a suitable surrogate for all other circulating pathogens is yet to be studied. In the case of samples collected from aircrafts, the pathogens are usually inactive as modern aircraft lavatory storage tanks are preloaded with bactericidal and viricidal agents to prevent disease spread. It is advisable to acquire an understanding of the target organism’s shelf life and the complexity of the wastewater before sample analysis. Based on the literature, a sample should be processed as soon as it reaches the laboratory. Under unforeseen circumstances, wastewater samples can be stored at 4 °C and the results should be adjusted based on the delay in analysis.
1.2. Advancements in Analytical Techniques for Detection of Pathogen from Wastewater
1.2.1. PCR Based Detection Method
The identification and characterization of pathogenic microbes from wastewater microbiomes pose several challenges by classical plating and enumeration methods. The process is even more cumbersome for virus detection (e.g., polio), as the wastewater sample must undergo a high-volume chemical concentration method (PEG/Dextran) followed by cell line studies and then molecular typing for the positive samples [77]. The process of culturing viruses requires class 3 facilities and highly trained personnel to perform the analysis. Molecular methods circumvent these challenges and have made it possible to analyze and categorize the diversity of hazardous microorganisms. Nucleic Acid Amplification Tests (NAATs) are useful for detecting pathogens in wastewater samples [78]. A Spanish study demonstrated the detection of SARS-CoV-2 in wastewater while patients’ nasal swab PCR tests produced a negative result [79]. This has led to the acceleration of molecular technology as a method of choice for wastewater analysis [80]. There has been a combination of new detection tools utilized for the quantification and analysis of various gene targets to improve the sensitivity and accuracy of pathogen detection, which has led to the discovery of new surveillance opportunities [81]. However, it is important to consider the differences in PCR efficiencies and how this can significantly affect the quantification accuracy and the limit of detection [82]. The sensitivity of different PCR techniques is summarized in Table 3 and the pathogens identified in wastewater through various PCR analysis techniques are shown in Table 4.
Table 3.
Sensitivity of different detection methods for WBE.
| S. No | Sensitivity | Method Used | Reference |
|---|---|---|---|
| 1 | 2.9–4.6 genome copies/reaction | RT-dPCR | [83] |
| 2 | 0.066 copies/μL | Reverse transcription-droplet digital PCR (RT-ddPCR) | [84] |
| 3 | 25 × 102 copies/μL | RT-ddPCR | [85] |
| 4 | 0.4 copies/μL | Reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) | [86] |
| 5 | 0.31 × 10−3 ng/μL | RT-LAMP | [87] |
| 6 | 0.0093–9.3 copies/μL | RT-LAMP | [88] |
| 7 | 105 copies/μL | Nested PCR | [89] |
| 8 | 1.67 plaque forming units (PFU) | NESTED PCR | [90] |
| 9 | 7.8 × 103 viruses/liter | NESTED PCR | [10] |
| 10 | 2.4 × 103 viruses/liter | NESTED PCR | [91] |
Table 4.
Summary of various pathogen detected from wastewater using different PCR and sequencing techniques.
| S. No | Targeted Organism | PCR Technique | Sequencing | Country | References |
|---|---|---|---|---|---|
| 1 | SARS-CoV-2 | Multiplex PCR | Nanopore sequencing and Illumina sequencing | Netherlands and Belgium | [92,93] |
| 2 | SARS-CoV-2 | qRT-PCR | Illumina sequencing | United Kingdom | [94] |
| 3 | SARS-CoV-2 | RT-ddPCR | metatranscriptomic sequencing-Illumina | United States | [95] |
| 4 | SARS-CoV-2 | qRT-PCR | Sanger Sequencing | United States | [96] |
| 5 | SARS-CoV-2 | qRT-PCR | - | Netherlands | [27] |
| 6 | SARS-CoV-2 | dd PCR | - | United States | [97,98] |
| 7 | SARS-CoV-2 | RT-ddPCR | NextSeq Illumina sequencing | Australia | [99] |
| 8 | Rotavirus A (RVA), AstV, NoV, AdV, and SV | RT-PCR | - | Japan | [12] |
| 9 | Influenza A and B virus, RSV, Mpox virus, human metapneumovirus (HMPV), NoV GII, and PMMoV | Droplet digital PCR (dd-PCR) | - | Central California, USA | [13] |
| 10 | Human AdV, HAV, NoV, and Salmonella enterica | Integrated cell culture-PCR (ICC-PCR) and qRT-PCR | - | United States | [100] |
| 11 | Enterovirus (EVs), NoV, AdV, Hepatitis A and E virus | qRT-PCR and Cell culture | - | Italy | [101] |
| 12 | NoV | RT-PCR | Illumina sequencing | South Africa | [102] |
| 13 | Influenza A virus | ddRT-PCR | Sanger Sequencing | United States | [103] |
| 14 | Mpox virus | qPCR | Sanger Sequencing | Canada | [104] |
| 15 | Mpox virus | qPCR | Sanger Sequencing | Netherlands | [105] |
| 16 | Campylobacter jejuni | qRT-PCR and Multiplex PCR | 16S rRNA | Canada | [106] |
| 17 | C. jejuni and C. coli | qPCR | - | Australia | [62] |
| 18 | Escherichia coli | qRT-PCR | Illumina sequencing | Germany | [107] |
| 19 | Escherichia coli | PCR | Illumina sequencing | Czech Republic | [108,109] |
| 20 | E. coli and Klebsiella spp. | PCR | ONT and Illumina sequencing | Switzerland | [110] |
| 21 | Influenza A(H5N1) | dPCR | - | USA | [111] |
| 22 | Polyomaviruses (KI, WU, and Merkel cell polyomavirus) | Nested PCR | - | Barcelona | [112] |
| 23 | Salmonella, Campylobacter, and NoV | qPCR and RT-qPCR | - | Oklahoma, USA | [113] |
| 24 | Salmonella | - | Illumina MiSeq | Hawaii | [114] |
Over the last few years, digital PCR (dPCR) has grown in popularity, especially for wastewater surveillance [84]. The principle of dPCR is to divide the sample into several independent partitions so that each contains few or no target sequences. Then, the dispersion of the target sequences in the partitions can be approximated using a Poisson distribution [115]. The ratio of the positive distributions (presence of fluorescence) to the total number makes it possible to determine the concentration of the target in the sample [80]. In a study made by Flood et al. (2021) [116], the authors revealed that the observed coefficient of variation (CV) for RT qPCR measures were much larger than the dPCR results for the absolute measurement of SARS-CoV-2 genetic markers in various wastewater matrices. In studies conducted by W Ahmed et al. (2022) [83,99], it was observed that untreated effluent from aircraft demonstrated the potential of dPCR to detect a higher number of positive samples compared to qPCR. Additionally the limit of detection using dPCR were approximately 2–5 times lower than those of observed with RT-qPCR. A cheaper and faster alternative method to dPCR is the NESTED PCR; this method was designed to improve the sensitivity and specificity of the reaction. It involves two sets of primers instead of one in two successive PCR reactions, as this improves the sensitivity and specificity. The advantage of this method is that it provides an exceptionally low probability of nonspecific amplification [117].
Another alternative technique that amplifies DNA with high specificity is the loop-mediated isothermal amplification (LAMP) technique. This technique has some advantages over qPCR and is used often in diagnostics [118]. LAMP PCR is one of the quickest, as the whole amplification can be performed under one set temperature, whereas PCR requires several cycles of different temperatures. Tumino et al. (2020) [119] compared the LAMP assay to a well-established TaqMan real-time PCR method for the detection of Mycoplasma agalactiae and showed that the LAMP assay was faster and more sensitive than the real-time PCR method (90% vs. 77%). Even though the LAMP-based method has a high sensitivity, its performance is affected when the infection prevalence is low. In areas where the infection number is low, the detection limit of 10 copies/25 µL may be a limitation. However, there were some noticeable improvements when the initial sample volume was increased from 1 to 5 µL [86]. Nonetheless, qualitative analysis now makes the most use of the RT-LAMP technique. As a result, its application is restricted to target detection and cannot offer information on the viral load. Nevertheless, as an early warning system, RT-LAMP is considered an ideal technique, as it provides information about the presence of SARS-CoV-2 in wastewater [120]. JE Ongerth et al. (2021) [121] used RT-LAMP in a quantitative application for the detection of SARS-CoV-2 in raw sewage. Primer sets targeting ORF1a, E- and N-gene regions were chosen to test for method performance characteristics for SARS-CoV-2 detection in the raw sewage of samples from a municipal sewage system serving >600,000 people in Australia; although the viral quantification was not consistent, it detected the virus in all the samples from each of the three independent interceptors near the treatment terminus. Haorui Cao et al. (2022) [122] developed a point-of-use detection method for SARS-CoV-2 using CRISPR/Cas12a and RT-LAMP in a paper device. The author reported an analytical sensitivity of 97.7% and an 82% semiquantitative accuracy. More recently, a high throughput microfluidic quantitative PCR system (HT-qPCR) was used to analyze wastewater for the presence of multiple pathogens and antibiotic resistance genes (ARGs) in a single run. By using this approach, more reactions can be achieved in a single plate, thereby reducing cost and improving TATs [123]. In another attempt, Rao et al. (2024) [124] used the PCR-Taqman array card (TAC) to detect infectious pathogens in wastewater; in this study, the author was able to quantify and analyze 35 pathogenic targets including bacteria, viruses, protozoa, and helminths simultaneously. Further, the author used two PCR platforms to validate the TAC, i.e., RT-qPCR quantstudio and dPCR QIAcuity four. In summary, PCR-based methods for the detection of pathogens from wastewater have immense potential in both in situ and ex situ testing, and they are constantly evolving to meet our testing capabilities.
1.2.2. Wastewater Concentration for Enhanced Biomarker Detection
The sensitivity and accuracy of PCR techniques highly rely on the quality of the nucleic acid extracted from the wastewater. Unlike clinical samples, wastewater is diluted several thousand-fold and requires a concentration step prior to extraction to enhance the detection limit. Conventional concentration procedures involve polyethylene glycol (PEG), aqueous two-phase partitioning, ultracentrifugation, electronegative membrane filtration, ultrafiltration, salt-based precipitation, adsorption, and tangential flow filtration systems, and are laborious and time-consuming [125,126,127,128,129,130]. Recently, a high throughput automated sample concentration method has been suggested to reduce process fluctuation due to handling and reduce the labor-intensiveness of the protocol. Kevill. J.L. et al. (2022) [131] compared a PEG-based concentration method with precipitation (ammonium sulfate) and ultrafiltration using CP Select (Innovaprep) at different storage temperatures, turbidity, and surfactant concentrations for SARS-CoV-2 and fecal indicator virus (Assphage) recovery. Although all three chosen methods were found to be suitable, the PEG method yielded higher recovery due to longer exposure. After the precipitation and CP select methods, Wishlist CP recoveries are marginally better in quality and more consistent. This may be because most WBE studies target non-enveloped enteric viruses, whereas SARS-CoV-2 is an enveloped virus with a lipid layer that is sensitive to organic chemicals [132]. A single-step concentration method for SARS-CoV-2 using polyaluminum chloride precipitation was studied by Wehrendt et al. (2021) [133], where the authors report a 25-fold increase in sensitivity, and the precipitated sample was stable for a week at 4 °C. Similarly, Katayama et al. (2023) [134] used coagulation using poly aluminum chloride and digestion using proteinase K followed by magnetic bead-based extraction (COPMAN) for the extraction of SARS-CoV-2 from wastewater. The authors compared COPMAN with PEG, ultrafiltration, and Episens-S-based concentration methods [135] and reported a higher sensitivity and recovery compared to other methods in SARSCoV-2-spiked wastewater samples (25.2% (1.0 × 103 copies/mL of HI-SARS-CoV-2) [136]. Furthermore, this method helps recover viruses from liquid and solid fractions, thereby covering a wide range of targets. A direct capture method using silica-based columns (pure yield) for concentration followed by the purification of nucleic acid was proposed by Mondel et al. (2021) [137]. The result showed a 20-fold increase in SARS-CoV-2 RNA when compared with classical PEG/NaCl concentration. Peinado et al. (2022) [138] used a pretreatment step with glycine buffer and showed an increased sensitivity and recovery of control virus (MGV) using a centricon-based ultrafiltration and using adsorption–precipitation (aluminum hydroxide). An automated nucleic acid concentration protocol using magnetic beads in a Kingfisher flex system was reported by Karthikeyan et al. (2021) [139]. The authors reported a 19% higher recovery than PEG and a 14% higher recovery than the electronegative filtration system. The higher recovery was due to the sensitivity of the magnetic bead (Ceres Biotech, Inc., Manassas, VA, USA.), and the automated system eliminated handling discrepancies between samples. Understanding the sample chemistry and target partitioning in wastewater helps in determining the ideal concentration method. The time required to process, resources required, process complications, and labor intensiveness are to be considered as wastewater surveillance involves high-volume sample processing. None of the methods are capable of 100% recovery, suggesting a process investigation to identify the unforeseen loss during sample processing.
1.2.3. Nucleic Acid Extraction from Wastewater Sample
Several commercial nucleic acid extraction systems have been used for wastewater samples. The following section outlines a few significant attempts and comments on their suitability for large-scale operations. A cost-effective direct capture of SARS-CoV-2 from wastewater was proposed by Oscar. N. Whitney (2021) [140], using silica, salt, sewage, and SARS-CoV-2 (4S). The method uses an in-house developed lysis procedure using NaCl and EDTA and extraction using a silica column. This method can be used to extract other fecal viruses (PMMoV), and the extract is stable during storage. The author reports a six-fold increase in yield compared to the ultrafiltration method and is comparatively economical to perform at 13 USD per sample. O’Brien et al. (2021) [141] compared four commercial RNA extraction kits for wastewater collected from a small college population. The Qiagen PowerViral DNA/RNA kit, New England BioLabs Monarch RNA Miniprep Kit, ZymoZymo Quick RNA-Viral Kit, and Quick-RNA Fecal/Soil Microbe Micro Kit were used for comparisons. This study revealed that the Zymo Quick-RNA fecal/soil microbes are effective in extracting RNA from the sample. The author advocates that the use of the preservatives and the specificity of the kit contributed to a higher yield. This approach of concentration followed by purification is time-consuming and laborious in terms of the precipitation–centrifugation of large volumes of samples. Boehm A.B. et al. (2023) [142] used the Chemagic Viral DNA/RNA 300 kit H96 for the Perkin Elmer Chemagic 36.0; the author used a solid fraction of the wastewater sample and reported SARS-CoV-2, influenza A and B virus, RSV, Mpox virus, HMPV, NoV GII, and pepper mild mottle virus. The author performed a PCR inhibitor removal step using the Zymo OneStep-96 PCR Inhibitor Removal kit [97]. In another study, Pérez-Cataluña et al. (2021) [143] compared manual spin column-based extraction with magnetic silica bead-based extraction using the Maxwell RSC Instrument in wastewater seeded with gamma-radiated SARS-CoV-2, porcine epidemic diarrhea virus (PEDV), and mengovirus (MgV). This study showed that aluminum-based precipitation followed by extraction using magnetic beads delivered acceptable sensitivity and reproducibility. Similarly, Qiu et al. (2022) [144] compared five widely used commercially available RNA extraction kits (Qiagen, Venlo, The Netherlands, Thermo Fisher, Waltham, MA, USA, and Promega, Madison, WI, USA) for SARS-CoV-2 extraction from wastewater. Among the chosen kits, MagMAX-96 viral RNA extraction kits using the Kingfisher Flex automated system showed the highest recovery [139].
Although several attempts have been made to establish a standard method for the extraction of RNA/DNA from wastewater, defining a single universal method may not be appropriate because of the diverse nature of wastewater. A myriad of parameters must be considered to establish a method suitable for the regional wastewater. The decision about which method to adopt thus depends on the use case for wastewater testing and resource availability, sensitivity, operational feasibility, and scalability.
1.2.4. Process Automation for Wastewater Analysis
The early detection of infectious diseases plays a crucial role in mitigating the disease’s spread. The lead time for early detection is usually less and varies between diseases and/or until the infected individual seeks clinical testing [145]. As a result, wastewater analysis requires a short turnaround time (TAT) and a high sample frequency for the surveillance of any given catchment area. The primary challenges and potential solutions to meet the TAT are highlighted in the following section.
A manual microbiological analysis approach using plating and culturing is not sufficient to meet the short TAT. An alternative approach involves the use of automated robots for plating and molecular diagnostics. Several automated systems, including microbial plating machines [146], liquid handlers, and automated nucleic extraction systems, have been extensively used for WBE. A comparative study on different automated solutions for microbial plating was conducted by Antony Croxatto et al. (2015) [147] on the InoqulA (BD (Becton, Dickinson Kiestra, Becton Drive Frankilin Lakes, NJ, USA)) and Walkaway specimen processing (WASP) systems (Copan) with manual plating using a defined bacterial consortium and a cloudy urine sample. The InoqulA system produced more discrete colonies at higher concentrations and more statistically significant results than the other chosen methods [148]. A detailed study conducted by Culbreath et al. (2021) [149] revealed the benefits of implementing automation systems in four different facilities with different sample loads. About a 13 to 93% increase in productivity and substantial efficiency and cost savings by using automated microbial plating machines was observed. Similarly, several studies were conducted using liquid handlers and automated nucleic acid extraction systems. Karthikeyan et al. (2021) [150] used Kingfisher Flex (Thermo Fisher Scientific, Waltham, MA, USA) robot systems for the concentration and extraction of nucleic acid from wastewater. The customized extraction plates and reagents were prepared using the Eppendorf ep-motion automated liquid handler, which has reduced process fluctuation and time. Banadaki et al. (2023) [151] developed an automated version of the exclusion-based sample preparation (ESP) RNA concentration and extraction method using PIPETMAX (Gilson), compared the manual extraction efficiency with automated extraction, and proved that automated extraction increased the LOD and sensitivity. In another study, Pérez-Cataluña et al. (2021) [143] compared two concentration and two extraction systems (i.e., manual spin column-based extraction and automated magnetic silica-based extraction using Maxwell RSC Instrument) and concluded that no significant effect was observed in the concentration step, whereas automation using Pure Food GMO and an authentication kit (Promega) showed slightly higher sensitivity than manual extraction. In a similar study conducted by Nicholas W. West et al. (2022) [152], a Chemagic™ 360 instrument with a 12-rod head to extract nucleic acid from raw wastewater was used; unlike the standard method, which involves concentration using PEG/ultrafiltration, the sample (45 mL) was centrifuged and the supernatant (10 mL) was used for RNA extraction. Although these systems require significant capital investment, they are cost-advantageous in the long term because they increase throughput and minimize the workforce, leading to a reduction in the cost per sample [149]. All the automation systems discussed above have some unique advantages over others; it is up to the end user to choose one that is more appropriate for their laboratory setup and sample volume.
1.2.5. The Prospects of Wastewater-Based Epidemiology (WBE)
Application of Machine Learning for Outbreak Prediction
The value of the WBE can only be comprehended when it relates to the per capita infection and baseline data from normal surveillance settings. Interpreting such a large dataset manually will not be possible as many variables and individual parameters are considered to interpret the results. In recent times, the application of machine learning (ML) and artificial intelligence (AI) in WBE has been explored due to the capability of self-improvement, identifying patterns in data, and making predictions with limited human interventions. The ML model uses statistical analyses of data to train the parameters, thereby helping in the prediction and identification of patterns in the given dataset. Riberio et al. (2020) [153] attempted to forecast the spread of SARS-CoV-2 using a variety of models, ranging from a simple linear regression model to auto-regressive integrated moving average advance (ARIMA) and support vector regression (SVR) models. The author recommends utilizing the SVR model over the other chosen method for accurate forecasting with minimal errors. In a similar study using a random forest approach, Liam Vaughan et al. (2023) [154] explored the difficulties in machine learning and time series forecasting for COVID-19. The datasets were obtained from the W-SPHERE website (Global Water Pathogens Project 2022). The authors emphasize that sample frequency, training set size, population mobility, and the inclusion of an excessive number of characteristics are the main factors that impact prediction accuracy. A transferable artificial neural network (ANN) model was created by Guangming Jiang et al. [155] in 2022 to forecast SARS-CoV-2 transmission and pandemic dynamics using data gathered from Utah, USA. Prevalence and incidence rates may be predicted with accuracy using the ANN model. Similarly, a model known as the susceptible-exposed-infectious-recovered (SEIR) model was established by Christopher S. McMahan et al. [156] in 2021 to aid in the association between the concentration of SARS-CoV-2 gene fragments isolated in wastewater and the number of infected individuals in the catchment region. The proposed model is further validated with clinical data. Although machine learning and AI technologies are viable techniques for disease outbreak prediction, training a model and verifying it using real-world scenarios are extremely challenging since the developed model must handle various uncertainties in the WBE study.
1.2.6. The Financial Aspects of WBE
The average cost of individual SARS-CoV-2 testing was around USD 148 whereas the cost of WBE is around USD 300 (2022 prices) and covers thousands of individuals in the sampling region [157]. Guerrero-Latorre et al. (2022) [158] established a WBE system that processes 46 samples weekly and biweekly from wastewater treatment plants (WWTPs). The author estimated an annual cost of EUR 20,000 for the weekly process of samples from a single WWTP. Similarly, two pilot laboratories in Nepal were the subject of a thorough cost estimation project by NgwiraI et al. in 2022 [159]. In Blantyre, the expected cost per sample was between USD 25 and 74, whereas in Kathmandu, it was between USD 120 and 175. The price is contingent on the quantity of samples processed; a higher sample count results in a lower cost per sample. In another study, to define the screening strategy in the Tokyo Olympic and Paralympic Village in 2021, a more extensive cost study was conducted, which compared two-step screening methods using PCR individual diagnosis, followed by an antigen test for positive cases against wastewater surveillance. The findings revealed that wastewater surveillance was a cost-effective and justifiable choice for monitoring public health [160]. Although the cost of WBE studies is low compared to clinical surveillance, overall, it can be a financial burden in low-income countries. Therefore, cost-effective methods of wastewater surveillance need to be developed.
2. Challenges in Processing Wastewater and Predictability of Disease Outbreak
Although WBE serves as a valuable tool for monitoring infectious diseases and outbreaks in the community, it is worth mentioning that there are still some major challenges in sample processing and the ability to predict outbreaks. For example, consider the following challenges faced in sampling.
Community-level wastewater surveillance will not capture communities or facilities served by de-centralized wastewater treatment;
Precise mapping of the sewer network is required to estimate the infection load in the catchment area;
A balance between accessibility, cost, and sensitivity is yet to be derived for wastewater sampling;
The effects of temperature and pH on various wastewater matrix are factors to be considered before sampling;
A standard protocol for sampling at different scales is yet to be established.
2.1. Varying Limit of Detection
-
6.
Low infection levels in the community may not be captured by wastewater surveillance [161];
-
7.
During the SARS-CoV-2 pandemic, researchers have been able to estimate fecal shedding rates of the virus; however, this has yet to be established for other pathogens.
2.2. Hurdles in Correlation with Public Health Data
-
8.
Population dynamics and effects of the floating population affect the accuracy of the WBE;
-
9.
The search for the optimal surrogate to validate pre-treatment procedures has become an additional obstacle. A surrogate that is stable during wastewater processing, has no toxicity, possesses similar structural features to the target pathogen, and is unaffected by the wastewater matrices [162] is needed;
-
10.
The inability of qPCR data to provide data on the virus’s lifecycle represents a significant obstacle. Therefore, it is essential to investigate the survivability of SARS-CoV-2 in wastewater samples and their half-life to comprehend the virus’s transmission between various environmental compartments;
-
11.
Challenges in correlating, sharing, and interpreting routine wastewater surveillance data across different agencies, such as policymakers and public health officials, limit the full potential of wastewater-based epidemiology (WBE);
-
12.
Establishing a link between pathogen detection and the clinical impact or location of cases is often unclear;
-
13.
The lack of updated standardized testing methods poses a challenge, as current approaches are often adapted from clinical diagnostics, which are yet to be validated for wastewater testing.
2.3. Complex Wastewater Matrix and Varying Environmental Conditions
-
14.
The complex matrix of urban wastewater containing detergents, xenobiotics, antibiotics, and the presence of PCR inhibitors [70], and varying composition [163], leads to frequent failures and makes the process cumbersome to optimize [164];
-
15.
WBE is not a comprehensive solution for the monitoring of all the circulating pathogens; the limits and drawbacks are to be considered. There is a lack of trained personnel in wastewater surveillance of infectious disease; in most cases, personnel trained in clinical laboratories are utilized for WBE.
3. Limitation of This Study
The article attempts to cover the entire process flow of WBE, starting from sampling to the use of advanced methods for the prediction of outbreaks; for this reason, selective studies from the literature were reviewed; the objective of this article is to provide an overview of wastewater-based epidemiological surveillance and its recent trends to the reader. The suggestions provided are based on the reviewed literature and the author’s experience.
4. Conclusions
Wastewater surveillance certainly holds the potential to be used as an early warning system and can help in establishing control measures in near-real-time. A variety of barriers that need to be addressed in implementing a WBE system were discussed in the study. An overview of recent advancements in analytical methods and factors to be considered in the interpretation and correlation of the WBE results were presented. The effects of different sampling regimes and storage conditions were compared. A quick summary of pathogens reported in wastewater and the different types of PCR used in quantifying the pathogen were reviewed. Possibilities for integrating an AI-based program for effective monitoring and to create a system that alerts the health authorities were discussed. Further research is needed to assess the impact of WBE in informing policy decisions and guiding public health interventions.
Author Contributions
W.E.: methodology, supervision, formal analysis, and reviewing; B.K.S.: methodology, resources, writing original draft—reviewing and editing; M.A., D.A., M.S., H.S., R.S., L.P., C.M.O., G.B. and S.B.S. are co-authors and contributed equally. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
Authors Bhuvanesh Kumar Shanmugam, Maryam AlQaydi, Degan Abdisalam, Monika Shukla, Helio Santos, Ranya Samour, Lawrence Petalidis, Grzegorz Brudecki, Wael Elamin were employed by the company ‘M42 healthcare’, UAE. Charles Matthew Oliver was employed by Mubadala Health, UAE, and Samara Bin Salem was employed by Abu Dhabi Quality and Conformity Council (ADQCC), UAE.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thomas K.V., Bijlsma L., Castiglioni S., Covaci A., Emke E., Grabic R., Hernandez F., Karolak S., Kasprzyk-Hordern B., Lindberg R.H., et al. Comparing illicit drug use in 19 European cities through sewage analysis. Sci. Total Environ. 2012;432:432–439. doi: 10.1016/j.scitotenv.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 2.Baz-Lomba J.A., Salvatore S., Gracia-Lor E., Bade R., Castiglioni S., Castrignanò E., Causanilles A., Hernandez F., Kasprzyk-Hordern B., Kinyua J., et al. Comparison of pharmaceutical, illicit drug, alcohol, nicotine and caffeine levels in wastewater with sale, seizure and consumption data for 8 European cities. BMC Public Health. 2016;16:1035. doi: 10.1186/s12889-016-3686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilaru P., Hill D., Anderson K., Collins B.M., Green H., Kmush L.B., Larsen D.A.L. Wastewater surveillance for infectious disease: A systematic review. Am. J. Epidemiol. 2023;192:305–322. doi: 10.1093/aje/kwac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tulchinsky T.H. Case Studies in Public Health. Academic Press; Cambridge, MA, USA: 2018. Chapter 5, John Snow, cholera, the Broad Street pump; waterborne diseases then and now; pp. 77–99. [DOI] [Google Scholar]
- 5.Metcalf T.G., Melnick J.L., Estes M.K. Environmental virology: From detection of virus in sewage and water by isolation to identification by molecular biology—A trip of over 50 years. Annu. Rev. Microbiol. 1995;49:461–487. doi: 10.1146/annurev.mi.49.100195.002333. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X., Estes M.K., Metcalf T.G. Detection of hepatitis A virus by hybridization with single-stranded RNA probes. Appl. Environ. Microbiol. 1987;53:2487–2495. doi: 10.1128/aem.53.10.2487-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijnen L., Medema G. Surveillance of influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J. Water Health. 2011;9:434–442. doi: 10.2166/wh.2011.019. [DOI] [PubMed] [Google Scholar]
- 8.Lee D.Y., Shannon K., Beaudett L.A. Detection of bacterial pathogens in municipal wastewater using an oligonucleotide microarray and real-time quantitative PCR. J. Microbiol. Methods. 2006;65:453–467. doi: 10.1016/j.mimet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Lépesová K., Kraková L., Pangallo D., Medveďová A., Olejníková P., Mackuľak T., Tichý J., Grabic R., Birošová L. Prevalence of antibiotic-resistant coliform bacteria, Enterococcus spp. and Staphylococcus spp. in wastewater sewerage biofilm. J. Glob. Antimicrob. Resist. 2018;14:145–151. doi: 10.1016/j.jgar.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Fong T.T., Phanikumar M.S., Xagoraraki I., Rose J.B. Quantitative Detection of Human Adenoviruses in Wastewater and Combined Sewer Overflows Influencing a Michigan River. Appl. Environ. Microbiol. 2010;76:715–723. doi: 10.1128/AEM.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Massih A., Kamel A., Zaki A.M., Aboudeif A., Emad C., Ramadan D., Gaber H., Bastorous H., Shaker M., Salah N., et al. Revisiting the overlooked role of recycled sewage water in high-income countries in adenoviral outbreaks such as the “2022 pediatric hepatitis’ outbreak”. Egypt. Pediatr. Assoc. Gaz. 2022;70:22. doi: 10.1186/s43054-022-00113-2. [DOI] [Google Scholar]
- 12.Hoque S.A., Saito H., Akino W., Kotaki T., Okitsu S., Onda Y., Kobayashi T., Hossian T., Khamrin P., Motomura K., et al. The Emergence and Widespread Circulation of Enteric Viruses Throughout the COVID-19 Pandemic: A Wastewater-Based Evidence. Food Environ. Virol. 2023;15:342–354. doi: 10.1007/s12560-023-09566-z. [DOI] [PubMed] [Google Scholar]
- 13.Boehm A.B., Hughes B., Duong D., Chan-Herur V., Buchman A., Wolfe M.K., White B.J. Wastewater concentrations of human influenza, metapneumovirus, parainfluenza, respiratory syncytial virus, rhinovirus, and seasonal coronavirus nucleic-acids during the COVID-19 pandemic: A surveillance study. Lancet Microbe. 2023;4:340–348. doi: 10.1016/S2666-5247(22)00386-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F.C., Li X.F., Deng Y.Q., Tong Y.G., Qin C.F. Excretion of infectious Zika virus in urine. Lancet Infect. Dis. 2016;16:641–642. doi: 10.1016/S1473-3099(16)30070-6. [DOI] [PubMed] [Google Scholar]
- 15.Lin K., Marr L.C. Aerosolization of Ebola Virus Surrogates in Wastewater Systems. Environ. Sci. Technol. 2017;51:2669–2675. doi: 10.1021/acs.est.6b04846. [DOI] [PubMed] [Google Scholar]
- 16.Bibby K., Fischer R.J., Casson L.W., Stachler E., Haas C.N., Munster V.J. Persistence of Ebola Virus in Sterilized Wastewater. Environ. Sci. Technol. Lett. 2015;2:245–249. doi: 10.1021/acs.estlett.5b00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snow J. On the Mode of Communication of Cholera. Edinb. Med. J. 1856;1:668–670. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson W.J. Isolation of Enteric Bacilli from Sewage and water and its bearing on epidemiology. Br. Med. J. 1933;2:560–562. doi: 10.1136/bmj.2.3794.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul J.R., Trask J.D. The virus of poliomyelitis in stools and sewage. JAMA. 1941;116:493. doi: 10.1001/jama.1941.02820060041009. [DOI] [Google Scholar]
- 20.Neefe J.R., Stokes J., Jr. Epidemic of infectious hepatitis apparently due to water borne agent: Epidemiologic observations and transmission experiments in human volunteers. JAMA. 1945;128:1063–1075. doi: 10.1001/jama.1945.02860320005003. [DOI] [Google Scholar]
- 21.Moore B. The detection of paratyphoid carriers in towns by means of sewage examination. Mon. Bull. Minist. Health Public Health Lab. Serv. 1948;7:241–248. [Google Scholar]
- 22.Clark E.M., Knowles D.S., Shimada F.T., Rhodes A.J., Ritchie R.C., Donohue W.L. Coxsackie virus in urban sewage, Recovery of virus in season of low incidence of reported poliomyelitis. Can. J. Public Health. 1951;42:103–107. [PubMed] [Google Scholar]
- 23.Foy H.M., Cooney M.K., Hatlen J.B. Adenovirus type 3 epidemic associated with intermittent chlorination of a swimming pool. Arch. Environ. Health. 1968;17:795–802. doi: 10.1080/00039896.1968.10665321. [DOI] [PubMed] [Google Scholar]
- 24.Steinmann J. Detection of Rota virus in Sewage. Appl. Environ. Microbiol. 1981;41:1043–1045. doi: 10.1128/aem.41.4.1043-1045.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor J.W., Gary G.W., Greenberg H.B. Norwalk-related viral gastroenteritis due to contaminated drinking water. Am. J. Epidemiol. 1981;114:584–592. doi: 10.1093/oxfordjournals.aje.a113224. [DOI] [PubMed] [Google Scholar]
- 26.Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th Hospital of the Chinese People’s Liberation Army. Water Sci. Technol. 2005;52:213–221. doi: 10.2166/wst.2005.0266. [DOI] [PubMed] [Google Scholar]
- 27.Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139:105689. doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Global Wastewater Monitoring Sources for SARS-CoV-2 (COVID-19 Virus) [(accessed on 2 October 2024)]. Available online: https://data.who.int/dashboards/covid19/wastewater#:~:text=Wastewater%20testing%20can%20be%20used%20to%20monitor%20the%20trends%20of.
- 31.Centers for Disease Control and Prevention; National Wastewater Surveillance System (NWSS Wastewater Monitoring in the U.S.|National Wastewater Surveillance System|CDC) [(accessed on 14 October 2024)]; Available online: https://www.cdc.gov/nwss/index.html.
- 32.EU Wastewater Observatory for Public Health EU4S. [(accessed on 2 October 2024)]. Available online: https://wastewater-observatory.jrc.ec.europa.eu/#/content/maps-dashboards-dashboards.
- 33.Yeager R., Holm R.H., Saurabh K., Fuqua J.L., Talley D., Bhatnagar A., Smith T. Wastewater Sample Site Selection to Estimate Geographically Resolved Community Prevalence of COVID-19: A Sampling Protocol Perspective. GeoHealth. 2021;5:e2021GH000420. doi: 10.1029/2021GH000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mac Mahon J., Criado Monleon A.J., Gill L.W., O’Sullivan J.J., Meijer W.G. Wastewater-based epidemiology (WBE) for SARS-CoV-2: A review focusing on the significance of the sewer network using a Dublin city catchment case study. Water Sci. Technol. 2022;86:1402–1425. doi: 10.2166/wst.2022.278. [DOI] [PubMed] [Google Scholar]
- 35.Sharara N., Endo N., Duvallet C., Ghaeli N., Matus M., Heussner J., Olesen S.W., Alm E.J., Chai P.R., Erickson T.B. Wastewater network infrastructure in public health: Applications and learnings from the COVID-19 pandemic. PLoS Glob. Public Health. 2001;1:e0000061. doi: 10.1371/journal.pgph.0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Roppolo Brazell L., Hinton K., Lontai J., Stark N., Young I., et al. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782:146749. doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee L., Valmond L., Thomas J., Kim A., Austin P., Foster M., Matthews J., Kim P., Newman J. Wastewater surveillance in smaller college communities may aid future public health initiatives. PLoS ONE. 2022;17:e0270385. doi: 10.1371/journal.pone.0270385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wartell B.A., Proano C., Bakalian L., Kaya D., Croft K., McCreary M., Lichtenstein N., Miske V., Arcellana P., Boyer J., et al. Implementing wastewater surveillance for SARS-CoV-2 on a university campus: Lessons learned. Water Environ. Res. 2022;94:e10807. doi: 10.1002/wer.10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong T.E., Thurston G.M., Barlow N., Cahill N.D., Carichino L., Maki K., Ross D., Schneider J. Evaluating the sensitivity of SARS-CoV-2 infection rates on college campuses to wastewater surveillance. Infect. Dis. Model. 2021;6:1144–1158. doi: 10.1016/j.idm.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koureas M., Kyritsi M., Vontas A., Kourentis L., Vasileiou C., Mouchtouri V.A., Hadjichristodoulou C. Pilot Implementation of SARS-CoV-2 Wastewater Surveillance on Cruise Ships Arriving at the Port of Piraeus from June to November 2021. Med. Sci. Forum. 2022;13:6. doi: 10.3390/msf2022013006. [DOI] [Google Scholar]
- 41.Li J., Hosegood I., Powell D., Tscharke B., Lawler J., Thomas K.V., Mueller J.F. A global aircraft-based wastewater genomic surveillance network for early warning of future pandemics. Lancet Glob. Health. 2023;11:e791–e795. doi: 10.1016/S2214-109X(23)00129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones D.L., Rhymes J.M., Wade M.J., Kevill J.L., Malham S.K., Grimsley J.M.S., Rimmer C., Weightman A.J., Farkas K. Suitability of aircraft wastewater for pathogen detection and public health surveillance. Sci. Total Environ. 2023;856:159162. doi: 10.1016/j.scitotenv.2022.159162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shingleton J.W., Lilley C.J., Wade M.J. Evaluating the theoretical performance of aircraft wastewater monitoring as a tool for SARS-CoV-2 surveillance. PLoS Glob. Public Health. 2023;3:e0001975. doi: 10.1371/journal.pgph.0001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bivins A., Kaya D., Ahmed W., Brown J., Butler C., Greaves J., Leal R., Maas K., Rao G., Sherchan S. Passive sampling to scale wastewater surveillance of infectious disease: Lessons learned from COVID-19. Sci. Total Environ. 2022;835:155347. doi: 10.1016/j.scitotenv.2022.155347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santarpia J.L., Klug E., Ravnholdt A., Kinahan S.M. Environmental sampling for disease surveillance: Recent advances and recommendations for best practice. J. Air Waste Manag. Assoc. 2023;73:434–461. doi: 10.1080/10962247.2023.2197825. [DOI] [PubMed] [Google Scholar]
- 46.Rafiee M., Isazadeh S., Mohseni-Bandpei A., Mohebbi S.R., Jahangiri-Rad M., Eslami A., Dabiri H., Roostaei K., Tanhaei M., Amereh F. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021;790:148205. doi: 10.1016/j.scitotenv.2021.148205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sikorski M.J., Levine M.M. Reviving the “Moore Swab”: A classic environmental surveillance tool involving filtration of flowing surface water and sewage water to recover typhoidal Salmonella bacteria. Appl. Environ. Microbiol. 2020;86:e00060-20. doi: 10.1128/AEM.00060-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cha G., Graham K.E., Zhu K.J., Rao G., Lindner B.G., Kocaman K., Woo S., D’amico I., Bingham L.R., Fischer J.M., et al. Parallel deployment of passive and composite samplers for surveillance and variant profiling of SARS-CoV-2 in sewage. Sci. Total Environ. 2023;866:161101. doi: 10.1016/j.scitotenv.2022.161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breulmann M., Kallies R., Bernhard K., Gasch A., Müller R.A., Harms H., Chatzinotas A., van Afferden M. A long-term passive sampling approach for wastewater-based monitoring of SARS-CoV-2 in Leipzig, Germany. Sci. Total Environ. 2023;887:164143. doi: 10.1016/j.scitotenv.2023.164143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schang C., Crosbie N.D., Nolan M., Poon R., Wang M., Jex A., John N., Baker L., Scales P., Schmidt J., et al. Passive sampling of SARS-CoV-2 for wastewater surveillance. Environ. Sci. Technol. 2021;55:10432–10441. doi: 10.1021/acs.est.1c01530. [DOI] [PubMed] [Google Scholar]
- 51.Habtewold J., McCarthy D., McBean E., Law I., Goodridge L., Habash M., Murphy H.M. Passive sampling, a practical method for wastewater-based surveillance of SARS-CoV-2. Environ. Res. 2022;204:112058. doi: 10.1016/j.envres.2021.112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson M., Qiu Y., Yu J., Lee B.E., McCarthy D.T., Pang X. Comparison of auto sampling and passive sampling methods for SARS-CoV-2 detection in wastewater. Pathogens. 2022;11:359. doi: 10.3390/pathogens11030359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayes E.K., Sweeney C.L., Anderson L.E., Li B., Erjavec G.B., Gouthro M.T., Krkosek W.H., Stoddart A.K., Gagnon G.A. A novel passive sampling approach for SARS-CoV-2 in wastewater in a Canadian province with low prevalence of COVID-19. Environ. Sci. Water Res. Technol. 2021;7:1576–1586. doi: 10.1039/D1EW00207D. [DOI] [Google Scholar]
- 54.Acer P.T., Kelly L.M., Lover A.A., Butler C.S. Quantifying the relationship between SARS-CoV-2 wastewater concentrations and building-level COVID-19 prevalence at an isolation residence: A passive sampling approach. Int. J. Environ. Res. Public Health. 2022;19:11245. doi: 10.3390/ijerph191811245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West N.W., Hartrick J., Alamin M., Vasquez A.A., Bahmani A., Turner C.L., Shuster W., Ram J.L. Passive swab versus grab sampling for detection of SARS-CoV-2 markers in wastewater. Sci. Total Environ. 2023;889:164180. doi: 10.1016/j.scitotenv.2023.164180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geissler M., Mayer R., Helm B., Dumke R. Food and environmental virology: Use of passive sampling to characterize the presence of SARS-CoV-2 and other viruses in wastewater. Food Environ. Virol. 2024;16:25–37. doi: 10.1007/s12560-023-09572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed W., Bivins A., Bertsch P.M., Bibby K., Gyawali P., Sherchan S.P., Simpson S.L., Thomas K.V., Verhagen R., Kitajima M., et al. Intraday variability of indicator and pathogenic viruses in 1-h and 24-h composite wastewater samples: Implications for wastewater-based epidemiology. Environ. Res. 2021;193:110531. doi: 10.1016/j.envres.2020.110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Augusto M.R., Claro I.C.M., Siqueira A.K., Sousa G.S., Caldereiro C.R., Duran A.F.A., de Miranda T.B., Bomediano Camillo L.M., Cabral A.D., de Freitas Bueno R. Sampling strategies for wastewater surveillance: Evaluating the variability of SARS-CoV-2 RNA concentration in composite and grab samples. J. Environ. Chem. Eng. 2022;10:107478. doi: 10.1016/j.jece.2022.107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J., Ahmed W., Metcalfe S., Smith W.J.M., Tscharke B., Lynch P., Sherman P., Vo P.H.N., Kaserzon S.L., Simpson S.L., et al. Monitoring of SARS-CoV-2 in sewersheds with low COVID-19 cases using a passive sampling technique. Water Res. 2022;218:118481. doi: 10.1016/j.watres.2022.118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy H. Persistence of pathogens in sewage and other water types. In: Rose J.B., Jiménez-Cisneros B., editors. Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management. Michigan State University; East Lansing, MI, USA: 2017. Global Water Pathogen Project. [DOI] [Google Scholar]
- 61.Ballesté E., Blanch A.R. Persistence of Bacteroides species populations in a river as measured by molecular and culture techniques. Appl. Environ. Microbiol. 2010;76:7608–7616. doi: 10.1128/AEM.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S., Shi J., Sharma E., Li X., Gao S., Zhou X., O’Brien J., Coin L., Liu Y., Sivakumar M., et al. In-sewer decay and partitioning of Campylobacter jejuni and Campylobacter coli and implications for their wastewater surveillance. Water Res. 2023;233:119737. doi: 10.1016/j.watres.2023.119737. [DOI] [PubMed] [Google Scholar]
- 63.Joseph-Duran B., Serra-Compte A., Sàrrias M., Gonzalez S., López D., Prats C., Català M., Alvarez-Lacalle E., Alonso S., Arnaldos M. Assessing wastewater-based epidemiology for the prediction of SARS-CoV-2 incidence in Catalonia. Sci. Rep. 2022;12:15073. doi: 10.1038/s41598-022-18518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar M., Jiang G., Kumar Thakur A., Chatterjee S., Bhattacharya T., Mohapatra S., Chaminda T., Kumar Tyagi V., Vithanage M., Bhattacharya P., et al. Lead time of early warning by wastewater surveillance for COVID-19: Geographical variations and impacting factors. Chem. Eng. J. 2022;441:135936. doi: 10.1016/j.cej.2022.135936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- 66.Aquino de Carvalho N., Stachler E.N., Cimabue N., Bibby K. Evaluation of Phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017;51:8692–8700. doi: 10.1021/acs.est.7b01296. [DOI] [PubMed] [Google Scholar]
- 67.Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities, and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markt R., Mayr M., Peer E., Wagner A.O., Lackner N., Insam H. Detection and stability of SARS-CoV-2 fragments in wastewater: Impact of storage temperature. Pathogens. 2021;10:1215. doi: 10.3390/pathogens10091215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddell S., Sherchan S.P., et al. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simpson A., Topol A., White B.J., Wolfe M.K., Wigginton K.R., Boehm A.B. Effect of storage conditions on SARS-CoV-2 RNA quantification in wastewater solids. PeerJ. 2021;9:e11933. doi: 10.7717/peerj.11933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huge B.J., North D., Mousseau C.B., Bibby K., Dovichi N.J., Champion M.M. Comparison of RT-dPCR and RT-qPCR and the effects of freeze–thaw cycle and glycine release buffer for wastewater SARS-CoV-2 analysis. Sci. Rep. 2022;12:20641. doi: 10.1038/s41598-022-25187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beattie R.E., Blackwood A.D., Clerkin T., Dinga C., Noble R.T. Evaluating the impact of sample storage, handling, and technical ability on the decay and recovery of SARS-CoV-2 in wastewater. PLoS ONE. 2022;17:e0270659. doi: 10.1371/journal.pone.0270659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu S.Y., Bayati M., Li C., Hsieh H.Y., Belenchia A., Klutts J., Zemmer S.A., Reynolds M., Semkiw E., Johnson H.Y., et al. Biomarkers selection for population normalization in SARS-CoV-2 wastewater-based epidemiology. Water Res. 2022;223:118985. doi: 10.1016/j.watres.2022.118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maal-Bared R., Qiu Y., Li Q., Gao T., Hrudey S.E., Bhavanam S., Ruecker N.J., Ellehoj E., Lee B.E., Pang X. Does normalization of SARS-CoV-2 concentrations by Pepper Mild Mottle Virus improve correlations and lead time between wastewater surveillance and clinical data in Alberta (Canada): Comparing twelve SARS-CoV-2 normalization approaches. Sci. Total Environ. 2023;856:158964. doi: 10.1016/j.scitotenv.2022.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Aoust P.M., Mercier E., Montpetit D., Jia J.J., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188:116560. doi: 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guidelines for Environmental Surveillance of Poliovirus Circulation, WHO/V&B/03.03. World Health Organization; Geneva, Switzerland: 2023. [Google Scholar]
- 78.Wittwer C.T., Makrigiorgos G.M. Principles and Applications of Molecular Diagnostics. Elsevier; Amsterdam, The Netherlands: 2018. 4-Nucleic Acid Techniques; pp. 47–86. [Google Scholar]
- 79.Chavarria-Miró G., Anfruns-Estrada E., Martínez-Velázquez A., Vázquez-Portero M., Guix S., Paraira M., Galofré B., Sánchez G., Pintó R.M., Bosch A. Time Evolution of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Wastewater during the First Pandemic Wave of COVID-19 in the Metropolitan Area of Barcelona, Spain. Appl. Environ. Microbiol. 2021;87:e02750-20. doi: 10.1128/AEM.02750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dewantoro A., Anggundari W., Prasetya B., Yopi Y. Review of digital PCR potential for surveillance of emerging disease from wastewater. IOP Conf. Ser. Earth Environ. Sci. 2021;926:012065. doi: 10.1088/1755-1315/926/1/012065. [DOI] [Google Scholar]
- 81.Corpuz M.V.A., Buonerba A., Zarra T., Hasan S.W., Korshin G.V., Belgiorno V., Naddeo V. Advances in virus detection methods for wastewater-based epidemiological applications. Case Stud. Chem. Environ. Eng. 2022;6:100238. doi: 10.1016/j.cscee.2022.100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svec D., Tichopad A., Novosadova V., Pfaffl M.W., Kubista M. How Good Is a PCR Efficiency Estimate: Recommendations for Precise and Robust qPCR Efficiency Assessments. Biomol. Detect. Quantif. 2015;3:9–16. doi: 10.1016/j.bdq.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed W., Bivins A., Metcalfe S., Smith W.J.M., Verbyla M.E., Symonds E.M., Simpson S.L. Evaluation of process limit of detection and quantification variation of SARS-CoV-2 RT-qPCR and RT-dPCR assays for wastewater surveillance. Water Res. 2022;213:118132. doi: 10.1016/j.watres.2022.118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ciesielski M., Blackwood D., Clerkin T., Gonzalez R., Thompson H., Larson A., Noble R. Assessing sensitivity and reproducibility of RT-ddPCR and RT-qPCR for the quantification of SARS-CoV-2 in wastewater. J. Virol. Methods. 2021;297:114230. doi: 10.1016/j.jviromet.2021.114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neault N., Baig A., Graber T., D’Aoust P., Mercier E., Alexandrov I., Crosby D., Mayne J., Pounds T., MacKenzie M., et al. SARS-CoV-2 protein in wastewater mirrors COVID-19 prevalence. medRxiv. 2020 doi: 10.1101/2020.09.01.20185280. PPR:PPR208645. [DOI] [Google Scholar]
- 86.Amoah I.D., Mthethwa N.P., Pillay L., Deepnarain N., Pillay K., Awolusi O.O., Kumari S., Bux F. RT-LAMP: A cheaper, simpler and faster alternative for the detection of SARS-CoV-2 in wastewater. Food Environ. Virol. 2021;13:447–456. doi: 10.1007/s12560-021-09489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Godinez A., Hill D., Dandaraw B., Green H., Kilaru P., Middleton F., Run S., Kmush B.L., Larsen D.A. High Sensitivity and Specificity of Dormitory-Level Wastewater Surveillance for COVID-19 during Fall Semester 2020 at Syracuse University, New York. Int. J. Environ. Res. Public Health. 2022;19:4851. doi: 10.3390/ijerph19084851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu Y., Wu X., Gu A., Dobelle L., Cid C.A., Li J., Hoffmann M.R. Membrane-based in-gel loop-mediated isothermal amplification (mgLAMP) system for SARS-CoV-2 quantification in environmental waters. Environ. Sci. Technol. 2021;56:862–873. doi: 10.1021/acs.est.1c04623. [DOI] [PubMed] [Google Scholar]
- 89.Oshiki M., Miura T., Kazama S., Segawa T., Ishii S., Hatamoto M., Yamaguchi T., Kubota K., Iguchi A., Tagawa T., et al. Microfluidic PCR Amplification and MiSeq Amplicon Sequencing Techniques for High-Throughput Detection and Genotyping of Human Pathogenic RNA Viruses in Human Feces, Sewage, and Oysters. Front. Microbiol. 2018;9:830. doi: 10.3389/fmicb.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kittigul L., Ekchaloemkiet S., Utrarachkij F., Siripanichgon K., Sujirarat D., Pungchitton S., Boonthum A. An efficient virus concentration method and RT-nested PCR for detection of rotaviruses in environmental water samples. J. Virol. Methods. 2005;124:117–122. doi: 10.1016/j.jviromet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Izquierdo-Lara R., Elsinga G., Heijnen L., Munnink B.B.O., Schapendonk C.M.E., Nieuwenhuijse D., Kon M., Lu L., Aarestrup F.M., Lycett S., et al. Monitoring SARS-CoV-2 Circulation and Diversity through Community Wastewater Sequencing, the Netherlands and Belgium. Emerg. Infect. Dis. 2021;27:1405–1415. doi: 10.3201/eid2705.204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munnink B.B., Sikkema R.S., Nieuwenhuijse D.F., Molenaar R.J., Munger E., Molenkamp R., van der Spek A., Tolsma P., Rietveld A., Brouwer M., et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 2021;371:172–177. doi: 10.1126/science.abe5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hillary L.S., Farkas K., Maher K.H., Lucaci A.O., Thorpe J., Distaso M.A., Gaze W.H., Paterson S., Burke T.A., Connor T.R., et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021;200:117214. doi: 10.1016/j.watres.2021.117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rothman J.A., Loveless T.B., Kapcia J., Adams E.D., Steele J.A., Zimmer-Faust A.G., Langlois K., Wanless D., Griffith M., Mao L., et al. RNA viromics of Southern California wastewater and detection of SARS-CoV-2 single-nucleotide variants. Appl. Environ. Microbiol. 2021;87:e01448-21. doi: 10.1128/AEM.01448-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5:00614–00620. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolfe M.K., Archana A., Catoe D., Coffman M.M., Dorevich S., Graham K.E., Kim S., Mendoza Grijalva L., Roldan-Hernandez L., Silverman A.I., et al. Scaling of SARS-CoV-2 RNA in Settled Solids from Multiple Wastewater Treatment Plants to Compare Incidence Rates of Laboratory-Confirmed COVID-19 in Their Sewersheds. Environ. Sci. Technol. Lett. 2021;8:398–404. doi: 10.1021/acs.estlett.1c00184. [DOI] [PubMed] [Google Scholar]
- 98.Wolfe M.K., Topol A., Knudson A., Simpson A., White B., Vugia D.J., Yu A.T., Li L., Balliet M., Stoddard P., et al. High-Frequency, High-Throughput Quantification of SARS-CoV-2 RNA in Wastewater Settled Solids at Eight Publicly Owned Treatment Works in Northern California Shows Strong Association with COVID-19 Incidence. mSystems. 2021;6:e00829-21. doi: 10.1128/msystems.00829-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K.A., et al. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: A surveillance tool for assessing the presence of COVID-19 infected travelers. J. Travel Med. 2020;27:taaa116. doi: 10.1093/jtm/taaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong K., Onan B.M., Xagoraraki I. Quantification of enteric viruses, pathogen indicators, and Salmonella bacteria in class B anaerobically digested biosolids by culture and molecular methods. Appl. Environ. Microbiol. 2010;76:6441–6448. doi: 10.1128/AEM.02685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iaconelli M., Muscillo M., Della Libera S., Fratini M., Meucci L., De Ceglia M., Giacosa D., La Rosa G. One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food Environ. Virol. 2017;9:79–88. doi: 10.1007/s12560-016-9263-3. [DOI] [PubMed] [Google Scholar]
- 102.Mabasa V.V., van Zyl W.B., Ismail A., Allam M., Taylor M.B., Mans J. Multiple novel human norovirus recombinants identified in wastewater in Pretoria, South Africa by next-generation sequencing. Viruses. 2022;14:2732. doi: 10.3390/v14122732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfe M.K., Duong D.H., Bakker K.M., Ammerman M.B., Mortenson L., Hughes B., Arts P.J., Lauring A.S., Fitzsimmons W.J., Bendall E.E., et al. Wastewater-based detection of two influenza outbreaks. Environ. Sci. Technol. Lett. 2022;9:687–692. doi: 10.1021/acs.estlett.2c00350. [DOI] [Google Scholar]
- 104.Mejia E.M., Hizon N.A., Dueck C.E., Lidder R., Daigle J., Wonitowy Q., Medina N.G., Mohammed U.P., Cox G.W., Safronetz D., et al. Detection of mpox virus in wastewater provides forewarning of clinical cases in Canadian cities. Sci. Total Environ. 2024;933:173108. doi: 10.1016/j.scitotenv.2024.173108. [DOI] [PubMed] [Google Scholar]
- 105.de Jonge E.F., Peterse C.M., Koelewijn J.M., van der Drift A.R., van der Beek R.F.H.J., Nagelkerke E., Lodder W.J. The detection of monkeypox virus DNA in wastewater samples in The Netherlands. Sci. Total Environ. 2022;852:158265. doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim J., Oh E., Banting G.S., Braithwaite S., Chui L., Ashbolt N.J., Neumann N.F., Jeon B. An improved culture method for selective isolation of Campylobacter jejuni from wastewater. Front. Microbiol. 2016;7:1345. doi: 10.3389/fmicb.2016.01345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahfouz N., Caucci S., Achatz E., Semmler T., Guenther S., Berendonk T.U., Schroeder M. High genomic diversity of multi-drug resistant wastewater Escherichia coli. Sci. Rep. 2018;8:8928. doi: 10.1038/s41598-018-27292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kutilova I., Medvecky M., Leekitcharoenphon P., Munk P., Masarikova M., Davidova-Gerzova L., Jamborova I., Bortolaia V., Pamp S.J., Dolejska M. Extended-spectrum beta-lactamase-producing Escherichia coli and antimicrobial resistance in municipal and hospital wastewaters in Czech Republic: Culture-based and metagenomic approaches. Environ. Res. 2021;193:110487. doi: 10.1016/j.envres.2020.110487. [DOI] [PubMed] [Google Scholar]
- 109.Davidova-Gerzova L., Lausova J., Sukkar I., Nesporova K., Nechutna L., Vlkova K., Chudejova K., Krutova M., Palkovicova J., Kaspar J. Hospital and community wastewater as a source of multidrug-resistant ESBL-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2023;13:1184081. doi: 10.3389/fcimb.2023.1184081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biggel M., Zurfluh K., Treier A., Nüesch-Inderbinen M., Stephan R. Characteristics of fosA-carrying plasmids in E. coli and Klebsiella spp. isolates originating from food and environmental samples. J. Antimicrob. Chemother. 2021;76:2004–2011. doi: 10.1093/jac/dkab119. [DOI] [PubMed] [Google Scholar]
- 111.Louis S., Mark-Carew M., Biggerstaff M., Yoder J., Boehm A.B., Wolfe M.K., Flood M., Peters S., Stobierski M.G., Coyle J., et al. Wastewater Surveillance for Influenza A Virus and H5 Subtype Concurrent with the Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in Cattle and Poultry and Associated Human Cases-United States. MMWR Morb. Mortal Wkly. Rep. 2024;73:804–809. doi: 10.15585/mmwr.mm7337a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bofill-Mas S., Rodriguez-Manzano J., Calgua B., Carratala A., Girones R. Newly described human polyomaviruses Merkel cell, KI and WU are present in urban sewage and may represent potential environmental contaminants. Virol. J. 2010;7:141. doi: 10.1186/1743-422X-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuhn K.G., Shukla R., Mannell M., Graves G.M., Miller A.C., Vogel J., Malloy K., Deshpande G., Florea G., Shelton K., et al. Using Wastewater Surveillance to Monitor Gastrointestinal Pathogen Infections in the State of Oklahoma. Microorganisms. 2023;11:2193. doi: 10.3390/microorganisms11092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diemert S., Yan T. Clinically unreported salmonellosis outbreak detected via comparative genomic analysis of municipal wastewater Salmonella isolates. Appl. Environ. Microbiol. 2019;85:e00139-19. doi: 10.1128/AEM.00139-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., et al. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224:121726. doi: 10.1016/j.talanta.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flood M.T., D’Souza N., Rose J.B., Aw T.G. Methods evaluation for rapid concentration and quantification of SARS-CoV-2 in raw wastewater using droplet digital and quantitative RT-PCR. Food Environ. Virol. 2021;13:303–315. doi: 10.1007/s12560-021-09488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: Comparison with norovirus cases. Environ. Sci. Technol. 2020;54:6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- 118.Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tumino S., Tolone M., Parco A., Puleio R., Arcoleo G., Manno C., Nicholas R.A.J., Loria G.R. Validation of loop-mediated isothermal amplification (LAMP) field tool for rapid and sensitive diagnosis of contagious agalactia in small ruminants. Animals. 2020;10:509. doi: 10.3390/ani10030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buddolla A., Kim S. Recent trends in the utilization of LAMP for the diagnosis of viruses, bacteria, and allergens in food. Recent Dev. Appl. Microbiol. Biochem. 2021;2:291–297. doi: 10.1016/B978-0-12-821406-0.00027-8. [DOI] [Google Scholar]
- 121.Ongerth J.E., Danielson R.E. RT qLAMP-direct detection of SARS-CoV-2 in raw sewage. J. Biomol. Tech. 2021;32:206–213. doi: 10.7171/jbt.21-32-03-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao H., Mao K., Ran F., Xu P., Zhao Y., Zhang X., Zhou H., Yang Z., Zhang H., Jiang G. Paper device combining CRISPR/Cas12a and reverse-transcription loop-mediated isothermal amplification for SARS-CoV-2 detection in wastewater. Environ. Sci. Technol. 2022;56:13245–13253. doi: 10.1021/acs.est.2c04727. [DOI] [PubMed] [Google Scholar]
- 123.Shrestha S., Malla B., Haramoto E. High-throughput microfluidic quantitative PCR system for the simultaneous detection of antibiotic resistance genes and bacterial and viral pathogens in wastewater. Environ. Res. 2024;255:119156. doi: 10.1016/j.envres.2024.119156. [DOI] [PubMed] [Google Scholar]
- 124.Rao G., Capone D., Zhu K., Knoble A., Linden Y., Clark R., Lai A., Kim J., Huang C.H., Bivins A., et al. Simultaneous detection and quantification of multiple pathogen targets in wastewater. PLoS Water. 2024;3:e0000224. doi: 10.1371/journal.pwat.0000224. [DOI] [Google Scholar]
- 125.Zheng X., Deng Y., Xu X., Li S., Zhang Y., Ding J., On H.Y., Lai J.C.C., Yau C., Chin A.W.H., et al. Comparison of virus concentration methods and RNA extraction methods for SARS-CoV-2 wastewater surveillance. Sci. Total Environ. 2022;824:153687. doi: 10.1016/j.scitotenv.2022.153687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sapula S.A., Whittall J.J., Pandopulos A.J., Gerber C., Venter H. An optimized and robust PEG precipitation method for detection of SARS-CoV-2 in wastewater. Sci. Total Environ. 2022;785:147270. doi: 10.1016/j.scitotenv.2021.147270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hjelmsø M.H., Hellmér M., Fernandez-Cassi X., Timoneda N., Lukjancenko O., Seidel M., Elsässer D., Aarestrup F.M., Löfström C., Bofill-Mas S., et al. Evaluation of Methods for the Concentration and Extraction of Viruses from Sewage in the Context of Metagenomic Sequencing. PLoS ONE. 2017;12:e0170199. doi: 10.1371/journal.pone.0170199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nour I., Hanif A., Alanazi F., Zakri A.M., Al-Ashkar I., Alhetheel A., Eifan S. Evaluation of three different concentration and extraction methods for recovery efficiency of human adenovirus and human rotavirus virus A. J. Virol. Methods. 2021;295:114212. doi: 10.1016/j.jviromet.2021.114212. [DOI] [PubMed] [Google Scholar]
- 129.Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755:142939. doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iqbal M., Tao Y., Xie S., Zhu Y., Chen D., Wang X., Huang L., Peng D., Sattar A., Shabbir M.A., et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Direct. 2016;18:18. doi: 10.1186/s12575-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kevill J.L., Kevill J.L., Pellett C., Farkas K., Brown M.R., Bassano I., Denise H., McDonald J.E., Malham S.K., Porter J., et al. A comparison of precipitation and filtration-based SARS-CoV-2 recovery methods and the influence of temperature, turbidity, and surfactant load in urban wastewater. Sci. Total Environ. 2021;808:151916. doi: 10.1016/j.scitotenv.2021.151916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Polo D., Quintela-Baluja M., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde J.L. Making waves: Wastewater-based epidemiology for COVID-19—Approaches and challenges for surveillance and prediction. Water Res. 2020;186:116404. doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wehrendt D.P., Massó M.G., Gonzales Machuca A., Vargas C.V., Barrios M.E., Campos J., Costamagna D., Bruzzone L., Cisterna D.M., Iglesias N.G., et al. A rapid and simple protocol for concentration of SARS-CoV-2 from sewage. J. Virol. Methods. 2021;297:114272. doi: 10.1016/j.jviromet.2021.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adachi Katayama Y., Hayase S., Ando Y., Kuroita T., Okada K., Iwamoto R., Yanagimoto T., Kitajima M., Masago Y. COPMAN: A novel high-throughput and highly sensitive method to detect viral nucleic acids including SARS-CoV-2 RNA in wastewater. Sci. Total Environ. 2022;856:158966. doi: 10.1016/j.scitotenv.2022.158966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ando H., Iwamoto R., Kobayashi H., Okabe S., Kitajima M. The Efficient and Practical virus Identification System with ENhanced Sensitivity for Solids (EPISENS-S): A rapid and cost-effective SARS-CoV-2 RNA detection method for routine wastewater surveillance. Sci. Total Environ. 2022;843:157101. doi: 10.1016/j.scitotenv.2022.157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barril P.A., Pianciola L.A., Mazzeo M., Ousset M.J., Jaureguiberry M.V., Alessandrello M., Sánchez G., Oteiza J.M. Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewater. Sci. Total Environ. 2021;756:144105. doi: 10.1016/j.scitotenv.2020.144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mondal S., Feirer N., Brockman M., Preston M.A., Teter S.J., Ma D., Goueli S.A., Moorji S., Saul B., Cali J.J. A direct capture method for purification and detection of viral nucleic acid enables epidemiological surveillance of SARS-CoV-2. Sci. Total Environ. 2021;795:148834. doi: 10.1016/j.scitotenv.2021.148834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Peinado B., Martínez-García L., Martínez F., Nozal L., Sánchez M.B. Improved methods for the detection and quantification of SARS-CoV-2 RNA in wastewater. Sci. Rep. 2022;12:7201. doi: 10.1038/s41598-022-11187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Karthikeyan S., Ronquillo N., Belda Ferre P., Alvarado D., Javidi T., Longhurst C.A., Knight R. High-throughput wastewater SARS-CoV-2 detection enables forecasting of community infection dynamics in San Diego County. mSystems. 2021;6:e00045-21. doi: 10.1128/msystems.00045-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Whitney O.N., Kennedy L.C., Fan V.B., Hinkle A., Kantor R., Greenwald H., Crits-Christoph A., Al-Shayeb B., Chaplin M., Maurer A.C., et al. Sewage, Salt, Silica, and SARS-CoV-2 (4S): An Economical Kit-Free Method for Direct Capture of SARS-CoV-2 RNA from Wastewater. Environ. Sci. Technol. 2021;55:4880–4888. doi: 10.1021/acs.est.0c08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Brien M.B., Rundell Z.C., Nemec M.D., Langan L.M., Back J.A., Lugo J.N. A Comparison of Four Commercially Available RNA Extraction Kits for Wastewater Surveillance of SARS-CoV-2 in a College Population. Sci. Total Environ. 2021;801:149595. doi: 10.1016/j.scitotenv.2021.149595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boehm A.B., Wolfe M.K., Wigginton K.R., Bidwell A., White B.J., Hughes B., Duong D., Chan-Herur V., Bischel H.N., Naughton C.C. Human viral nucleic acids concentrations in wastewater solids from Central and Coastal California, USA. Sci. Data. 2023;10:396. doi: 10.1038/s41597-023-02297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G. Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 2021;758:143870. doi: 10.1016/j.scitotenv.2020.143870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu Y., Yu J., Pabbaraju K., Lee B.E., Gao T., Ashbolt N.J., Hrudey S.E., Diggle M., Tipples G., Maal-Bared R., et al. Validating and optimizing the method for molecular detection and quantification of SARS-CoV-2 in wastewater. Sci. Total Environ. 2022;812:151434. doi: 10.1016/j.scitotenv.2021.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Olesen S.W., Imakaev M., Duvallet C. Making waves: Defining the lead time of wastewater-based epidemiology for COVID-19. Water Res. 2021;202:117433. doi: 10.1016/j.watres.2021.117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailey A.L., Ledeboer N., Burnham C.D. Clinical Microbiology Is Growing Up: The Total Laboratory Automation Revolution. Clin. Chem. 2017;65:634–643. doi: 10.1373/clinchem.2017.274522. [DOI] [PubMed] [Google Scholar]
- 147.Croxatto A., Dijkstra K., Prod’hom G., Greub G. Comparison of Inoculation with the InoqulA and WASP Automated Systems with Manual Inoculation. J. Clin. Microbiol. 2015;53:2298–2307. doi: 10.1128/JCM.03076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iversen J., Stendal G., Gerdes C.M., Meyer C.H., Andersen C.Ø., Frimodt-Møller N. Comparative Evaluation of Inoculation of Urine Samples with the Copan WASP and BD Kiestra InoqulA Instruments. J. Clin. Microbiol. 2016;54:328–332. doi: 10.1128/JCM.01718-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Culbreath K., Piwonka H., Korver J., Noorbakhsh M. Benefits derived from full laboratory automation in microbiology: A tale of four laboratories. J. Clin. Microbiol. 2021;59:e01969-20. doi: 10.1128/JCM.01969-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karthikeyan S., Nguyen A., McDonald D., Zong Y., Ronquillo N., Ren J., Zou J., Farmer S., Humphrey G., Henderson D., et al. Rapid, large-scale wastewater surveillance and automated reporting system enable early detection of nearly 85% of COVID-19 cases on a university campus. mSystems. 2021;6:e00793-21. doi: 10.1128/msystems.00793-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dehghan Banadaki M., Torabi S., Strike W.D., Noble A., Keck J.W., Berry S.M. Improving wastewater-based epidemiology performance through streamlined automation. J. Environ. Chem. Eng. 2023;11:109595. doi: 10.1016/j.jece.2023.109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.West N.W., Vasquez A.A., Bahmani A., Khan M.F., Hartrick J., Turner C.L., Shuster W., Ram J.L. Sensitive detection of SARS-CoV-2 molecular markers in urban community sewersheds using automated viral RNA purification and digital droplet PCR. Sci. Total Environ. 2022;847:157547. doi: 10.1016/j.scitotenv.2022.157547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riberio M.H.D.M., da Silva R.G., Mariani V.C., Coelho L.D.S. Short-term forecasting COVID-19 cumulative confirmed cases: Perspectives for Brazil. Chaos Solitons Fractals. 2020;135:109853. doi: 10.1016/j.chaos.2020.109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vaughan L., Zhang M., Gu H., Rose J.B., Naughton C.C., Medema G., Allan V., Roiko A., Blackall L., Zamyadi A. An exploration of challenges associated with machine learning for time series forecasting of COVID-19 community spread using wastewater-based epidemiological data. Sci. Total Environ. 2023;858:159748. doi: 10.1016/j.scitotenv.2022.159748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang G., Wu J., Weidhaas J., Li X., Chen Y., Mueller J., Li J., Kumar M., Zhou X., Arora S., et al. Artificial neural network-based estimation of COVID-19 case numbers and effective reproduction rate using wastewater-based epidemiology. Water Res. 2022;218:118451. doi: 10.1016/j.watres.2022.118451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McMahan C., Self S., Rennert L., Kalbaugh C., Kriebel D., Graves D., Colby C., Deaver J., Popat S., Karanfil T. COVID-19 wastewater epidemiology: A model to estimate infected populations. Lancet Planet Health. 2021;5:874–881. doi: 10.1016/S2542-5196(21)00230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Safford H.R., Shapiro K., Bischel H.N. Opinion: Wastewater analysis can be a powerful public health tool-if it’s done sensibly. Proc. Natl. Acad. Sci. USA. 2022;119:e2119600119. doi: 10.1073/pnas.2119600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guerrero-Latorre L., Collado N., Abasolo N., Anzaldi G., Bofill-Mas S., Bosch A., Bosch L., Busquets S., Caimari A., Canela N., et al. The Catalan Surveillance Network of SARS-CoV-2 in Sewage: Design, implementation, and performance. Sci. Rep. 2022;12:16704. doi: 10.1038/s41598-022-20957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ngwira L.G., Sharma B., Shrestha K.B., Dahal S., Tuladhar R., Manthalu G., Chilima B., Ganizani A., Rigby J., Kanjerwa O., et al. Cost of wastewater-based environmental surveillance for SARS-CoV-2: Evidence from pilot sites in Blantyre, Malawi and Kathmandu, Nepal. PLoS Glob. Public Health. 2022;2:e0001377. doi: 10.1371/journal.pgph.0001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoo B.K., Iwamoto R., Chung U., Sasaki T., Kitajima M. Economic evaluation of wastewater surveillance combined with clinical COVID-19 screening tests, Japan. Emerg. Infect. Dis. 2023;29:1608–1617. doi: 10.3201/eid2908.221775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Betancourt W.Q., Schmitz B.W., Innes G.K., Prasek S.M., Pogreba Brown K.M., Stark E.R., Foster A.R., Sprissler R.S., Harris D.T., Sherchan S.P., et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total Environ. 2021;779:146408. doi: 10.1016/j.scitotenv.2021.146408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Price B.L., Gilbert P.B., van der Laan M.J. Estimation of the optimal surrogate based on a randomized trial. Biometrics. 2018;74:1271–1281. doi: 10.1111/biom.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barceló D. Wastewater-Based Epidemiology to monitor COVID-19 outbreak: Present and future diagnostic methods to be in your radar. Case Stud. Chem. Eng. 2020;2:100042. doi: 10.1016/j.cscee.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Belouhova M., Peykov S., Stefanova V., Topalova Y. Comparison of Two Methods for SARS-CoV-2 Detection in Wastewater: A Case Study from Sofia, Bulgaria. Water. 2023;15:658. doi: 10.3390/w15040658. [DOI] [Google Scholar]